Back to Journals » International Journal of General Medicine » Volume 9
Cost-effectiveness of amlodipine compared with valsartan in preventing stroke and myocardial infarction among hypertensive patients in Taiwan
Authors Chan L, Chen C, Hwang J, Yeh S, Shyu K, Lin R, Li Y, Liu L, Li J Z , Shau W, Weng T
Received 8 December 2015
Accepted for publication 27 February 2016
Published 31 May 2016 Volume 2016:9 Pages 175—182
DOI https://doi.org/10.2147/IJGM.S102095
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lung Chan,1 Chen-Huan Chen,2 Juey-Jen Hwang,3 San-Jou Yeh,4 Kou-Gi Shyu,5 Ruey-Tay Lin,6 Yi-Heng Li,7 Larry Z Liu,8 Jim Z Li,9 Wen-Yi Shau,10 Te-Chang Weng,10
1Department of Neurology, Shuang-Ho Hospital, School of Medicine, College of Medicine, Taipei Medical University, New Taipei, 2Department of Internal Medicine, Faculty of Medicine, National Yang-Ming University, 3Division of Cardiology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, 4Division of Cardiology, Department of Internal Medicine, Chang Gung Memorial Hospital, Taoyuan, 5Division of Cardiology, Department of Internal Medicine, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, 6Department of Neurology, Kaohsiung Medical University Hospital, Kaohsiung, 7Division of Cardiology, Department of Internal Medicine, National Cheng Kung University Hospital, Tainan, Taiwan; 8Pfizer Inc, New York, NY, USA; 9Pfizer Inc, San Diego, CA ,USA; 10Pfizer Ltd., New Taipei City, Taiwan
Abstract: Hypertension is a major risk factor for strokes and myocardial infarction (MI). Given its effectiveness and safety profile, the calcium channel blocker amlodipine is among the most frequently prescribed antihypertensive drugs. This analysis was conducted to determine the costs and quality-adjusted life years (QALYs) associated with the use of amlodipine and valsartan, an angiotensin II receptor blocker, in preventing stroke and MI in Taiwanese hypertensive patients. A state transition (Markov) model was developed to compare the 5-year costs and QALYs for amlodipine and valsartan. Effectiveness data were based on the NAGOYA HEART Study, local studies, and a published meta-analysis. Utility data and costs of MI and stroke were retrieved from the published literature. Medical costs were based on the literature and inflated to 2011 prices; drug costs were based on National Health Insurance prices in 2014. A 3% discount rate was used for costs and QALYs and a third-party payer perspective adopted. One-way sensitivity and scenario analyses were conducted. Compared with valsartan, amlodipine was associated with cost savings of New Taiwan Dollars (NTD) 2,251 per patient per year: costs were NTD 4,296 and NTD 6,547 per patient per year for amlodipine and valsartan users, respectively. Fewer cardiovascular events were reported in patients receiving amlodipine versus valsartan (342 vs 413 per 10,000 patients over 5 years, respectively). Amlodipine had a net gain of 58 QALYs versus valsartan per 10,000 patients over 5 years. Sensitivity analyses showed that the discount rate and cohort age had a larger effect on total cost and cost difference than on QALYs. However, amlodipine results were more favorable than valsartan irrespective of discount rate or cohort age. When administered to Taiwanese patients for hypertension control, amlodipine was associated with lower cost and more QALYs compared with valsartan due to a lower risk of stroke and MI events.
Keywords: cost-effectiveness, pharmacoeconomic, Markov model, CCB, ARB
Introduction
Hypertension is the leading cause of cardiovascular morbidity and mortality worldwide and has an associated severe economic burden.1 In 2001, ~54% of stroke, 47% of ischemic heart disease, and 25% of other cardiovascular events worldwide were caused by elevated blood pressure.2 By 2025, the worldwide prevalence of hypertension is predicted to be 1.56 billion, an increase of 60% since 2000.3
Myocardial infarction (MI) and stroke are fatal and costly cardiovascular diseases. It is well known that hypertension substantially increases the risk of MI.4 In subjects with moderate hypertension, a small decrease in blood pressure over a period of 3–4 years lowers the incidence of cardiac events by 35%.5 A meta-analysis of randomized controlled trials showed that well-controlled blood pressure in hypertensive individuals was associated with a 30%–40% reduction in the risk of stroke.6,7 A reduction of blood pressure by 10 mmHg in individuals with hypertension has been shown to lower the risk of cardiovascular events ~17% in males and ~30% in females.8
A number of classes of antihypertensive agents with different mechanisms of action are available. The most widely used are thiazide diuretics, angiotensin-converting enzyme inhibitors (ACEIs), calcium channel blockers (CCBs), β-blockers, and angiotensin II receptor blockers (ARBs). ARBs such as losartan and valsartan are relatively newer antihypertensive agents that have improved tolerance and affirmative efficacy. They are primarily prescribed for individuals who are intolerant to ACEIs. ARBs do not adversely affect kidney function, even in subjects with chronic renal insufficiency;9 however, because of their short half-lives, many require twice-daily dosing to maintain blood pressure control. This can substantially increase the costs of treatment.9 Another widely used class of antihypertensives is the CCBs, which are well tolerated. Compared with other classes of antihypertensive drugs, CCBs do not cause withdrawal syndrome, have low associated incidences of drug discontinuation and switching,10 and are especially suitable for elderly hypertensive patients with stable angina pectoris or diabetes mellitus because they can be administered concurrently with other drugs such as antibiotics, nonsteroidal anti-inflammatory drugs, and glucose-lowering agents. CCBs reduce the risk of fatal stroke by 44%–55% and that of stroke-related dementia by 50%.11 CCBs account for upward of 60% of all antihypertensive drugs prescribed in Taiwan.12 Dihydropyridine CCBs, for example, amlodipine and aranidipine, comprise over 85% of all CCBs prescribed.13 Amlodipine is the most frequently prescribed CCB, given its favorable pharmacodynamic and pharmacokinetic properties. It has a long half-life, high bioavailability, and long duration of action, enabling once-daily dosing. Amlodipine reduces the risk of cardiovascular events (including cerebral circulatory disorders) in line with the degree of severity of hypertension.14 Studies in Europe, North America, and the People’s Republic of China showed that use of amlodipine was associated with improved clinical outcome and lower total cost compared with traditional treatments for hypertension or coronary artery disease.15,16 Additionally, amlodipine reduces the number of hospitalizations and need for invasive surgical procedures compared with traditional treatments.
In a study of hypertensive patients in East Asia and the Pacific region, deaths and disability-adjusted life years attributed to stroke comprised the highest cause of all cardiovascular endpoints assessed.2 The prevalence of hypertension in Taiwan is ~22%.17 Taiwan has one of the leading stroke mortality rates in Asia; >7.0% of all deaths are caused by cardiovascular diseases.18 In 2010, the health care cost associated with cardiovascular diseases was New Taiwan Dollars (NTD) 84.9 billion, accounting for 10.3% of the total annual health care spend.19
The cost-effectiveness of amlodipine in the hypertensive population in Taiwan has not been examined. The primary objective of this economic evaluation was to evaluate the cost-effectiveness of amlodipine (5/10 mg) compared with an ARB (valsartan; 80/160 mg) in preventing stroke and MI in the Taiwanese setting (in hypertensive patients with glucose intolerance) from the government payer’s perspective.
Methods
Overview
Risk of MI and stroke in patients receiving valsartan was assessed using the NAGOYA HEART Study,20 sex-specific risk ratio for stroke and MI was assessed using a Taiwanese cohort,21 and odds ratios for stroke and MI in patients receiving amlodipine versus ARBs were obtained from the meta-analysis study by Wang et al.22 Mortality risk was obtained from local studies23–25 and the Ministry of Health and Welfare statistics for 2009.18
A state transition (Markov) model, based on the model used by Wu et al,16 was constructed to compare the expected costs and outcomes of patients treated with amlodipine against those treated with valsartan. Costs of antihypertensive medications were based on the National Health Insurance (NHI) drug reimbursement price in 2014.26 Costs of cardiovascular events, including nonfatal stroke and MI as well as their follow-up management, were based on a local study using longitudinal NHI database.27 Utility data for patients without MI or stroke were obtained from the 2008 population health status data for the People’s Republic of China,28 and for patients with MI or stroke, data was obtained from a systematic review.29
The analysis was carried out from a third-party payer perspective in a Taiwanese setting. Medical costs (ie, MI and stroke-associated costs) were inflated to 2011 prices in NTD. The discount rate applied to both costs and outcomes was 3%.
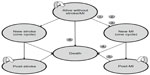
Model structure
Markov modeling is a widely used methodology in pharmacoeconomic studies because of its ability to follow patients as they move between health states over time. As indicated in Figure 1, the Markov model has six main states of health: 1) alive without stroke/MI, 2) MI, 3) post-MI, 4) stroke, 5) post-stroke, and 6) death. In Figure 1, each oval represents a health state with specific associated costs and quality-adjusted life years (QALYs). Costs and QALYs were accrued at the end of each cycle, depending on the health state of the individual at that time point. Each arrow represents a transition from one health state to another with a certain probability. The transitions occur at yearly cycles. The model considered costs and outcomes in a 5-year time period, which was chosen to capture costs and outcomes in a typical time window considered by decision makers for similar chronic conditions while reducing the uncertainty associated with extrapolation to longer terms. The simulation was conducted with 10,000 hypertensive patients in each of the amlodipine and valsartan groups.
The model incorporated the incidence of stroke and MI in the general hypertensive population, and the amlodipine-treated and valsartan-treated populations to estimate the total time spent in different states of health. The demographic profile, including age and sex of the population, was factored into the modeling analysis; thus, the particular risks associated with different age or sex groups were accounted for. The estimation of total time in different states of health was combined with costs and quality-of-life data to calculate the total cost and QALY in each treatment group.
Model inputs
Treatment effects and transition probability
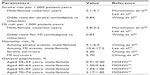
Risk of stroke or MI events in valsartan users was taken from the NAGOYA HEART Study,20 and risk of stroke or MI in males versus females was taken from the Chin-Shan Community Cardiovascular Cohort (see Supplementary materials, Figure S1A and B, for calculations of clinical parameter values).21 The odds ratios for stroke or MI events in patients receiving amlodipine or ARBs was assessed by Wang et al,22 who conducted a meta-analysis to examine the effects of treatment with amlodipine or ARBs for prevention of stroke and MI in patients with hypertension, coronary artery disease, or diabetic nephropathy. Twelve clinical trials that enrolled a total of 94,338 participants were included in the meta-analysis. The risk of stroke and MI was 16% and 17% lower, respectively, among patients taking amlodipine versus those taking ARBs.22 Data on mortality risk after stroke was obtained from a hospital-based study conducted in Taiwan,23 and data after MI was taken from the NHI Research Database study24 and the Taiwan Acute Coronary Syndrome Full Spectrum Registry.25 The overall mortality rate in the Taiwanese population was obtained from Department of Health figures.18 Mortality rates in the general population in different sex- and age-groups were retrieved from statistics provided by the Taiwanese government.18 The risk of stroke, MI, or mortality for each annual cycle was assumed to be the same over the 5-year time period studied. Risks of MI and stroke and mortality rates are summarized in Table 1.
  | Table 1 Summary of clinical data used in the base-case analysis |
Resource use and cost inputs
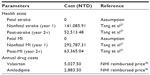
Costs of antihypertensive medications were based on Taiwan NHI reimbursement fee schedule. Event costs for fatal/nonfatal stroke and MI and annual costs of follow-up management of second year and onward were taken from a study using longitudinal NHI database.27 As of December 2014, the NHI prices were NTD 12.5 for valsartan 80 mg (assumed 33% market share) and NTD 14.4 for valsartan 160 mg (67%), with an average cost of NTD 13.8. The price for amlodipine (5 or 10 mg) was NTD 7.9. Annual costs of valsartan and amlodipine were estimated at NTD 5,027.5 and 2,883.5, respectively. Cost estimates are presented in Table 2.
  | Table 2 Summary of direct costs of each health state used in the base-case analysis |
Health utilities inputs
Health utilities were taken from an extensive literature review conducted to identify estimates derived from similar racial/cultural populations with values for specific sex- and age-groups. Health utilities for the Taiwanese population with the health conditions evaluated were not available in the published literature. For health utilities of the elderly population living without stroke or MI, we used the data of the Chinese mainland population.28 Health utilities of post-stroke or post-MI patients were based on data provided in a review study by Ara et al.30 Different utility scores were applied to different sex- and age-groups without stroke or MI and to the first year of a stroke or MI event and then subsequent years. The utility scores are presented in Table 3.
  | Table 3 Utility estimates of the health states by age and sex |
Analyses
Results of base-case analysis were based on a cohort of hypertensive patients aged 65 years old and 50% males. Valsartan was the selected ARB comparator. A deterministic model was run and the total costs in the valsartan and amlodipine groups during the 5-year time period were calculated and compared. QALYs in the valsartan and amlodipine groups during the 5-year time period were calculated and compared separately. Microsoft Office Excel 2013 (Microsoft Corporation, Redmond, WA, USA) was used for modeling and computations.
Sensitivity analysis
To test the robustness of the results, extensive one-way sensitivity and scenario analyses were performed to examine the change in incremental cost, outcome, and the incremental cost-effectiveness ratio, if applicable. In the sensitivity analyses, sex composition of hypertensive patients, age of treatment initiation, and discount rate were altered to examine the impact of these changes on the results. Values of the following input parameters were altered: 1) percentage of male patients was altered from 50% to 30%, 40%, 60%, and 70%; 2) age of the cohort was altered from 65 years to 55, 60, and 70 years; and 3) discount rate for cost and QALY was altered to 1%, 2%, 4%, 5%, 6%, 7%, and 8%.
Results
Base-case analysis
The base-case analysis showed that in a cohort of 10,000 patients over a period of 5 years, both drug (NTD 2,549 vs NTD 4,425) and event costs per patient-year (NTD 1,747 vs NTD 2,123) were lower for amlodipine compared with valsartan, resulting in a cost saving of NTD 2,251 for amlodipine per patient per year (Figure 2). In total, over a period of 5 years, 71 additional cardiovascular events were prevented with amlodipine (342 events were accumulated with amlodipine in 5 years vs 413 for valsartan) (Figure 2). Amlodipine prevented 25 additional nonfatal MI events compared with valsartan (118 vs 143, respectively) and 39 additional nonfatal stroke events (189 vs 228, respectively). Furthermore, amlodipine prevented five additional fatal MI events compared with valsartan (20 vs 25, respectively) and two additional fatal stroke events (15 vs 17, respectively). Amlodipine accrued more QALYs than valsartan (31,903 vs 31,845, respectively), resulting in a gain of 58 QALYs for amlodipine. This cost-effectiveness analysis showed that amlodipine is dominant because it is associated with lower costs and better outcomes.
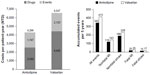  | Figure 2 Cost-effectiveness analysis in a cohort of 10,000 patients over 5 years. |
Sensitivity analysis
The results of the one-way sensitivity analyses are also presented in a tornado diagram (Figure 3), which shows that the risk reduction between valsartan and amlodipine in stroke had the largest impact on the incremental cost-effectiveness ratio. Changes in drug cost and the risk reduction between valsartan and amlodipine in MI also had a large effect on the incremental cost estimation, whereas changes in sex composition had a smaller effect. Among the range of parameters analyzed, all results consistently showed lower costs and better outcomes with amlodipine.
Discussion
Hypertension is a leading and highly prevalent risk factor for cardiovascular diseases that results in increased risk of morbidity and mortality. Given that morbidity and mortality due to cardiovascular diseases have increased in most Asia-Pacific countries, including Taiwan,29 the assessment, control, and modification of risk factors such as hypertension are imperative. Furthermore, data suggest that in the Asian population, the incidence of stroke and the mortality rates from stroke, are higher than those for MI (see Supplementary materials for stroke/MI rates in Taiwan and Japan). This differs from data from Western countries, where the incidence of MI and mortality rates from MI are higher than those for stroke.31,32
Despite the availability of different classes of antihypertensive medications, blood pressure control remains suboptimal in a large proportion of individuals with hypertension. The use of antihypertensive therapy would be an important and effective strategy for controlling hypertension and reducing the risk of adverse cardiovascular events such as stroke in Asian countries.33 CCBs such as amlodipine provide additional clinical benefit compared with ARBs.34 A meta-analysis of the efficacy of amlodipine compared with ARBs for the prevention of stroke/MI in patients with hypertension, coronary artery disease, or diabetic nephropathy showed that amlodipine provided significantly more protection against stroke than ARBs.22 In pursuing improved management of hypertension, however, both cost and effectiveness need to be considered, but economic studies comparing amlodipine and ARBs in the Taiwanese setting are lacking in this regard. In our cost-effectiveness study, amlodipine and valsartan were compared from the Taiwanese government payer perspective.
Our study showed that amlodipine was a better treatment option than valsartan, as it was associated with lower cost, higher QALYs gained, and more cardiovascular events prevented, especially in stroke. The findings of this study are consistent with those from international cost-effectiveness studies of amlodipine in patients with hypertension, coronary artery disease, or diabetic nephropathy; in that, they report that amlodipine also prevented more stroke events than ACEI and other older drug classes.15,16 In a recent (2013) Chinese study in patients with hypertension and high cardiovascular risk, the incremental cost-effectiveness ratios for amlodipine compared with ARBs in stroke/MI were renminbi –162,297 per QALY gained (valsartan) and renmibi –17,529 per QALY gained (irbesartan).16
To ensure the robustness of the results, sensitivity analyses were performed. In keeping with the International Society of Pharmacoeconomics and Outcomes Research recommendations,35 one-way sensitivity analysis was performed to explore the impact of different assumptions across their broadly adopted ranges on the results of the model. Scenario analyses with varying sex composition, cohort age, and discount rate simultaneously confirmed the robustness of the conclusions.
Limitations
The current study has several limitations. There is a lack of formal Taiwanese data on MI/stroke incidence in patients receiving long-term antihypertensive therapy with amlodipine or ARBs. Therefore, in our study, we used data from Japanese populations to estimate the risk of fatal/nonfatal stroke and MI in a Taiwanese population20; these risk estimates–although from an Asian rather than a Western population–may not accurately reflect the risk levels of the Taiwanese population. In addition, QALYs were only available for ischemic stroke and intracerebral hemorrhage (ie, not for MI).36 Further limitations of this study were that the model structure did not allow for comparison of dose escalation or antihypertensive combination therapy and that the analyses were restricted to first stroke or MI event only after initiation of treatment. It should also be noted that the aforementioned results are subject to the price change of amlodipine and/or valsartan. Hence, results of this study should be interpreted with caution and additional consideration may be needed in applying the findings to the real-world situation.
Conclusion
Based on available current data informing our probability, cost, and utility estimates, amlodipine has not only better effectiveness but also is predicted to be cost saving compared with valsartan, a commonly prescribed ARB in Taiwan, warranting its consideration as an agent of choice in treating hypertensive patients in the Taiwanese setting. It lowers the acute care costs associated with stroke and MI episodes as well as costs of follow-up disease management.
Acknowledgments
The authors thank Jou-Wei Lin for analysis and interpretation of data. The study was sponsored by Pfizer. Editorial assistance was provided by Anne Jakobsen, MSc, and Helen Jones, PhD, of Engage Scientific and was funded by Pfizer.
Disclosure
JZL, T-CW, and W-YS are employees of Pfizer. LZL was an employee of Pfizer during the study and manuscript development. C-HC has been a consultant for Novartis and has received speaker honoraria from Novartis and Pfizer. The other authors report no conflicts of interest in this work.
References
Lim SS, Vos T, Flaxman AD et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. | |
Lawes CM, Vander Hoorn S, Rodgers A; International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371(9623):1513–1518. | |
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. | |
Fang J, Madhavan S, Cohen H, Alderman MH. Measures of blood pressure and myocardial infarction in treated hypertensive patients. J Hypertens. 1995;13(4):413–419. | |
Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A; Fever Study Group. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens. 2005;23(12):2157–2172. | |
Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. | |
Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):331–337. | |
Glynn RJ, L’Italien GJ, Sesso HD, Jackson EA, Buring JE. Development of predictive models for long-term cardiovascular risk associated with systolic and diastolic blood pressure. Hypertension. 2002; 39(1):105–110. | |
Barreras A, Gurk-Turner C. Angiotensin II receptor blockers. Proc (Bayl Univ Med Cent). 2003;16(1):123–126. | |
Wong MC, Jiang JY, Lam AT, Fung H, Griffiths S, Mercer SW. Patterns of antihypertensive prescribing, discontinuation and switching among a Hong Kong Chinese population from over one million prescriptions. J Hum Hypertens. 2008;22(10):714–716. | |
Zhang JF. Medication use among hypertension patients in a Chinese hospital. China Pract Med. 2009;5(22):111–112. | |
Medical and Pharmaceutical Industry Technology and Development Center, Taiwan, ROC. Overview of the Anti-Hypertensive Drugs Market [in Chinese]. Available from: http://www.pitdc.org.tw/member/%E4%B8%AD%E8%A5%BF%E8%97%A5%E5%B8%82%E5%A0%B4/%E8%A5%BF%E8%97%A5/%E9%AB%98%E8%A1%80%E5%A3%93%E6%B2%BB%E7%99%82%E8%97%A5%E5%B8%82%E5%A0%B4%E6%A6%82%E8%BF%B0-%E4%B8%8A.pdf. Accessed January 25, 2016. | |
Chen WX, Tan X. A review of the utilization of oral calcium channel blockers. Curr Pharm Today (Chinese). 2009;19(5):31–33. | |
Pfizer Inc. Norvasc tablets [US package insert]. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=562. Accessed December 10, 2014. | |
de Portu S, Mantovani LG. Amlodipine: a pharmacoeconomic review. J Med Econ. 2009;12(1):60–68. | |
Wu Y, Zhou Q, Xuan J, et al. A cost-effectiveness analysis between Norvasc angiotensin II receptor blockers in stroke and myocardial infarction prevention among hypertension patients in China. Value Health Reg Issues. 2013;2:75–80. | |
Singh RB, Suh IL, Singh VP, et al. Hypertension and stroke in Asia: prevalence, control and strategies in developing countries for prevention. J Hum Hypertens. 2000;14(10–11):749–763. | |
Department of Health, Executive Yuan, R.O.C. (Taiwan). 2010 Statistics for Causes of Death. Available from: http://www.mohw.gov.tw/MOHW_Upload/doc/2010-statistics%20of%20cause%20of%20death.pdf. Accessed January 29, 2014. | |
Department of Health EY, R.O.C., (Taiwan). 2010 Statistical Annual Report of Medical Care, National Health Insurance. Available from: http://www.mohw.gov.tw/EN/Ministry/Statistic_P.aspx?f_list_no=474&fod_list_no=3477&doc_no=27787. Accessed November 13, 2015. | |
Muramatsu T, Matsushita K, Yamashita K, et al; NAGOYA HEART Study Investigators. Comparison between valsartan and amlodipine regarding cardiovascular morbidity and mortality in hypertensive patients with glucose intolerance: NAGOYA HEART Study. Hypertension. 2012;59(3):580–586. | |
Lee Y, Lin RS, Sung FC, et al. Chin-Shan community cardiovascular cohort in Taiwan-baseline data and five-year follow-up morbidity and mortality. J Clin Epidemiol. 2000;53(8):838–846. | |
Wang JG, Li Y, Franklin SS, Safar M. Prevention of stroke and myocardial infarction by amlodipine and angiotensin receptor blockers: a quantitative overview. Hypertension. 2007;50(1):181–188. | |
Chang KC, Lee HC, Tseng MC, Huang YC. Three-year survival after first-ever ischemic stroke is predicted by initial stroke severity: a hospital-based study. Clin Neurol Neurosurg. 2010;112(4):296–301. | |
Lee CH, Cheng CL, Yang YH, et al. Trends in the incidence and management of acute myocardial infarction from 1999 to 2008: get with the guidelines performance measures in Taiwan. J Am Heart Assoc. 2014;3(4):e001066. | |
Chiang FT, Shyu KG, Wu CJ, et al. Predictors of 1-year outcomes in the Taiwan Acute Coronary Syndrome Full Spectrum Registry. J Formos Med Assoc. 2014;113(11):794–802. | |
National Health Insurance Administration MoHaW. National Health Insurance reimbursed drug price [in Chinese]. Available from: http://www.nhi.gov.tw/query/query1.aspx?menu=21&menu_id=713&webdata_id=3510&WD_ID=851. Accessed December 31, 2014. | |
Tang CH, Chuang PY, Chen CA, Fang YC. Medical costs of cardiovascular diseases in Taiwan. Value Health Reg Issues. 2014;17(7):A759–A760. | |
Sun S, Chen J, Johannesson M, et al. Population health status in China: EQ-5D results, by age, sex and socio-economic status, from the National Health Services Survey 2008. Qual Life Res. 2011;20(3):309–320. | |
Reid CM, Yan B, Wan Ahmad WA, et al. The Asia-Pacific Evaluation of Cardiovascular Therapies (ASPECT) collaboration – improving the quality of cardiovascular care in the Asia Pacific region. Int J Cardiol. 2014;172(1):72–75. | |
Ara R, Tumur I, Pandor A, et al. Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation. Health Technol Assess. 2008;12(21):iii, xi–xiii, 1–212. | |
Hata J, Kiyohara Y. Epidemiology of stroke and coronary artery disease in Asia. Circ J. 2013;77(8):1923–1932. | |
Chiang CE, Wang TD, Ueng KC, et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J Chin Med Assoc. 2015;78(1):1–47. | |
Chen X, Zhou L, Zhang Y, et al. Risk factors of stroke in Western and Asian countries: a systematic review and meta-analysis of prospective cohort studies. BMC Public Health. 2014;14:776. | |
Thorvaldsen P, Kuulasmaa K, Rajakangas AM, Rastenyte D, Sarti C, Wilhelmsen L. Stroke trends in the WHO MONICA project. Stroke. 1997;28(3):500–506. | |
Sullivan SD, Avey SG, Flynn JA, et al. The AMCP format for formulary submissions (version 3.0): a format for submission of clinical and economic evidence of pharmaceuticals in support of formulary consideration. Available from: http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=9127. Accessed November 13, 2015. | |
Lee HY, Hwang JS, Jeng JS, Wang JD. Quality-adjusted life expectancy (QALE) and loss of QALE for patients with ischemic stroke and intracerebral hemorrhage: a 13-year follow-up. Stroke. 2010;41(4):739–744. | |
Ministry of Health and Welfare. 2009 statistics of causes of death. Available from: http://www.mohw.gov.tw/EN/Ministry/Statistic.aspx?f_list_no=474&fod_list_no=3481. Accessed August 1, 2015. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.