Back to Journals » ClinicoEconomics and Outcomes Research » Volume 14
Cost-Effectiveness and Budget Impact of Comprehensive Anemia Management, The First Pillar of Patient Blood Management, on the Turkish Healthcare System
Authors Tatar M, Alkış N, Yıldırım Güçlü Ç, Bermede O, Erdemli B, Günaydın S
Received 16 February 2022
Accepted for publication 7 May 2022
Published 30 May 2022 Volume 2022:14 Pages 415—426
DOI https://doi.org/10.2147/CEOR.S360944
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Giorgio Colombo
Mehtap Tatar,1 Neslihan Alkış,2 Çiğdem Yıldırım Güçlü,2 Onat Bermede,2 Bülent Erdemli,3 Serdar Günaydın4
1Polar Health Economics and Policy, Ankara, Turkey; 2Department of Anesthesiology and Intensive Care Unit, Faculty of Medicine, Ankara University, Ankara, Turkey; 3Department of Orthopaedic Surgery and Traumatology, Faculty of Medicine, Ankara University, Ankara, Turkey; 4Department of Cardiovascular Surgery, University of Health Sciences, Ankara City Hospital Campus, Ankara, Turkey
Correspondence: Mehtap Tatar, Polar Health Economics and Policy, Mustafa Kemal Mah. Dumlupınar Bulvarı No:266 Tepe Prime İş Merkezi A Blok No 18, 06800 Çankaya, Ankara, Turkey, Tel +90 532 5538324, Email [email protected]
Purpose: Patient blood management (PBM) is a patient-centered, evidence-based, multidisciplinary approach aimed at optimizing hemoglobin concentration, ensuring the continuity of hemostasis and minimizing blood loss in patients undergoing surgery. The aims of this study were: (1) to explore the cost-effectiveness of comprehensive anemia management, the first pillar of PBM, in non-cardiac and cardiac surgery from the Turkish Social Security Institution’s (SSI’s) perspective; and (2) to explore the potential budget impact of PBM for coronary artery bypass grafting (CABG) and hip and knee arthroplasty to the SSI.
Methods: Cost-effectiveness and budget impact models were developed based on the avoided postoperative adverse events following implementation of the first pillar of PBM for non-cardiac and cardiac surgical patients. The probabilities of adverse events (sepsis with and without pneumonia, renal failure, myocardial infarction and stroke) were taken from a recent meta-analysis and the costs of treating these adverse events to the SSI were estimated through expert views and the use of SSI guidelines.
Results: The PBM arm dominated the control arm for both non-cardiac and cardiac surgeries in terms of cost-effectiveness in the simulated cohort of patients and was associated with improved outcomes and lower costs (1768 and 1244 avoided adverse events, and incremental cost reductions for non-cardiac and cardiac surgery of 7504 Turkish lira [TRY] and 6102 TRY, respectively). The budget impact analysis showed that PBM is a potential cost-saving option for the SSI, with savings of up to 196,937,705 TRY (€ 12,841,697) for hip and knee arthroplasty and 24,642,504 TRY (€ 1,606,861) for CABG surgery.
Conclusion: PBM is a cost-effective option with a potential of cost-saving for cardiac and non-cardiac surgery in Turkey.
Keywords: patient blood management, cost-effectiveness analysis, budget impact, Turkey; Turkish healthcare system
Plain Language Summary
Patient blood management (PBM) is an approach to treating patients with the goal of optimizing blood hemoglobin levels, ensuring the ability of the blood to clot is maintained and blood loss in patients undergoing surgery is kept to a minimum. The aims of this study were: (1) to understand if managing anemia in patients (the first pillar of PBM) is cost-effective in patients undergoing hip and knee surgery or cardiac surgery from the view of the Turkish Social Security Institution (SSI); and (2) to explore the potential savings to the SSI if these PBM measures were used for patients undergoing hip and knee surgery or cardiac surgery. Models were used to assess the number of complications (adverse events) avoided after surgery (hip and knee or cardiac) if patients were treated with PBM. Data from a recent study was used to determine the chance of experiencing these complications, and the costs of treating the complications were estimated by clinical experts and Turkish SSI guidelines. Together, these were used to determine the cost-savings that could be made if PBM was used. This study found that using PBM was more cost-effective and linked to improved outcomes for patients compared with not using PBM. The costs saved to the Turkish SSI were estimated to be up to 196,937,706 TRY (€12,841,697) for hip and knee surgery and 24,642,504 TRY (€1,606,861) for cardiac surgery. This study suggests that PBM could be a cost-effective and cost-saving option for cardiac and non-cardiac surgery in Turkey.
Introduction
Patient blood management (PBM) is a patient-centered, evidence-based multidisciplinary approach that aims to optimize hemoglobin concentration, maintain hemostasis and minimize blood loss in patients undergoing surgery.1–3 The available evidence indicates that anemia, bleeding and exposure to allogeneic products are risk factors for perioperative morbidity and mortality,4–11 which are associated with decreased quality of life and survival for patients. Moreover, these place an economic burden on health-care systems through prolonged length of stay in hospital (LOS), increased risk of adverse events and increased use of allogeneic blood products. There is growing research and evidence exploring the benefits and economic value of a PBM program for major surgery. According to a recent meta-analysis,1 implementation of multimodal PBM measures resulted in an overall 39% decrease in transfusion rates. The highest reduction was observed in orthopedic surgery (55%), followed by cardiac surgery (50%). There was also a decrease in LOS (0.45 days in orthopedic surgery and 1.34 days in cardiac surgery), number of adverse events (20%) and mortality (11%). It is acknowledged that three types of surgery – orthopedic (especially hip and knee arthroplasties), cardiac and colorectal – result in significant perioperative bleeding. Kurian et al12 stated that hospitals with established PBM programs may have a 50–75% reduction in blood use in patients undergoing orthopedic and cardiac surgery compared with those without PBM programs.
Since the endorsement of PBM by the World Health Assembly (WHA 63.12) in 2010,13 several PBM programs have been introduced at both national and institutional levels, and an extensive body of literature has been developed regarding their implementation and implications.2,14–16 The PBM approach is comprised of three pillars:1,17 (1) comprehensive anemia management; (2) minimization of hospital-acquired anemia such as through unnecessary blood loss; and (3) harnessing and optimizing the patient-specific physiological tolerance of anemia.
There are currently very few programs and institutions that implement all three pillars of the PBM approach; a systematic review and meta-analysis identified just 17 studies that addressed all three PBM pillars.1 Other studies have focused solely on the implementation of the first pillar in different health-care settings: Abdullah et al found that implementing the first PBM pillar is a feasible and sensible first step in health-care settings in the Asia-Pacific region,4 while Drabinski et al reported on the epidemiological and economic benefits of implementing the first PBM pillar in the German healthcare system18. The study we report here focuses on the first pillar of PBM and the potential impacts of its implementation on the Turkish healthcare system.
PBM in Turkey
The Ministry of Health (MoH) is the agency responsible for blood and blood products in Turkey. The MoH embarked on a project titled “Technical Assistance for Improving Blood Transfusion Management in Turkey” in March 2019 with assistance from the European Union.19 The main goal of the project was defined as ‘establishing a strong PBM system in Turkey with special reference to developing the required infrastructure for the system’. In 2021, the project was at the stage of developing guidelines and documents for PBM and training health-care workers. In 2019, The Turkish Society of Cardiovascular Surgery, Turkish Society of Cardiology and Society of Cardio-Vascular-Thoracic Anaesthesia and Intensive Care published a consensus report on PBM in cardiac surgery.20 To date, these are the only structured guidelines that focus on blood-conservation strategies with recommendations in line with global guidelines and practice.
The most relevant and recent research related to the use of blood products in Turkey was undertaken by Ünal et al21. The aims of the Turkish National Perioperative Transfusion Study (TULIP-TS) were: (1) to evaluate perioperative practices in patients undergoing elective major surgery; (2) to estimate the incidence of and indications for perioperative transfusion; and (3) to assess the impact of transfusion on patients’ outcomes. Preoperative anemia was identified in one-third of patients, a similar rate to that seen in other studies.11,17 During the perioperative course, a quarter of the patients received at least one unit of a blood component. The majority of transfusions were made in cardiovascular/thoracic (45.9%) and orthopedic (14.6%) surgery (see Table S1 in the supplementary materials for more details).
There are few published studies with results from institutional implementation of PBM in Turkey.22–25 Sert et al25 initiated a partial PBM program in a Turkish training and research hospital and compared outcomes of cardiac surgery in patients receiving and those not receiving PBM. There was a significant decrease in the use of blood/blood products and costs in those receiving PBM. After the PBM program was introduced, the cost of erythrocyte suspension per patient decreased from 627 Turkish lira (TRY) to 140 TRY, while the cost of fresh frozen plasma per patient decreased from 184 TRY to 36 TRY. In another single-institution study,22–24 a structured blood conservation program based on training surgeons in PBM was implemented in the cardiovascular surgery clinics of a training and research hospital. A substantial reduction in the use of blood/blood products was observed between 2015 and 2017 (whole blood: 140 units in 2015 to 0 units in 2017; fresh frozen plasma: 1667 units in 2015 to 817 units in 2017, packed red blood cells: 2 units in 2015 to 0 units in 2017). The PBM program was extended to all surgical clinics and the hospital-wide blood/blood product usage decreased by 3% in 2018.
This study is the first attempt to investigate the cost-effectiveness and potential budget impact of PBM in the Turkish healthcare context. The aims of the study are: (1) to explore the cost-effectiveness of PBM in non-cardiac and cardiac surgery; and (2) to explore the potential budget impact of implementing the first pillar of PBM in coronary artery bypass grafting (CABG) and hip and knee arthroplasty from the Turkish Social Security Institution’s (SSI’s) perspective.
Materials and Methods
Cost-effectiveness and budget impact models were designed based on the risk of postoperative adverse events. Only the first pillar of PBM, that is, diagnosing and treating anemia in patients before surgery, was included. The number and costs of avoided adverse events by implementing the first pillar of the PBM approach were analyzed. Both the cost-effectiveness and budget impact analyses were designed from the SSI’s perspective and all costs were calculated based on the reimbursement prices of products and services.
Preoperative anemia treatment was made with intravenous (IV) ferric carboxymaltose (FCM) (two 500 mg per 10 mL vials before surgery), as there is strong evidence that treatment with IV iron before surgery increases hemoglobin levels and decreases a patient’s need for blood products.26–29
Cost-Effectiveness Analysis
A decision tree model with probabilities of adverse events was developed to assess the cost-effectiveness of PBM in non-cardiac and cardiac surgery, with a simulated cohort of 10,000 patients in Turkey (Figure 1); the time horizon of the model was a hospitalization period of up to 30 days for no specific year. TreeAge Pro (TreeAge Software, LLC, Williamstown, MA, USA) was used to build and run the model. The endpoints of the study were postoperative adverse events (sepsis with or without pneumonia, acute renal failure, acute myocardial infarction and acute stroke) avoided by implementation of PBM. Data on endpoints were obtained from the results of the Kleinerüschkamp et al study,8 and given as “incremental cost per avoided postoperative complication”. The probabilities of the postoperative adverse events for both the PBM and control arms for non-cardiac and cardiac surgery are shown in Table 1.
 |
Table 1 Postoperative Adverse Event Probabilities |
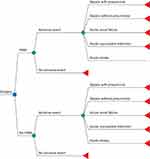 |
Figure 1 PBM cost-effectiveness decision tree model: general outlook. Abbreviation: PBM, patient blood management. |
The major cost parameters in the model were the cost of PBM and the cost of treating adverse events, based on expert opinion using health-care resources for the treatment of these events. A healthcare resource utilization tool was developed for each adverse event, to be filled in by experts. After identifying the type, duration and frequency of resources used in the treatment of each adverse event, SSI guidelines and price tariffs were used to calculate the cost to the SSI of treating these adverse events. Cost of treating MI, stroke and renal failure was calculated for three years to reflect the long-term burden of these on the health-care system. A 3% discount rate was used. In the PBM arm, only the cost of anemia management (tests for diagnosis and treatment with two vials of IV FCM) were included (Table 2).
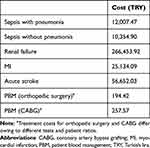 |
Table 2 Treatment Costs of Adverse Events and Cost of PBM (2021) |
In the absence of a threshold for evaluating the results of a cost-effectiveness analysis, the World Health Organization’s recommendation for willingness to pay (WTP) for developing countries, based on gross domestic product (GDP) per capita, was used in the final analysis.30 The GDP per capita for Turkey was calculated as $8599 (US dollars, equivalent to 70,000 TRY: 1$ = 8.14 TRY, Central Bank of Turkey, March 29, 2021) for 2020 by the Turkish Statistical Institute.31
Sensitivity Analysis
One-way sensitivity analyses were conducted in order to observe the response of the model’s results to uncertainties in cost-effectiveness model parameters. The following analyses were conducted: (1) differing costs per patient of PBM (range: 155.54–233.30 TRY for non-cardiac surgery and 206.06–309.08 TRY for cardiac surgery) and (2) number of adverse events avoided (range: 1414–2122 for non-cardiac surgery and 995–1492 for cardiac surgery). For costs, the sensitivity analysis model was run with 1000 iterations of 10 different PBM costs (TreeAge Pro). For adverse events avoided, the impact of a 20% change in this rate was analyzed. The cost of PBM was considered the single most important input that could have an impact on the model results. For this reason, the cost of PBM was changed within a range of 20% to assess the sensitivity of the model’s results to the change in the most important input.
Budget Impact Analysis
The budget impact model was based on the costs of treating postoperative adverse events and the cost of receiving PBM. Hip and knee arthroplasties and CABG were selected, as there is strong evidence that PBM has the highest impact on patients undergoing these surgeries.1 The results for cardiac surgery from Kleinerüschkamp et al8 were used as a proxy to estimate the budget impact of implementing PBM in CABG. Similarly, results for non-cardiac surgery were used as a proxy for hip and knee arthroplasty. Budget impact analyses covered only hip and knee arthroplasties and CABG in MoH hospitals; surgeries conducted in university and private hospitals were excluded. Potential cost savings in the budget impact model were based only on the number and cost of avoided postoperative adverse events. Potential savings from shortened LOS and reduced use of blood products were not included. Two clinical scenarios were modeled in the budget impact analysis: scenario 1 assumed that all patients were treated for anemia; scenario 2 assumed that half of patients identified had iron deficiency anemia (IDA), and only half of those would be treated for the condition.
In 2017, there were 12,237 CABG surgeries and 77,780 hip and knee arthroplasties in MoH hospitals. These figures do not cover operations in university and private hospitals. The MoH owned 60% of all hospital beds in 2017.28 According to the latest study by Ünal et al,21 33% of these would have preoperative anemia (4038 patients undergoing CABG and 25,667 patients having hip and knee arthroplasty). For both categories of surgery, in the first scenario it was assumed that all patients would receive 1000 mg of IV FCM (two vials), at a public price of 254.11 TRY per vial before the operation. In the second scenario, based on the findings of Drabinski et al,18 it was assumed that 50% of the patients with preoperative anemia would have IDA (2019 patients undergoing CABG and 12,834 patients having hip and knee arthroplasty). It was further assumed, based on expert opinion, that 50% of patients with IDA would receive 1000 mg of IV FCM treatment (6417 patients having hip and knee arthroplasty and 1010 patients undergoing CABG). The number of postoperative adverse events in each arm was calculated by applying the probabilities established in the Kleinerüschkamp et al study.8
Results
Cost-Effectiveness Analysis
PBM was found to dominate the control arm in both non-cardiac and cardiac surgery and provided better outcomes with lower costs (Table 3). With the implementation of PBM, the incremental cost was –7504 TRY for non-cardiac surgery and –6102 TRY for cardiac surgery. As can be seen, the cost of implementing PBM was lower than the cost of not implementing PBM. In addition, PBM implementation versus control was associated with 1768 and 1244 avoided adverse events for non-cardiac and cardiac surgery, respectively. Based on the 70,000 TRY WTP threshold, PBM was shown to be a highly cost-effective option compared with control in Turkey. The probabilities of postoperative adverse events within total adverse events were 0.3088 for the control arm and 0.132 for the PBM arm for non-cardiac surgery, and 0.1873 for the control arm and 0.0629 for the PBM arm for cardiac surgery. As the probabilities reveal, PBM implementation was more effective than the control option in terms of avoiding postoperative adverse events (Table 1).
 |
Table 3 Cost-Effectiveness Analysis Results |
Sensitivity Analysis
The cost-effectiveness one-way sensitivity analysis of costs of PBM and number of adverse events avoided found that the PBM arm dominated the control arm in all iterations, indicating the robustness of the model. All results showed that implementing the first pillar of PBM had a cost-saving impact on the SSI’s budget under all scenarios, through reduced costs for PBM and avoided adverse events. The potential savings would be higher if the surgeries undertaken at university and private hospitals were considered, and if other potential cost-saving items such as reduced LOS and reduced use of blood products were included. Results of the sensitivity analysis are shown in Tables S2 and S3.
Budget Impact Model
The overall cost savings related to avoided post-surgical adverse events following hip and knee arthroplasty in Turkey in 2017 were 196,937,705 TRY (€12,841,697) for scenario 1 and 49,234,426 TRY (€3,210,424) for scenario 2 (Table 4). For CABG surgeries in Turkey during 2017, the overall cost savings related to avoided post-surgical adverse events were 24,642,504 TRY (€1,606,861) for scenario 1 and 6,153,207 TRY (€401,232) for scenario 2 (Table 5).
 |
Table 4 Budget Impact of PBM in Hip and Knee Arthroplasty in Turkey Based on Treatment of Post-Operative Adverse Events (2017) – Scenarios 1 and 2 |
 |
Table 5 Budget Impact of PBM in CABG Operations in Turkey Based on Treatment of Postoperative Adverse Events (2017) – Scenarios 1 and 2 |
Discussion
The unnecessary use of blood products is known to be associated with a range of risk factors and burdens to individual patients and to health-care systems as a whole. Apart from the direct cost of using these products, the negative outcomes in terms of postoperative adverse events are widely acknowledged. Transmissible infectious diseases, transfusion reactions and potential effects of immunomodulation are all known risk factors to patients, and allogeneic blood transfusions have been linked with unfavorable outcomes including increased risk of mortality and various morbidities.32–38 There is growing evidence in the literature that PBM is a cost-saving and cost-effective option in enhancing the optimal use of blood products, for the benefit of both patients and health-care systems.15,29,39–42
This study is, to our knowledge, the first attempt to analyze the cost-effectiveness and cost-saving potential of PBM with a simulated model in Turkey. Our study concluded that PBM was a cost-effective option and has cost-saving potential in hip and knee arthroplasties and CABG in Turkey. This finding is in line with examples from other health-care systems,15,43,44 and is similar to results reported in earlier literature; it concludes that implementing just the first pillar of PBM can be a cost-effective option and may provide considerable cost-saving opportunities to the SSI. In the cost-effectiveness analysis, the incremental cost per avoided postoperative adverse event was used as the decision criterion. In both interventions, PBM dominated the control arm; in other words, more adverse events were avoided, and costs were lower with PBM than without. Moreover, PBM was found to be a highly cost-effective intervention at the 70,000 TRY WTP threshold. In addition, the findings were robust to scenario changes in the sensitivity analyses.
It should be noted here that our results cover only the avoided costs of treating postoperative adverse events resulting from the implementation of the first pillar of PBM. Potential cost reductions due to reduced use of blood products and decreased LOS are not included in the analysis. Available evidence indicates that these two outcomes of a PBM program are very important contributors to cost savings.43 For instance, Kotze et al45 investigated the economic impact of PBM in 281 patients undergoing major orthopedic surgery and concluded that there were savings of €160,000, as the LOS was decreased by 0.7 days and the readmission rate was decreased by 5%. Froessler et al46 estimated the economic consequences of perioperative administration of FCM versus usual care in patients with IDA and concluded that FCM resulted in cost savings to hospitals. According to their estimations, the average cost per case treated with FCM and usual care were €2461 and €3246, respectively. The cost-effectiveness and budget impact of FCM has also been analyzed for the Italian healthcare system,44 in which FCM was compared with placebo for the management of iron deficiency with chronic heart failure. FCM was dominant over placebo with an annual cost saving of €20–97 million. In another study exploring the cost-effectiveness of using FCM in optimizing preoperative hemoglobin in knee arthroplasty,47 the results supported FCM as a cost-effective option. Mehra et al48 conducted a prospective interventional cohort study with 101,794 patients, in which there was a 27% decrease in allogeneic blood transfusion after the implementation of a PBM program; this resulted in savings of direct transfusion costs totaling more than $2,000,000 in 1 year. Meybohm et al17 performed a cost-benefit analysis to assess the economic impact of a PBM program, based on the findings of Althoff et al1. The implementation of PBM measures in 235,779 surgical patients resulted in decreased red blood cell utilization and decreased LOS, with the mean cost of transfusion per patient reduced from €68.62 to €32.41. Similarly, there was a decrease in LOS by 0.45 days, which resulted in cost savings of €114.43. This evidence suggests that if there were enough data in Turkey about reduced use of blood products and decreased LOS, then the cost-saving impact of the PBM program would be greater.
The budget/economic impact of PBM was assessed using two scenarios for categories of surgery. The first scenario yielded 24,642,504 TRY (€1,606,861) and 196,37,705 TRY (€12,841,697) cost savings for CABG and hip and knee arthroplasties, respectively, in Turkey. The second scenario yielded 6,153,207 TRY (€401,232) cost saving for CABG and 49,234,426TRY (€3,210,424) cost saving for hip and knee arthroplasties. The hospital budget of the SSI for the same year was 44,543,769,000 TRY.49 This indicates that the potential savings from scenario 1 for both hip and knee arthroplasties and CABG comprised 0.5% of the SSI’s hospital budget in 2017; for scenario 2, the potential savings comprised 0.12% of the budget.
There are some limitations to this study. First, the probabilities of postoperative adverse events were taken from the meta-analysis results of the Kleinerüschkamp et al study.8 Therefore, there is a likelihood that any limitations of that study will be carried over into this study as well. Second, the costs of treating adverse events were calculated based on expert opinion on treatment resources. In the absence of cost data, this was considered the only way to estimate costs from the SSI’s perspective; it should be noted that there are other published examples where cost of treatment was also obtained from expert opinions.44 Finally, the budget impact analysis results covered only surgical procedures conducted at MoH hospitals and excluded those carried out at university and private hospitals. This will certainly have led to underestimation of cost savings associated with the implementation of PBM. The SSI reimburses cardiac and non-cardiac surgeries included in this analysis by a fixed package price; this may have an impact on the total cost of these surgeries and hence on the interpretation of the cost-effectiveness of PBM implementation.
Conclusion
In this cost-effectiveness and budget impact analysis modeling study, the implementation of PBM was associated with a decreased rate of adverse events in both cardiac and non-cardiac surgical patients. PBM could be explored as a cost-effective and cost-saving option in major surgeries in Turkey. The SSI can play a leading role by promoting, regulating and implementing policy for the inclusion of PBM in hospital-based process improvement initiatives, with the goal of improving patient safety and clinical outcomes through the use of PBM.
Abbreviations
CABG, coronary artery bypass grafting; FCM, ferric carboxymaltose; GDP, gross domestic product; IDA, iron deficiency anemia; IV, intravenous; LOS, length of stay (in hospital); MoH, Ministry of Health; PBM, patient blood management; SSI, Turkish Social Security Institution; TRY, Turkish lira; TULIP-TS, Turkish National Perioperative Transfusion Study; WTP, willingness to pay.
Data Sharing Statement
All data are available within the main manuscript and supplementary materials.
Ethics Approval and Consent to Participate
No ethics approval required for study.
Acknowledgments
Medical writing assistance was provided by Rebecca Hornby, PhD, and Gemma Carter, PhD, of Oxford PharmaGenesis, Oxford, UK, and was funded by Vifor Pharma.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Vifor Pharma.
Disclosure
MT provides consultancy to Vifor Pharma. OB, BE, SG, CYG and NA report no conflicts of interest in this work.
References
1. Althoff FC, Neb H, Herrmann E, et al. Multimodal patient blood management program based on a three-pillar strategy: a systematic review and meta-analysis. Ann Surg. 2019;269(5):794–804. doi:10.1097/SLA.0000000000003095
2. Freedman J, Luke K, Escobar M, Vernich L, Chiavetta JA. Experience of a network of transfusion coordinators for blood conservation (Ontario Transfusion Coordinators [ONTraC]). Transfusion. 2008;48(2):237–250. doi:10.1111/j.1537-2995.2007.01515.x
3. Liumbruno GM, Grazzini G, Rafanelli D. Post-operative blood salvage in patient blood management: is it really cost-effective and safe? Blood Transfus. 2013;11(2):175–177. doi:10.2450/2013.0001-13
4. Abdullah HR, Ang AL, Froessler B, et al. Getting patient blood management Pillar 1 right in the Asia-Pacific: a call for action. Singapore Med J. 2020;61(6):287–296. doi:10.11622/smedj.2019037
5. Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009;208(5):931–937. doi:10.1016/j.jamcollsurg.2008.11.019
6. Chatterjee S, Wetterslev J, Sharma A, Lichstein E, Mukherjee D. Association of blood transfusion with increased mortality in myocardial infarction: a meta-analysis and diversity-adjusted study sequential analysis. JAMA Intern Med. 2013;173(2):132–139. doi:10.1001/2013.jamainternmed.1001
7. Jakobsen CJ, Ryhammer PK, Tang M, Andreasen JJ, Mortensen PE. Transfusion of blood during cardiac surgery is associated with higher long-term mortality in low-risk patients. Eur J Cardiothorac Surg. 2012;42(1):114–120. doi:10.1093/ejcts/ezr242
8. Kleineruschkamp A, Meybohm P, Straub N, Zacharowski K, Choorapoikayil S. A model-based cost-effectiveness analysis of patient blood management. Blood Transfus. 2019;17(1):16–26. doi:10.2450/2018.0213-17
9. Hofmann A, Ozawa S, Farrugia A, Farmer SL, Shander A. Economic considerations on transfusion medicine and patient blood management. Best Pract Res Clin Anaesthesiol. 2013;27(1):59–68. doi:10.1016/j.bpa.2013.02.001
10. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg. 2016;264(2):203–211. doi:10.1097/SLA.0000000000001747
11. Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378(9800):1396–1407. doi:10.1016/S0140-6736(11)61381-0
12. Kurian DJ, Guinn NR, Hunting J, Kurian DJ, Guinn NR, Hunting Jet al. Preoperative blood management strategy for elective hip and knee arthroplasty. J Healthc Qual. 2019;41(6):376–383. doi:10.1097/JHQ.0000000000000207
13. World Health Organisation. Sixty-third world health assembly - availability, safety and quality of blood products, WHA63.12; 2010. Available from: https://apps.who.int/gb/ ebwha/ pdf_files/ WHA63/ A63_R12- en.pdf.
14. Leahy MF, Roberts H, Mukhtar SA, et al. A pragmatic approach to embedding patient blood management in a tertiary hospital. Transfusion. 2014;54(4):1133–1145. doi:10.1111/trf.12362
15. Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57(6):1347–1358. doi:10.1111/trf.14006
16. Theusinger OM, Kind SL, Seifert B, Borgeat L, Gerber C, Spahn DR. Patient blood management in orthopaedic surgery: a four-year follow-up of transfusion requirements and blood loss from 2008 to 2011 at the Balgrist University Hospital in Zurich, Switzerland. Blood Transfus. 2014;12(2):195–203. doi:10.2450/2014.0306-13
17. Meybohm P, Straub N, Fullenbach C, et al. Health economics of patient blood management: a cost-benefit analysis based on a meta-analysis. Vox Sang. 2020;115(2):182–188. doi:10.1111/vox.12873
18. Drabinski T, Zacharowski K, Meybohm P, Ruger AM, Ramirez de Arellano A. Estimating the epidemiological and economic impact of implementing preoperative anaemia measures in the German healthcare system: the health economic footprint of patient blood management. Adv Ther. 2020;37(8):3515–3536. doi:10.1007/s12325-020-01372-4
19. Turkish Ministry of Health and European Union. Technical assistance for improving the blood transfusion management system in Turkey; 2019. Available from: https://hastakanyonetimi.saglik.gov.tr/.
20. Ertugay S, Kudsioglu T, Sen T, Patient Blood Management Study Group Members. Consensus report on patient blood management in cardiac surgery by Turkish Society of Cardiovascular Surgery (TSCVS), Turkish Society of Cardiology (TSC), and Society of Cardio-Vascular-Thoracic Anaesthesia and Intensive Care (SCTAIC). Turk Gogus Kalp Damar Cerrahisi Derg. 2019;27(4):429–450. doi:10.5606/tgkdc.dergisi.2019.01902
21. Unal D, Senayli Y, Polat R, et al. Peri-operative blood transfusion in elective major surgery: incidence, indications and outcome - an observational multicentre study. Blood Transfus. 2020;18(4):261–279. doi:10.2450/2020.0011-20
22. Budak AB, McCusker K, Gunaydin S, A cardiopulmonary bypass based blood management strategy in adult cardiac surgery. Heart Surg Forum. 2017;20(5):E195–E198. doi:10.1532/hsf.1792
23. Gunaydin S, Spahn DR, Ozisik K, et al. Building a patient blood management program in a large-volume tertiary hospital setting: problems and solutions. Turk Gogus Kalp Damar Cerrahisi Derg. 2020;28(3):560–569. doi:10.5606/tgkdc.dergisi.2020.19701
24. Lafci A, Gokcinar D, Dag O, Gunertem E, Gunaydin S. The effect of “patient blood management” education on the number of red blood cell transfusions in patients undergoing cardiac surgery: a 5-year retrospective study. Turk J Clin Lab. 2019;1:98–103.
25. Sert GS, Cavus M, Kemerci P, et al. The results of cardiac surgery in terms of patient blood management in our hospital. Turk J Anaesthesiol Reanim. 2019;47(5):402–406. doi:10.5152/TJAR.2019.02058
26. Bisbe E, Basora M, Colomina MJ, Spanish Best Practice in Peri-operative Anaemia Optimisation Panel. Peri-operative treatment of anaemia in major orthopaedic surgery: a practical approach from Spain. Blood Transfus. 2017;15(4):296–306. doi:10.2450/2017.0177-16
27. Calleja JL, Delgado S, Del Val A, et al. Ferric carboxymaltose reduces transfusions and hospital stay in patients with colon cancer and anemia. Int J Colorectal Dis. 2016;31(3):543–551. doi:10.1007/s00384-015-2461-x
28. Cladellas M, Farre N, Comin-Colet J, et al. Effects of preoperative intravenous erythropoietin plus iron on outcome in anemic patients after cardiac valve replacement. Am J Cardiol. 2012;110(7):1021–1026. doi:10.1016/j.amjcard.2012.05.036
29. Munoz M, Gomez-Ramirez S, Martin-Montanez E, Naveira E, Seara J, Pavia J. Cost of post-operative intravenous iron therapy in total lower limb arthroplasty: a retrospective, matched cohort study. Blood Transfus. 2014;12(1):40–49. doi:10.2450/2013.0088-13
30. Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–124. doi:10.2471/BLT.14.138206
31. Turkiye iStatistik Kurumu. Periodic gross domestic product. Available from: https:// www.tuik.gov.tr/.
32. Goodnough LT, Shander A, Riou B. Patient blood management. Anesthesiology. 2012;116(6):1367–1376. doi:10.1097/ALN.0b013e318254d1a3
33. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406–3417. doi:10.1182/blood-2008-10-167643
34. Perkins HA, Busch MP. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress. Transfusion. 2010;50(10):2080–2099. doi:10.1111/j.1537-2995.2010.02851.x
35. Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753–765. doi:10.1111/j.1537-2995.2009.02518.x
36. Shander A, Fink A, Javidroozi M, et al. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25(3):232–246 e253. doi:10.1016/j.tmrv.2011.02.001
37. Vamvakas EC. Establishing causation in transfusion medicine and related tribulations. Transfus Med Rev. 2011;25(2):81–88. doi:10.1016/j.tmrv.2010.11.010
38. Glance LG, Dick AW, Mukamel DB, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114(2):283–292. doi:10.1097/ALN.0b013e3182054d06
39. Goodnough LT, Shieh L, Hadhazy E, Cheng N, Khari P, Maggio P. Improved blood utilization using real-time clinical decision support. Transfusion. 2014;54(5):1358–1365. doi:10.1111/trf.12445
40. Trentino KM, Mace HS, Symons K, et al. Screening and treating pre-operative anaemia and suboptimal iron stores in elective colorectal surgery: a cost effectiveness analysis. Anaesthesia. 2021;76(3):357–365. doi:10.1111/anae.15240
41. Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545–551. doi:10.1007/s00590-013-1225-y
42. Wan S, Sparring V, Cabrales DA, Jansson KA, Wikman A. Clinical and budget impact of treating preoperative anemia in major orthopedic surgery-a retrospective observational study. J Arthroplasty. 2020;35(11):3084–3088. doi:10.1016/j.arth.2020.06.018
43. Gross I, Seifert B, Hofmann A, Spahn DR. Patient blood management in cardiac surgery results in fewer transfusions and better outcome. Transfusion. 2015;55(5):1075–1081. doi:10.1111/trf.12946
44. Rognoni C, Gerzeli S. Ferric carboxymaltose for patients with heart failure and iron deficiency in Italy: cost-effectiveness and budget impact. J Comp Eff Res. 2019;8(13):1099–1110. doi:10.2217/cer-2019-0074
45. Kotze A, Carter LA, Scally AJ. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108(6):943–952. doi:10.1093/bja/aes135
46. Froessler B, Rueger AM, Connolly MP. Assessing the costs and benefits of perioperative iron deficiency anemia management with ferric carboxymaltose in Germany. Risk Manag Healthc Policy. 2018;11:77–82. doi:10.2147/RMHP.S157379
47. Basora M, Pereira A, Coca M, Tio M, Lozano L. Cost-effectiveness analysis of ferric carboxymaltose in pre-operative haemoglobin optimisation in patients undergoing primary knee arthroplasty. Blood Transfus. 2018;16(5):438–442. doi:10.2450/2018.0031-18
48. Mehra T, Seifert B, Bravo-Reiter S, et al. Implementation of a patient blood management monitoring and feedback program significantly reduces transfusions and costs. Transfusion. 2015;55(12):2807–2815. doi:10.1111/trf.13260
49. Sosyal Guvenlik Kurumu. Republic of Turkey Social Security Institution. Available from: http:// www.sgk.gov.tr/ wps/ portal/ sgk/ en/detail/social_security_system.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
