Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
Cost-Effectiveness Of Culture-Based Versus Empirical Antibiotic Treatment For Hospitalized Adults With Community-Acquired Pneumonia In Indonesia: A Real-World Patient-Database Study
Authors Purba AKR , Ascobat P, Muchtar A, Wulandari L , Dik JW , d'Arqom A , Postma MJ
Received 25 July 2019
Accepted for publication 26 September 2019
Published 29 November 2019 Volume 2019:11 Pages 729—739
DOI https://doi.org/10.2147/CEOR.S224619
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Video abstract presented by Abdul Khairul Rizki Purba.
Views: 141
Abdul Khairul Rizki Purba,1–5 Purwantyastuti Ascobat,3 Armen Muchtar,3 Laksmi Wulandari,6 Jan-Willem Dik,4 Annette d’Arqom,2,7 Maarten J Postma1,2,5,8
1Unit of Global Health, Department of Health Sciences, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; 2Department of Pharmacology and Therapy, Faculty of Medicine, Universitas Airlangga-Soetomo Hospital, Surabaya, Indonesia; 3Department of Pharmacology and Therapeutics, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia; 4Department of Medical Microbiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; 5Department of Pharmacy, Unit of Pharmacotherapy, -Epidemiology And -Economics (PTE2), University of Groningen, Groningen, The Netherlands; 6Department of Pulmonology and Respiratory Medicine, Faculty of Medicine, Universitas Airlangga-Soetomo Hospital, Surabaya, Indonesia; 7Faculty of Science, Faculty of Medicine Ramatibodhi Hospital, Faculty of Dentistry, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand; 8Department of Economics, Econometrics and Finance, University of Groningen, Faculty of Economics & Business, University of Groningen, Groningen, The Netherlands
Correspondence: Abdul Khairul Rizki Purba
Universitair Medisch Centrum Groningen, Hanzeplein 1, Groningen, RB 9700, The Netherlands
Tel +31 697 52 411
Email [email protected]
Objective: This study analyzes the cost-effectiveness of culture-based treatment (CBT) versus empirical treatment (ET) as a guide to antibiotic selection and use in hospitalized patients with community-acquired pneumonia (CAP).
Patients and methods: A model was developed from the individual patient data of adults with CAP hospitalized at an academic hospital in Indonesia between 2014 and 2017 (ICD-10 J.18x). The directed antibiotic was assessed based on microbiological culture results in terms of the impact on hospital costs and life expectancy (LE). We conducted subgroup analyses for implementing CBT and ET in adults under 60 years, elderly patients (≥ 60 years), moderate-severe CAP (PSI class III-V) cases, and ICU patients. The model was designed with a lifetime horizon and adjusted patients’ ages to the average LE of the Indonesian population with a 3% discount each for cost and LE. We applied a sensitivity analyses on 1,000 simulation cohorts to examine the economic acceptability of CBT in practice. Willingness to pay (WTP) was defined as 1 or 3 times the Indonesian GDP per capita (US$ 3,570).
Results: CBT would effectively increase the patients’ LE and be cost-saving (dominant) as well. The ET group’s hospitalization cost had the greatest influence on economic outcomes. Subgroup analyses showed that CBT’s dominance remained for Indonesian patients aged under 60 years or older, patients with moderate-severe CAP, and patients in the ICU. Acceptability rates of CBT over ET were 74.9% for 1xWTP and 82.8% for 3xWTP in the base case.
Conclusion: Both sputum and blood cultures provide advantages for cost-saving and LE gains for hospitalized patients with CAP. CBT is cost-effective in patients all ages, PSI class III or above patients, and ICU patients.
Keywords: microbiological culture, empirical treatment, life expectancy, cost-effectiveness, community-acquired pneumonia
Introduction
Community-acquired pneumonia (CAP) is an infectious disease with a clinical severity ranging from mild to life-threatening. CAP has high mortality rates and is costly to treat.1 In the US in 2012, the total direct medical costs related to CAP were estimated at US$ 7,220 to US$ 11,443.2 In European countries, the cost was estimated to be between €1,201 and €1,586 per patient. These high costs have been partially attributed to multiple antibiotic resistance.3
Established international guidelines of the British Thoracic Society (BTS), American Thoracic Society (ATS), and National Institute for Health and Care Excellence (NICE) concur that empirical treatment of CAP with antibiotics is urgent considering the elevated mortality rates, the culture timing, and unspecified results from clinical and radiology methods for determining bacterial infections.4–6 Additionally, abstaining from microbiological evaluation in clinical practices for empirical treatment of CAP patients potentially leads to antibiotic resistance and overuse, which indirectly contributes to hospitalization costs.7–9 At present, the implementation of rapid, advanced molecular microbiology methods for CAP diagnostics has largely been developed.10 The extended use of these promising novel approaches, however, remains unavailable and costly in low-middle income countries (LMICs), which carry higher burdens of the disease. Controversies surrounding the recommendation for the use of microbiology tests in mild-moderate hospitalized cases and outpatient CAP management have been described by previous research since clinical improvement could be achieved by empirical antibiotics alone.11,12 The evaluation of sputum cultures from respiratory samples is recommended for inpatient CAP treatment, aiming to select appropriate antibiotics and prevent further antibiotic resistance.4,6,13
When immediately employed, early directed therapy after evaluating culture results is an essential strategy to prevent high rates of antibiotic resistance. The implementation of culture analysis in developing countries with a high prevalence of CAP, such as Indonesia, should be considered alongside the assessment of culture analysis impact on costs and patient life expectancy (LE). Since 2014, Indonesia has employed a new universal health coverage program, which the health ministry contracted to a single public insurance company (Badan Penyelenggara Jaminan Sosial or BPJS). The universal health coverage also involves the implementation of a national social security system in managing expenditure related to reimbursement and costing in the national healthcare system, inclusive of cost-effectiveness considerations. Hitherto, the lack of evidence for cost-effectiveness has hampered the implementation of culture analysis as a diagnostic tool guiding the management of CAP. Therefore, the culture analysis approach has rarely been adopted in hospitals or in communities. Considering the current limitations of the evidence regarding the value of culture analysis prior to empirical-based antibiotic administration in CAP patients, we performed a cost-effectiveness analyses on the implementation of culture-based treatment (CBT) compared to empirical treatment only (ET) in hospitalized CAP patients.
Materials And Methods
Decision-Tree Model Overview
A decision-tree model was designed from individual patient data of hospitalized CAP patients in Indonesia. The model is shown in Figure 1. We built the model based on a payer perspective. The decision-tree compared two options: i) patients with empirical antibiotic treatment (ET) during hospitalization and ii) patients with CBT for whom medications were adjusted based on microbiological evaluation in terms of the culture and antimicrobial susceptibility tests. The culture analysis was conducted on samples from respiratory tracts (sputum) and blood draws. One package was produced for positive cultures demonstrating antimicrobial susceptibility after identification of the microorganisms. We assumed that the level of competencies and advances of the laboratory staff were equal in handling the specimens and reporting the results. The CBT group were treated with empirical antibiotics based on the Indonesian guidelines at 24 hrs post-admission. Thereafter, culture results were provided, and the patients were treated with the directed therapy involving a second round of antibiotics.14 The second round of antibiotics could be different from the previous empirical treatment (antibiotic change or AC) or be the same as (no antibiotic change or NC). We included in the AC group patients with negative cultures with no microorganism growth, for whom the physicians subsequently stopped the use of antibiotics. In the ET group, we assumed that the treatment using antibiotics was empirical during hospitalization. The termination states of the decision-tree for both CBT and ET were presented as recovery or death. Patients with no sputum available for culture tests were assumed to undergo empirical therapy during hospitalization, and we included such patients in the ET group. As there was no specific indication to the physician examining the culture of patients’ sputum, we assumed that the decision to perform ET or CBT was random.
 |
Figure 1 Decision-tree model of the culture-based treatment (CBT) and empirical treatment (ET) groups in the management of CAP. |
Data Input
Individual patient data were derived from 351 retrospective records of adult patients admitted on referral to the academic hospital of Dr. Soetomo, Indonesia, from 2014 to 2017. This hospital is a central hospital for the eastern part of Indonesia and has roughly 1,514 beds. The Center of Research and Development of Dr. Soetomo Hospital, under the ethical committee, approved the study proposal with no. 480/Panke.KKE/X/2014. The committee decided that the study did not require a review in terms of patient consent since the study was performed with retrospective observational design. We obtained the clinical data from the Department of Medical Record and all relevant costs from the Finance Department, Dr. Soetomo Hospital. The data were anonymous and confidentially. The study met the agreement with Indonesian research conduct and the Declaration of Helsinki (Ethical Principles for Medical Research Involving Human Subject version 2013).15
We included hospitalized CAP cases that had both blood and sputum culture with a pneumonia severity index (PSI) class from I to V. We input data of patients whose average age was 57.6 years (min-max: 19–91 years); they were predominantly males (78.3%). We performed subgroup analyses for the implementation of CBT on patients under 60 and aged ≥60, PSI class III and above, and in the intensive care unit (ICU). We defined the age of 60 as the breakpoint for the aging population in Indonesia, according to the United Nations Population Fund (UNFPA 2014).16 We addressed PSI class III in subgroup analyses since this cutoff increases the risk of 30-day mortality by ten times compared to class II.17
Calculation Of The Outcomes
We assessed the LE outcome in our model by adjusting the patients’ ages to the average LE for the Indonesian population between 2010 and 2015 using data provided by the United Nations Department of Economic and Social Affairs, Population Division, 2017.18 The average LE for each group of these ages for men and women is separately presented in Figure 2. The model-time horizon was the lifetime where recovery patients would have LE greater than their ages. Considering the discount rate launched by the Health Ministry of Indonesia, we applied a 3% discount each for costs and LE.19
 |
Figure 2 Average remaining life expectancy of Indonesian males and females for the patient model (calculated from the United Nations Department of Economic and Social Affairs, Population Division, 2017. World Population Prospects).17 |
Hospitalization costs were valued in US$ with 2016 adjustments. The conversion rate to an Indonesia Rupiah (IDR) was equal to US$ 13,308.33 according to the Organization for Economic Cooperation and Development (OECD).20 The costs from data were formulated as C, which is calculated from the average of the individual cost of administration (Ca), pharmacy costs (Cp), laboratory and radiology (Cl) expenses, cost of bed (Cb), and medical staff costs (Cm). The incremental cost-effectiveness ratio (ICER) in terms of total hospitalization costs and LE was calculated with the following Formula 1 below. Notably, in case the numerator was negative (cost-saving), and the denominator was positive (improving LE), then the ICER indicated dominant.
Formula 1 Incremental cost-effectiveness ratio (ICER) calculation based on Ca, Cp, Cl, Cb, and Cm
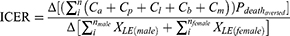
Table 1 shows the costs and LE employed gamma and exponential distribution, respectively. According to the World Health Organization – Choosing Interventions for Cost-Effectiveness criterion (WHO-CHOICE), a particular intervention is highly cost-effective when the ICER is less than the willingness-to-pay (WTP), defined as the Indonesian cost-effectiveness threshold of gross domestic product (GDP) per capita. If the ICER is between 1 and 3 times the GDP per capita, then the intervention is cost-effective.21 This definition was also applied in a previous study which was performed in Indonesia.22 For these thresholds, we applied the Indonesian GDP per capita in 2016 at US$ 3,570.23
 |
Table 1 The Total Hospitalization Costs And Life-Expectancy Parameters For The Decision-Tree Model |
Sensitivity Analyses
We performed one-way tornado sensitivity analyses using TreeAge Pro 2019 to address the robustness of the model ICERs on the changes of the upper and lower limits of the parameter values. The parameters included in the test were costs and the probability of death being averted. The outcome of LE was conclusive as it was based on the data presented by WHO for the Indonesian population.18 We also conducted random probabilistic sensitivity analyses using a Monte Carlo simulation with 1,000 random cohorts on the respective corresponding distributions. We addressed a cost-effectiveness acceptability curve to draw relationships between ICER and the Indonesian affordability threshold. Indeed a relationship between the probabilistic sensitivity analysis (PSA) and univariate analysis exists in terms of input. Notably, we used the range for the input in the PSA and in the univariate analysis.
Results
Baseline Characteristic Of The Patients
Concerning the change of antibiotics after culture analysis among patients in the CBT group, we determined that the AC and NC probabilities were 55.8% and 44.2%, respectively. The probability of antibiotic changes was based on microbiological evaluations and was not influenced by age or severity. This was subsequently analyzed with beta distributions. Patients’ baseline characteristics of the patients and the probabilities of antibiotic changes and deaths being averted in the AC, NC, and ET groups are presented in Table 2.
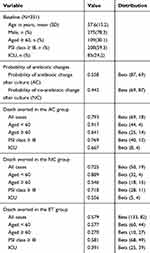 |
Table 2 Baseline Variables And Probabilities For The Inputs On The Decision-Tree Model And Univariate Analysis From Patient Data |
Cost-Effectiveness Analyses
The results of cost-effectiveness analyses are shown in Table 3. The implementation of CBT over ET from a payer perspective was dominant, where CBT was cost-saving (US$ 1,066,885) and improved LE (247 years) in all cases. In subgroup analyses, CBT was the most cost-saving, with an incremental cost of US$ 3,828,006 for adults 60 years or older. Moreover, in the elderly group (aged ≥ 60), in terms of health effects, it was found that 637 life-years were saved by CBT versus ET. Even in the other categorizations, CBT performed better for both hospitalization costs and LE outcomes for patients with PSI class ≥ III or ICU patients, and the cost-effectiveness outcomes were cost-saving (dominant) compared to ET.
 |
Table 3 Cost-Effectiveness Ratio For ET Versus CBT In 1,000 Patients With CAP |
Univariate And Probabilistic Sensitivity Analyses
Hospitalization costs for the ET group are shown on the top of the Tornado diagrams (Figure 3), which indicate that they had the strongest effect on the ICERs out of all parameters. When hospitalization costs for the ET group increased, the ICERs decrease. In contrast, when hospitalization costs for AC and NC groups (CBT) increased, the ICERs correspondingly increased. The parameters of death averted in the CBT and ET groups were only slightly influential on ICER calculations, especially in patients with moderate-severe CAP (PSI class III-V). Tornado diagram evaluation is also provided in calculation results in Supplementary Table S1.
The results of probabilistic sensitivity analyses on ICER values with 1,000 cohorts (Monte-Carlo simulations) between CBT and ET are shown in Figure 4. Correspondingly, Figure 5 shows the acceptability probabilities at varying WTP levels. In all cases, the probabilities of cost-effectiveness acceptability in 1xWTP and 3xWTP were 75% and 83%, respectively. In the subgroup analyses of patients all ages with moderate to severe CAP (PSI class ≥ III), and in the ICU, CBT would be a cost-effective or even cost-saving approach compared to 1xGDP per capita (US$ 3,570). CBT for patients 60 years or older represented the most cost-effective option, with acceptability values between 81% for 1xWTP and 89% for 3xWTP. The results of calculations of Monte-Carlo simulations and cost-effectiveness acceptability are available in Supplementary Table S2 and S3, respectively.
Discussion
Our findings demonstrate that treatment based on the evaluation of cultures followed by antimicrobial susceptibility testing (CBT) has advantages in terms of both cost and LE for CAP patients. CBT was dominant, not exceeding the GDP threshold in terms of cost-effectiveness (three times GDP) and even being cost-saving. Therefore, this method can reasonably be implemented for adults in general hospitals in Indonesia. Without culture analysis, treatments for CAP are more expensive and result in lost life-years, particularly for geriatric patient cases and high-severity classes of patients.24–28 Notably, the incidence of CAP among elderly people is high since they frequently present with pre-existing immunosuppressive conditions and comorbid conditions.29,30 According to ATS/BTS, the severity of CAP infection among males was higher than females.31 This is the reason that males need hospitalization more than females, as reflected in the input data for our study. Also, some previous prospective studies demonstrated this issue.31–33
In non-ICU hospitalization, culture analysis is one of the procedures recommended by the Infectious Diseases Society of America (IDSA) and ATS to guide antibiotic use for CAP patients.4 The implementation of the guidelines with the threshold of US$ 100,000/QALY (quality-adjusted life years) leads to cost-savings of US$ 799 to US$ 1,379 per patient and improved quality of life, particularly in elderly people.34 In the ICU, it is essential to considered CBT implementation since the use of antibiotic is costly. A previous study conducted in an LMIC reported that antibiotic utilization for CAP patients in ICU was determined as the highest proportion (38%) of total hospitalization costs.35
The assessment of severity using the PSI score resulted in higher sensitivity of sputum culture analyses. Gram staining and sputum culture procedures are very useful for inpatient treatment plans and ICU patients, guiding CAP diagnosis and antimicrobial selection.36 The organisms from the microbiological culture are more likely to accurately represent respiratory tract pathogens in CAP patients.37 Furthermore, the implementation of blood and sputum culture analysis should be considered in patients with moderate and severe CAP with poor clinical prognosis and who are considerably susceptible to bacteremia.38,39
Culture analysis is part of diagnostic stewardship that supports antimicrobial identification and infection prevention programs. CBT should be part of such integrated management within a theragnostic approach, involving multi-disciplines, and leading personalized management, improvement of prescriptions for treatment, improved clinical outcomes, and decreasing costs.40,41 An issue of concern is the potential contamination of biological specimens. Contaminated blood and sputum cultures could extend costs and have adverse effects.42,43 The role of the clinical microbiologist is critical at this point for screening assessments of the quality of the sample. Recently, several advanced methods to reduce contaminations have been developed, such as matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) and multiplex nucleic acid amplification test (NAAT).44,45 However, these methods are costly.
To our knowledge, this study is the first cost-effectiveness analysis for culture analysis in CAP patients considering real-world data and the impact of the effect of culture analysis in terms of changes in antibiotic treatments, particularly in an LMIC setting. The utility impact was accounted for the overall population as well as relevant subgroups of patients aged ≥ 60, with PSI class ≥ III and in the ICU. This approach provided the necessary level of granularity. However, this study has some limitations. First, community data from primary healthcare was not available. There is a lack of sample data related to CAP in the community since facilities for clinical diagnostics and bacterial identification remain unavailable, and these are not routine procedures, especially in remote areas. In clinical practice, we assumed randomness in the decision to do ET or CBT, as confirmed by the clinicians. However, we could not exclude that in some specific cases some aspect, may still have influential this decision and so probably introducing some bias in our analysis. Second, this study used a retrospective dataset; patients with incomplete data related to ICER outcomes could not be analyzed. In further investigations comparing the effects of treatments, randomized controlled clinical trials are necessary to obtain additional results. As such, our study can be considered as hypothesis generating rather than providing final evidence. Lastly, we did not include costs associated with the side effects of the treatments in the present research. Common undesirable side effects of antibiotic treatment, such as diarrhea, Clostridium difficile infections, and allergic reactions can extend the length of hospitalization and increase costs.46
Conclusion
In conclusion, CAP patients undergoing treatments based on culture analyses (CBT) were estimated to receive benefits in terms of cost-savings and increased LE than those with empirical treatment (ET) during their hospitalization; CBT was labeled dominant compared to ET. Notably, the implementation of CBT provides considerable advantages for patients all ages as well as for higher severity classes. Most notably in severe CAP cases, cultures can adequately guide antibiotic selection for patients with PSI class III or above or who are hospitalized in ICUs.
Abbreviations
AC, antibiotic change; ATS, American Thoracic Society; BTS, British Thoracic Society; Ca, cost of administration; CAP, Community-Aquired Pneumonia; Cb, cost for bed; CBT, Culture-based treatment; Cl, costs for laboratory and radiology; Cm, costs for medical staff; Cp, costs for pharmacy; ET, Empirical treatment; GDP, Gross Domestic Product; ICD, International Classification of Diseases; ICER, Incremental Cost Effectiveness Ratio; ICU, Intensive Care Unit; IDSA, Infectious Diseases Society of America; LE, Life Expectancy; LMICs, Low-Middle Income Countries; MALDI-TOF, Matrix-Assisted Laser Desorption/Ionization-Time Of Flight; NAAT, Nucleic Acid Amplification Test; NC, no antibiotic change; NICE, National Institute for Health and Care Excellence; OECD, Organization for Economic Cooperation and Development; PSI, Pneumonia Severity Index; QALY, Quality-Adjusted Life Years; UNFPA, United Nations Population Fund; WHO, World Health Organization; WHO-CHOICE, World Health Organization – Choosing Interventions for Cost-Effectiveness criterion; WTP, Willingness-to-Pay.
Acknowledgments
We would like to thank Simon van der Pol who was involved in discussions on LE calculations of the patients. The work was supported by a grant from the Directorate General of Higher Education (DIKTI), Ministry of Research, Technology and Higher Education of the Republic of Indonesia [No.224/D3.2/PG/2016].
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Outside of this work, Prof. Postma reports receiving various grants and honoraria from both public (EU, WHO) and private (pharmaceutical companies) parties. Prof. Postma is also shareholder of Pharmacoeconomics Advice Groningen and Ingress Health, and advisor to Asc Academics Groningen and Health-Ecore. The other authors report no conflicts of interest in this work.
References
1. Feldman C, Anderson R. Community-acquired pneumonia: still a major burden of disease. Curr Opin Crit Care. 2016;22(5):477–484. doi:10.1097/MCC.0000000000000340
2. Bonafede MM, Suaya JA, Wilson KL, Mannino DM, Polsky D. Incidence and cost of CAP in a large working-age population. Am J Manag Care. 2012;18(7):380–387.
3. Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–79. doi:10.1136/thx.2009.129502
4. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi:10.1086/511159
5. Eccles S, Pincus C, Higgins B, et al. Diagnosis and management of community and hospital acquired pneumonia in adults: summary of NICE guidance. BMJ. 2014;349(December):1–5. doi:10.1136/bmj.g6722
6. Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(Suppl 3):iii1–iii55. doi:10.1136/thx.2009.121434
7. Martin M, Moore L, Quilici S, Decramer M, Simoens S. A cost-effectiveness analysis of antimicrobial treatment of community-acquired pneumonia taking into account resistance in Belgium. Curr Med Res Opin. 2008;24(3):737–751. doi:10.1185/030079908X273336
8. Martin M, Quilici S, File T, Garau J, Kureishi A, Kubin M. Cost-effectiveness of empirical prescribing of antimicrobials in community-acquired pneumonia in three countries in the presence of resistance. J Antimicrob Chemother. 2007;59(5):977–989. doi:10.1093/jac/dkm033
9. Kuti JL, Capitano B, Nicolau DP. Cost-effective approaches to the treatment of community-acquired pneumonia in the era of resistance. Pharmacoeconomics. 2002;20(8):513–528. doi:10.2165/00019053-200220080-00002
10. Torres A, Lee N, Cilloniz C, Vila J, Van der Eerden M. Laboratory diagnosis of pneumonia in the molecular age. Eur Respir J. 2016;48(6):1764–1778. doi:10.1183/13993003.01144-2016
11. Lee JS, Primack BA, Mor MK, et al. Processes of care and outcomes for community-acquired pneumonia. Am J Med. 2011;124(12):
12. Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet (London, England). 2015;386(9998):1097–1108. doi:10.1016/S0140-6736(15)60733-4
13. Woodhead M. New guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2011;38(6):1250–1251. doi:10.1183/09031936.00105211
14. Indonesian Society of Respirology. Guideline for diagnosis and management of community pneumonia in Indonesia. Available from: https://www.scribd.com/doc/125419923/Pnemonia-Komuniti-Pdpi. Published 2003.
15. Ethical principles for medical research involving human subjects version 2013.
16. UNFPA. Indonesia on the threshold of population ageing; 2014. Available from: https://indonesia.unfpa.org/sites/default/files/pub-pdf/BUKU_Monograph_No1_Ageing_03_Low-res.pdf.
17. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118(4):384–392. doi:10.1016/j.amjmed.2005.01.006
18. The World Bank. Life expectancy at birth, total (years). Available from: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=ID. Published 2019.
19. Indonesian Health Technology Assessment Committee (InaHTAC), Ministry of Health of the Republic of Indonesia. Health Technology Assessment (HTA) Guideline. Available from: http://adphealth.org/upload/resource/FINAL_HTA_ENG_-1.pdf. Published 2017.
20. Organization for Economic Cooperation and Development (OECD). Available from: https://data.oecd.org/conversion/exchange-rates.htm. Published 2018. Accessed April 22, 2019.
21. Marseille E, Larson B, Kazi DS, Khan JG, Rosen S. Policy and Practice, Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118-124. doi:10.2471/BLT.14.138206
22. Setiawan D, Dolk FC, Suwantika AA, Westra TA, WIlschut JC, Postma MJ. Cost-utility analysis of human papillomavirus vaccination and cervical screening on cervical cancer patient in Indonesia. Value Heal Reg Issues. 2016;9:84–92. doi:10.1016/j.vhri.2015.10.010
23. The World Bank. GDP per capita (current US$). Available from: https://data.worldbank.org/indicator/ny.gdp.pcap.cd?end=2017&start=2016&year_high_desc=false. Published 2019.
24. Olasupo O, Xiao H, Brown JD. Relative clinical and cost burden of community-acquired pneumonia hospitalizations in older adults in the United States-a cross-sectional analysis. Vaccines. 2018;6:3. doi:10.3390/vaccines6030059
25. Konomura K, Nagai H, Akazawa M. Economic burden of community-acquired pneumonia among elderly patients: a Japanese perspective. Pneumonia (Nathan Qld). 2017;9:19. doi:10.1186/s41479-017-0042-1
26. Choi MJ, Song JY, Noh JY, et al. Disease burden of hospitalized community-acquired pneumonia in South Korea: analysis based on age and underlying medical conditions. Medicine (Baltimore). 2017;96(44):e8429. doi:10.1097/MD.0000000000008429
27. Vissink CE, Huijts SM, de Wit GA, Bonten MJM, Mangen M-J-J. Hospitalization costs for community-acquired pneumonia in Dutch elderly: an observational study. BMC Infect Dis. 2016;16:466. doi:10.1186/s12879-016-1783-9
28. Lee JY, Yoo CG, Kim H-J, Jung KS, Yoo KH. Disease burden of pneumonia in Korean adults aged over 50 years stratified by age and underlying diseases. Korean J Intern Med. 2014;29(6):764–773. doi:10.3904/kjim.2014.29.6.764
29. Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68(11):1057–1065. doi:10.1136/thoraxjnl-2013-204282
30. Cilloniz C, Polverino E, Ewig S, et al. Impact of age and comorbidity on cause and outcome in community-acquired pneumonia. Chest. 2013;144(3):999–1007. doi:10.1378/chest.13-0062
31. Falagas ME, Mourtzoukou EG, Vardakas KZ. Sex differences in the incidence and severity of respiratory tract infections. Respir Med. 2007;101(9):1845–1863. doi:10.1016/j.rmed.2007.04.011
32. Gutierrez F, Masia M, Mirete C, et al. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J Infect. 2006;53(3):166–174. doi:10.1016/j.jinf.2005.11.006
33. Rivero-Calle I, Pardo-Seco J, Aldaz P, et al. Incidence and risk factor prevalence of community-acquired pneumonia in adults in primary care in Spain (NEUMO-ES-RISK project). BMC Infect Dis. 2016;16(1):645. doi:10.1186/s12879-016-1974-4
34. Egger ME, Myers JA, Arnold FW, Pass LA, Ramirez JA, Brock GN. Cost effectiveness of adherence to IDSA/ATS guidelines in elderly patients hospitalized for community-aquired pneumonia. BMC Med Inform Decis Mak. 2016;16:34. doi:10.1186/s12911-016-0270-y
35. Kateel R, Adhikari P, Rajm S. Cost and antibiotic utilization of pneumonia patients in intensive care unit. J Appl Pharm. 2016;6(02):87–90. doi:10.7324/JAPS.2016.60212
36. García-Vázquez E, Marcos MA, Mensa J, et al. Assessment of the usefulness of sputum culture for diagnosis of community-acquired pneumonia using the PORT predictive scoring system. Arch Intern Med. 2004;164(16):1807–1811. doi:10.1001/archinte.164.16.1807
37. Afshar N, Tabas J, Afshar K, Silbergleit R. Blood cultures for community-acquired pneumonia: are they worthy of two quality measures? A systematic review. J Hosp Med. 2009;4(2):112–123. doi:10.1002/jhm.382
38. Metersky ML, Ma A, Bratzler DW, Houck PM. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169(3):342–347. doi:10.1164/rccm.200309-1248OC
39. Campbell SG, Marrie TJ, Anstey R, Dickinson G, Ackroyd-Stolarz S. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community-acquired pneumonia: a prospective observational study. Chest. 2003;123(4):1142–1150. doi:10.1378/chest.123.4.1142
40. Dik JH, Poelman R, Friedrich AW, Niesters HGM, Rossen JWA, Sinha B. Integrated Stewardship model comprising antimicrobial, infection prevention, and diagnostic stewardship (AID Stewardship). J Clin Microbiol. 2017;55(11):3306–3307. doi:10.1128/JCM.01283-17
41. Dik J-WH, Hendrix R, Poelman R, et al. Measuring the impact of antimicrobial stewardship programs. Expert Rev Anti Infect Ther. 2016;14(6):569–575. doi:10.1080/14787210.2016.1178064
42. McAdam AJ. Reducing contamination of blood cultures: consider costs and clinical benefits. Clin Infect Dis. 2017;65(2):206–207. doi:10.1093/cid/cix306
43. Alahmadi YM, Aldeyab MA, McElnay JC, et al. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect. 2011;77(3):233–236. doi:10.1016/j.jhin.2010.09.033
44. Altun O, Botero-Kleiven S, Carlsson S, Ullberg M, Ozenci V. Rapid identification of bacteria from positive blood culture bottles by MALDI-TOF MS following short-term incubation on solid media. J Med Microbiol. 2015;64(11):1346–1352. doi:10.1099/jmm.0.000168
45. Food and Drug Administration. Medical devices; immunology and microbiology devices; classification of multiplex nucleic acid assay for identification of microorganisms and resistance markers from positive blood cultures. Final order. Fed Regist. 2015;80(101):30153–30155.
46. Heimann SM, Cruz Aguilar MR, Mellinghof S, Vehreschild MJGT. Economic burden and cost-effective management of Clostridium difficile infections. Med Mal Infect. 2018;48(1):23–29. doi:10.1016/j.medmal.2017.10.010
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



