Back to Journals » Therapeutics and Clinical Risk Management » Volume 10
Cost-effectiveness analysis on the use of rFSH + rLH for the treatment of anovulation in hypogonadotropic hypogonadal women
Authors Papaleo E, Alviggi C, Colombo G, Pisanelli C, Ripellino C, Longobardi S, Canonico PL
Received 13 February 2014
Accepted for publication 23 April 2014
Published 25 June 2014 Volume 2014:10 Pages 479—484
DOI https://doi.org/10.2147/TCRM.S62351
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Enrico Papaleo,1 Carlo Alviggi,2 Giorgio Lorenzo Colombo,3,4 Claudio Pisanelli,5,6 Claudio Ripellino,7 Salvatore Longobardi,8 Pier Luigi Canonico9
1Centro Scienze della Natalità, Gynecological-Obstetrics Department, San Raffaele Hospital, Vita-Salute San Raffaele, Milan, Italy; 2Department of Neuroscience, Reproductive Sciences and Odontostomatology, University “Federico II” of Naples, Naples, Italy; 3Department of Drug Sciences, University of Pavia, Pavia, Italy; 4SAVE Studi Analisi Valutazioni Economiche, Milan, Italy; 5ACO San Filippo Neri, Rome, Italy; 6Società Italiana Di Farmacia Ospedaliera, Milan, Italy; 7CSD Medical Research Srl, Milan, Italy; 8Medical Department, Merck Serono SpA, Rome, Italy; 9Department of Pharmaceutical Sciences, University of Piemonte Orientale, Novara, Italy
Background: Hypogonadotropic hypogonadal women are characterized by ovarian functionality deficiency, caused by low concentrations of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). To recover reproduction functionality, recommended therapies for ovarian induction involve injections of FSH and LH medications.
Objective: Since important differences exist between recombinant and urinary gonadotropin therapies in terms of efficacy and cost, the objective of this study was to develop a cost-effectiveness model to compare recombinant FSH (rFSH) + recombinant LH (rLH) and highly purified human menopausal gonadotropin (HP-HMG).
Methods: A Markov model was developed, considering three cycles of therapy; probability of pregnancy and miscarriage were considered, and the efficacy was evaluated in terms of pregnancy occurrence. The perspective of the model was that of the Italian Health Service, so only direct cost (drugs, specialist visits, patient examinations, and hospitalizations) were included.
Results: rFSH + rLH is associated with a higher total cost (€3,453.50) and higher efficacy (0.87) compared with HP-HMG (€2,719.70 and 0.50). rFSH + rLH generated an incremental cost effectiveness ratio equal to €2,007.30 compared to HP-HMG; the average cost per pregnancy is estimated to be €3,990.00 for recombinant strategy and €5,439.80 for urinary strategy. Results of probabilistic sensitivity analysis were consistent with the abovementioned findings.
Conclusion: Despite the higher acquisition cost in comparison to HP-HMG, rFSH + rLH resulted in a higher pregnancy rate, which makes it the recommended choice when considering cost-effectiveness of LH in supporting FSH-induced follicular gonadotropins in hypogonadotropic hypogonadal women.
Keywords: HP-HMG, hypogonadotropic hypogonadism, gonadotropin
Introduction
Hypogonadotropic hypogonadism (HH) in women is characterized by lack or reduced function of the ovaries. It can be defined by inadequately low serum concentrations of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), due to gonadotropin-releasing hormone deficiency, which is the primary regulator of the reproductive function.
HH can be divided into two main categories: acquired or syndromic HH and idiopathic or isolated HH. The former is more common and is caused by several pathological processes with postnatal onset, including pituitary tumors, infections, head trauma, severe weight loss (anorexia nervosa), excessive chronic physical exercise, severe emotional stress, or medications (gonadotropin-releasing hormone agonists/antagonists, narcotics, chemotherapy), while the latter includes various congenital syndromes, such as Kallmann syndrome and normosmic idiopathic HH. Genetic causes of hypogonadism have been identified in several genes.1–3
Characteristic of HH is the absence of usual sexual modifications during puberty or, if presented after puberty, possible secondary amenorrhea. The main consequence of this disease is anovulation, which causes infertility during the reproductive period.4
In the United States, the incidence of Kallmann syndrome in women is one case per 50,000, while for normosmic idiopathic HH, it is estimated to be around one case per 70,000 to one case per 100,000.5
In order to recover reproductive functionality, convenient treatment options for ovulation induction (OI) include daily injection of exogenous gonadotropins, which are indicated when OI cannot be achieved with less complex methods.6 Before starting, all potential coexisting causes of infertility (Fallopian tube disorders, endometriosis, uterine abnormalities, cervical stenosis, and poor semen quality in men) must be excluded, since gonadotropin therapy is costly and has significant risks.2
The two key gonadotropic hormones, LH and FSH, were discovered in the early 20th century and then extracted in 1950 from menopausal urine to create human menopausal gonadotropin (HMG), which has an equal ratio of FSH- to LH-like activity.7–9 In the last 2 decades, scientific progress has enabled the involvement of genetic engineering in the development of recombinant gonadotropins, producing highly purified and effective products and allowing for the choice between two types of gonadotropins, differently derived, for practitioners.10,11
In HH women, unlike in other female infertile subjects for whom FSH therapy alone is indicated, both LH and FSH are required to reach an adequate follicular response.12
Since important differences exist between recombinant and urinary gonadotropin therapies in terms of efficacy and cost, the objectives of this study were to perform a comparison between recombinant FSH (rFSH) + recombinant LH (rLH) and HMG in severe LH- and FSH-deficient anovulatory women (ie, those with HH) and develop a cost-effectiveness analysis (CEA).
Materials and methods
Clinical evidence
The pregnancy data on which the economic evaluation was based were retrieved from a single study conducted by Carone et al.4 The aim of this two-arm, randomized, open-label study4 was to compare the efficacy of rFSH + rLH in a 2:1 ratio to highly purified HMG (HP-HMG) urinary extract, containing LH-like activity, in 35 women with severe LH and FSH deficiency (World Health Organization [WHO] type I anovulation [HH]).13 Results demonstrated that the pregnancy rate in the rFSH + rLH group was 55.6% compared to 23.3% in the HP-HMG group (P=0.01).4 Authors based the analysis only on this study, since other publications that compare rFSH + rLH with HP-HMG in women with HH are not available. Nevertheless, in order to account for the uncertainty due to unavailability of relevant data, probability distributions were applied to the model’s efficacy parameters.
Model design
A Markov model was developed to assess the clinical and economical outcomes of the two treatment strategies in Italy; the analysis involved medical experts’ feedback in order to reflect the local management of patients in the Italian health care system context.
A representation of the Markov model, developed using TreeAge Pro 2011 software (TreeAge Software, Inc., Williamstown, MA, USA), is depicted in Figure 1.
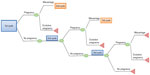  | Figure 1 Structure of the Markov model. |
In this model, the population starts the first cycle therapy with recombinant or urinary gonadotropins following pregnancy evaluation. If a woman becomes pregnant, the possibility of miscarriage is considered. Since the time horizon of this Markov model was set up for three consecutive cycles, women who do not become pregnant during the first series of treatment or have a miscarriage undergo a second cycle of therapy, maintaining the same treatment of the previous cycle. The same process applies to the third cycle.
According to the study conducted by Carone et al, a therapy cycle is described as gonadotropin treatment for a maximum of 16 days and OI by a single administration of human chorionic gonadotropin (hCG) on the day after the last gonadotropin treatments, when the leading follicle has reached a mean diameter of ≥17 mm.4 Furthermore, both Carone et al and the authors considered OI, with recombinant or urinary treatment, to be associated with timed sexual intercourse.4 Efficacy (outcome) for the present CEA was evaluated in terms of pregnancy development.
For each of the three cycles, transition probabilities (pregnancy rates and miscarriage rates) were derived from Carone et al and are presented in Table 1.4 These probabilities were assigned to both the recombinant and the urinary branch.
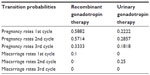  | Table 1 Model input data: transition probabilities |
Costs
The perspective of the economic evaluation was that of the Italian National Health Service (Servizio Sanitario Nazionale [SSN]); therefore, this analysis considered only direct medical costs including those of drugs, specialist visits, patient examinations, and hospitalizations for medical abortions.
All costs are expressed in euros and have been updated to 2013 prices. No discounting was applied due to the short time horizon employed in the model.
Treatment doses of HP-HMG and rFSH + rLH were assumed based on the average number of vials reported in the Carone et al study4 in which the FSH daily dose was fixed at 150 IU, based upon clinical experience, to ensure that all patients would be above their threshold needs.4,12–15 Unit costs of €119.73 per vial of rFSH + rLH, containing 150 IU of FSH activity and 75 IU of LH activity, and €26.57 per vial of HP-HMG, containing 75 IU of FSH activity and 75 IU of LH activity, were obtained from the prices database available on the Codifa database.16 Treatment doses and costs are shown in Table 2. According to Carone et al the authors have assumed that hypothetical patients have been treated with a dose of 10,000 IU of hCG by a single administration on the day after the last HP-HMG or rFSH + rLH treatment, in order to induce ovulation.4
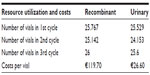  | Table 2 Model input data: treatment doses and costs |
Specialist visits and patient examinations were assessed with the help of medical experts to reflect standard clinical practice in Italy. Medical experts suggested a list of examinations that, according to standard clinical practice, are usually undertaken before the treatment starts and a set of examinations that are performed during each cycle of treatment (Table 3); we decided on four clinical consultations for the model, comprising examinations, during the stimulation period.
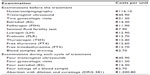  | Table 3 Model input data: other health costs |
Regional outpatient exam pricelists (available in their respective region websites) were used for retrieving resource utilization costs based on the health service perspective of 16 Italian regions. Population-based weighted regional data were used to feed the model.
It was assumed in our study that 50% of spontaneous abortions have implied dilation and curettage; this is why a mean cost derived from population-based weighted regional data on Diagnosis-Related Group price lists (DRG 381) was applied.
Regarding efficacy data, we applied probability distributions on cost inputs in order to account for uncertainty in the model.
CEA and probabilistic sensitivity analysis (PSA)
CEA is a method of assessing health gains in relation to the costs of different health interventions.
The main outcomes of CEA were mean therapy costs and number of developed pregnancies per therapy, considering the three-treatment-cycle horizon studied, and the incremental cost-effectiveness ratios (ICERs) that should be interpreted as the additional cost required to gain an additional unit of health outcome (pregnancy) when providing one treatment rather than another one.
Probabilities and treatment costs were subject to uncertainty, so it was necessary to test reliability of the results.
For this reason, we assessed uncertainty in the model using PSA, where the value for each parameter was determined independently from a probability distribution, and the results recalculated for each simulation. Ten thousand simulations were performed for PSA.
Gamma distributions were used for costs while beta distributions were applied for all the probabilities of the model.
Results
The results of the CEA are shown in Table 4.
  | Table 4 Results: costs, effectiveness, and incremental cost-effectiveness ratio (ICER) of the base case scenario |
HP-HMG is associated with a lower total cost (€2,719.70) than rFSH + rLH (€3,453.50); the recombinant therapy is €733.80 more expensive than urinary therapy due to the higher cost of drugs. The efficacy (ie, the pregnancy probability) associated with HP-HMG treatment is 0.50, whereas it is 0.87 for rFSH + rLH; in other words, there will be 50 pregnancies per 100 patients treated with HP-HMG and 87 pregnancies per 100 patients treated with rFSH + rLH.
The average cost per pregnancy was estimated to be €3,990.00 in the case of recombinant strategy and €5,439.80 in the case of urinary strategy, underlining a lower cost for pregnancy for rFSH + rLH.
Furthermore, rFSH + rLH generated an ICER equal to €2,007.30 compared to HP-HMG, which is the additional cost required for rFSH + rLH to gain an additional pregnancy in comparison with HP-HMG.
Finally, the results of the PSA are presented in the incremental CE scatter plot in Figure 2, which displays the distribution of differences in costs on the vertical axis and the distribution of differences in effects on the horizontal axis. Each one of the 10,000 simulations is represented as a point on the plot by means of coordinates based on distributions defined for each parameter.
  | Figure 2 Results of the probabilistic sensitivity analysis: Incremental cost-effectiveness, recombinant vs urinary gonadotropin therapy. |
Nearly all of the points indicate that the differences in costs and in efficacy are positive, indicating that rFSH + rLH is more expensive (up to €1.800 more than HP-HMG) but also more effective (from 18% to 57% more than HP-HMG). In conclusion, all the points in the scatter plot indicate that recombinant therapy is slightly more expensive compared to urinary therapy but is more effective.
Discussion
The main consequence of women suffering from HH is infertility during reproductive age. The absence of ovarian function determines the need for a gonadotrophin treatment to restore ovulation and, subsequently, fertility. Cost-effectiveness of OI in anovulatory, infertile patients may be related directly to pregnancy as a primary endpoint. The results of this pharmacoeconomic study indicate that the average cost per pregnancy is lower for treatment with rFSH + rLH than for treatment with HP-HMG because of the strong impact of the efficacy of the recombinant therapy with respect to the urinary therapy (87 pregnancies per 100 patients compared to 50 pregnancies per 100 patients). The ICER value for rFSH + rLH compared to HP-HMG was estimated to be €2,007.30; however, from a cost-effectiveness point of view, the preferred strategy depends on INHS willingness to pay: if the SSN is willing to pay less than €2,007.30 for one extra pregnancy, HP-HMG is to be preferred; if the SSN is willing to pay €2,007.30 or more for one extra pregnancy, rFSH + rLH is to be preferred. Since no national or international thresholds have ever been defined regarding ICER per pregnancy, no clear indications on the willingness to pay per pregnancy can be supported by the authors. The PSA results, based on 10,000 simulations, confirmed the findings observed in the base case analyses, giving robustness to the model.
This analysis is the first CEA comparing rFSH + rLH and HP-HMG for women with HH in Italy. Currently available evidence indicates that rFSH alone may not be sufficient to promote optimum follicular growth in severely gonadotropin-deficient women.12,17
Several studies have indicated that rFSH and rLH could be seen as an optimum preparation in terms of safety and clinical efficacy in HH patients.4,18–20
In the study by Carone et al, which was considered as the basis of this analysis, the authors showed the superiority of LH compared to hCG in supporting FSH-induced follicular development in WHO group I anovulatory women.4 The two sources of LH used, rFSH + rLH and HP-HMG, gave statistically different results in terms of pregnancy rate (P=0.01).
A Spanish multicenter study including 38 hypogonadotropic anovulatory (WHO group I) women undergoing 84 OI cycles in which patients received 150 IU/day rFSH and 75 IU/day rLH, confirmed that combined rFSH and rLH treatment induces follicular growth (sufficient follicular growth was observed in 79 [94%] out of 84 initiated cycles), ovulation, and pregnancy (cumulative pregnancy rate following three cycles of stimulation with follitropin alfa and lutropin alfa was 39.5%) in a good proportion of hypogonadotropic anovulatory patients and is well tolerated.18
Another study, by Kaufmann et al, demonstrated that 16 of 31 hypogonadotropic hypogonadal women with profound gonadotropin deficiency treated with lutropin alfa and follitropin alfa reached clinical pregnancy (59.3%).19
A review published by Bosch described pharmacological and clinical aspects of rFSH + rLH through the analysis of the Phase I, II, and III trials and postmarketing clinical randomized trials performed since the initial assays from 1998 to 2010.20 The 2:1 combination of rhFSH and rhLH has been seen as an optimum preparation in terms of safety and clinical efficacy in HH patients.
Studies based on pharmacoeconomic models fed with data reported in the literature and on different assumptions (such as that presented in this paper) suffer from biases and limitations and cannot be a substitute for direct real-life comparisons. The lack of data in literature comparing rFSH + rLH and HP-HMG in women with HH, which led us to base this pharmacoeconomic evaluation on a single trial, is an important limitation of the present study. Nevertheless, statistical distributions applied on all parameters of the model allowed us to be confident on model results. The fact that we considered only OI associated with timed sexual intercourse could be another potential limitation.
Nonetheless, in the application of any medically assisted procreation technique both recombinant and urinary branches are affected; thus, the choice of a different technique have an impact on efficacy and costs of both recombinant and urinary treatments. Furthermore, more complex techniques of medically assisted procreation, such as in vitro fertilization, depend also on the quality of the laboratory that is an aspect not present in low-technology infertility treatments; thus, OI alone allows for assessement of ovulatory quality to based on different gonadotropins without laboratory biases. A similar approach, ie, considering OI with timed sexual intercourse, was employed by Revelli et al.21
Another potential limitation is represented by the estimated national costs, which were based on mean costs weighted by the populations of 16 Italian regions. Nevertheless, since the Italian health care system decentralizes the administrative decisions at regional level, creating several pricelists for each region, applying a weighted mean to the regional data is a common way of estimating national cost data.22 Moreover, in this case, the mean costs have been varied by means of probability distributions, with a PSA, in order to account for uncertainty in the model.
Despite their limitations, pharmacoeconomic models play a fundamental role in establishing priorities in the allocation of resources in a specific therapeutic area, supplying decision-makers within health care systems with useful tools for making more rational and effective decisions.23
Conclusion
The results of this CEA indicate that combination therapy with rFSH + rLH is a cost-efficient treatment strategy for the Italian health service compared to HP-HMG in the treatment of infertility in hypogonadotropic hypogonadal women. In fact, recombinant treatment provides a lower average cost per pregnancy and an incremental cost per pregnancy of €2,007.30. Although therapy with rFSH + rLH has a relatively high acquisition cost, it is associated with a higher pregnancy rate. Hence, rFSH + rLH might be a better choice than HP-HMG when assessing cost-effectiveness of LH in supporting FSH-induced follicular gonadotropins in hypogonadotropic hypogonadal women.
Disclosure
This study was financially supported by Merck Serono SpA, which was not responsible for creation of the study documents, the data analysis, data interpretation, or writing of the manuscript. SL is an employee of Merck Serono SpA. CR is an employee of CSD Medical Research Srl. EP, CA, GC, CP, and PLC have received honoraria for manuscript writing by Merck Serono SpA. The authors report no other conflicts of interest in this work. All authors were responsible for data interpretation and reviewed and approved the final manuscript.
References
Skałba P, Guz M. Hypogonadotropic hypogonadism in women. Endokrynol Pol. 2011;62(6):560–567. | |
Practice Committee of American Society for Reproductive Medicine. Use of exogenous gonadotropins in anovulatory women: a technical bulletin. Fertil Steril. 2008;90(Suppl 5):S7–S12. | |
Silveira LF, Latronico AL. Approach to the patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2013;98:1781–1788. | |
Carone D, Caropreso C, Vitti A, Chiappetta R. Efficacy of different gonadotropin combinations to support ovulation induction in WHO type I anovulation infertility: clinical evidences of human recombinant FSH/human recombinant LH in a 2:1 ratio and highly purified human menopausal gonadotropin stimulation protocols. J Endocrinol Invest. 2012;35(11):996–1002. | |
Popat V. Gonadotropin-releasing hormone deficiency in adults. The European Journal of Endocrinology [online]. JE Publishing; 2012. Available from: http://ej-endocrinology.org/gnrh.html. Accessed September 13, 2013. | |
Huirne JA, Lambalk CB, van Loenen AC, et al. Contemporary pharmacological manipulation in assisted reproduction. Drugs. 2004;64:297–322. | |
Smith MG. On the interruption of pregnancy in the rat by the injection of ovarian follicular extract. Bull Johns Hopkins Hosp. 1926;39(4):203–214. | |
Fevold HL, Hisaw FL, Leonard SL. The gonad-stimulating and the luteinizing hormones of the anterior lobe of the hypophysis. Am J Physiol. 1931;97:291–301. Available from: http://ajplegacy.physiology.org/content/ajplegacy/97/2/291.full.pdf. Accessed May 22, 2014. | |
Lunenfeld B. Historical perspectives in gonadotrophin therapy. Hum Reprod Update. 2004;10(6):453–467. | |
Loumaye E, Campbell R, Salat-Baroux J. Human follicle-stimulating hormone produced by recombinant DNA technology: a review for clinicians. Hum Reprod Update. 1995;1(2):188–199. | |
Hull M, Corrigan E, Piazzi A, Loumaye E. Recombinant human luteinising hormone: an effective new gonadotropin preparation. Lancet. 1994;344:334–335. | |
[No authors listed]. Recombinant human luteinizing hormone (LH) to support recombinant human follicle-stimulating hormone (FSH)-induced follicular development in LH- and FSH-deficient anovulatory women: a dose-finding study. The European Recombinant Human LH Study Group. J Clin Endocrinol Metab. 1998;83(5):1507–1514. | |
Rowe PJ, Combaire FH, Hargreave TB, et al. WHO manual for the standardized investigation and diagnosis of the infertile couple. Cambridge University Press, 2001. | |
Bühler K, Naether O. A 2:1 formulation of follitropin alfa and lutropin alfa in routine clinical practice: a large, multicentre, observational study. Gynecol Endocrinol. 2011;27(9):650–654. | |
Bühler KF, Fischer R. Recombinant human LH supplementation versus supplementation with urinary hCG-based LH activity during controlled ovarian stimulation in the long GnRH-agonist protocol: a matched case-control study. Gynecol Endocrinol. 2012;28(5):345–350. | |
Codifa [database on the Internet]. Milan: EDRA LSWR SpA. Available from: http://www.codifa.it. Accessed July 25, 2013. Italian. | |
Hugues JN, Soussis J, Calderon I, Balasch J, Anderson RA, Romeu A; Recombinant LH Study Group. Does the addition of recombinant LH in WHO group II anovulatory women over-responding to FSH treatment reduce the number of developing follicles? A dose-finding study. Hum Reprod. 2005;20(3):629–635. | |
Burgués S; Spanish Collaborative Group on Female Hypogonadotrophic Hypogonadism. The effectiveness and safety of recombinant human LH to support follicular development induced by recombinant human FSH in WHO group I anovulation: evidence from a multicentre study in Spain. Hum Reprod. 2001;16(12):2525–2532. | |
Kaufmann R, Dunn R, Vaughn T, et al. Recombinant human luteinizing hormone, lutropin alfa, for the induction of follicular development and pregnancy in profoundly gonadotrophin-deficient women. Clin Endocrinol (Oxf). 2007;67(4):563–569. | |
Bosch E. Recombinant human follicular stimulating hormone and recombinant human luteinizing hormone in a 2:1 ratio combination. Pharmacological characteristics and clinical applications. Expert Opin Biol Ther. 2010;10(6):1001–1009. | |
Revelli A, Poso F, Gennarelli G, Moffa F, Grassi G, Massobrio M. Recombinant versus highly-purified, urinary follicle-stimulating hormone (r-FSH vs HP-uFSH) in ovulation induction: a prospective, randomized study with cost-minimization analysis. Reprod Biol Endocrinol. 2006;4:38. | |
Mencacci C, Aguglia E, Biggio G, et al. C-QUALITY: cost and quality-of-life pharmacoeconomic analysis of antidepressants in major depressive disorder in Italy. Adv Ther. 2013;30(7):697–712. | |
Colombo GL, Di Matteo S, Maggiolo F. Antiretroviral therapy in HIV-infected patients: a proposal to assess the economic value of the single-tablet regimen. Clinicoecon Outcomes Res. 2013;5:59–68. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
