Back to Journals » ClinicoEconomics and Outcomes Research » Volume 10
Cost-effectiveness analysis of voriconazole, fluconazole, and amphotericin B for invasive fungal infections following allogeneic hematopoietic stem cell transplantation in Mexico
Authors Morfín-Otero R , Alvarado-Ibarra M , Rodriguez-Noriega E, Resendiz-Sanchez J , Patel DA , Stephens JM, Di Fusco M, Mendoza CF, Charbonneau C
Received 21 November 2017
Accepted for publication 21 June 2018
Published 6 September 2018 Volume 2018:10 Pages 511—520
DOI https://doi.org/10.2147/CEOR.S157642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Samer Hamidi
Rayo Morfín-Otero,1,2 Martha Alvarado-Ibarra,3 Eduardo Rodriguez-Noriega,1,2 Jesus Resendiz-Sanchez,4 Dipen A Patel,5 Jennifer M Stephens,5 Manuela Di Fusco,6 Carlos F Mendoza,6 Claudie Charbonneau7
1Institute of Infectious and Experimental Pathology, University Center for Health Science, University of Guadalajara, Guadalajara, Jalisco, Mexico; 2Division of Infectious Diseases, Civil Hospital of Guadalajara, Fray Antonio Alcalde, Guadalajara, Jalisco, Mexico; 3Hematology Service, National Medical Center ‘November 20’, Mexico City, Mexico; 4Laboratory of Mycology, Children’s Hospital of Mexico, Mexico City, Mexico; 5Pharmerit International, Bethesda, MD, USA; 6Pfizer Inc, Mexico City, Mexico; 7Pfizer International Operations, Paris, Île-de-France, France
Background: Patients receiving allogeneic hematopoietic stem cell transplantation (alloHSCT) are at high risk of invasive fungal infections (IFIs), which are associated with high mortality and economic burden. The cost-effectiveness of prophylaxis for the prevention of IFIs in alloHSCT recipients in Mexico has not yet been assessed.
Methods: This analysis modeled a hypothetical cohort of 1,000 patients to estimate costs and outcomes for patients receiving prophylaxis for IFIs following alloHSCT, from the perspective of institutional payers in Mexico. The main prophylaxis agents currently used in Mexican clinical practice are voriconazole, fluconazole, and amphotericin B (AmB). The model accounted for event rates of IFIs during each treatment, assuming IFI causality due to invasive aspergillosis, invasive candidiasis, or other IFIs, and that the outcome for patients during follow-up was IFI-related death, death from other causes, or survival. Clinical efficacies were obtained from published literature; costs were based on local sources. Cost-effectiveness was assessed using incremental cost-effectiveness ratios (ICERs). Univariate (assessing the impact of varying each model parameter) and probabilistic sensitivity analyses were performed.
Results: Voriconazole was associated with the lowest number of breakthrough IFIs, IFI-related deaths, and total number of deaths. Total costs were lower for fluconazole (Mexican pesos [MXN] 72,944; US $4,079) than voriconazole (MXN 101,413; US $5,671) or AmB (MXN 110,529; US $6,180). Voriconazole had better clinical outcomes and lower costs than AmB and could be considered cost-effective compared with fluconazole in line with the local ICER threshold. Drug costs, monitoring costs, and duration of prophylaxis were most sensitive to variation from univariate sensitivity analysis. Findings from the probabilistic sensitivity analysis were consistent with the base-case results.
Conclusion: Voriconazole had the most favorable clinical outcomes, but overall prophylaxis costs were higher than with fluconazole. Overall, based on local ICER thresholds (MXN 184,665; US $10,326), voriconazole was considered a cost-effective option for prophylaxis of IFI in Mexico.
Keywords: allogeneic hematopoietic stem cell transplantation, triazole, invasive aspergillosis, invasive candidiasis, incremental cost-effectiveness ratio, prophylaxis
Introduction
Patients receiving allogeneic hematopoietic stem cell transplantation (alloHSCT) are at high risk of developing invasive fungal infections (IFIs),1 with reported rates following alloHSCT of ~6%–14% in US clinical practice.2,3 Morbidity and mortality due to IFIs are high, with a reported 90-day mortality of 57% following the first post-transplant IFI2; consequently, the economic burden of IFIs in this patient group is considerable.4,5 A recent evaluation of the costs of diagnosis and treatment of IFIs in the UK estimated a cost of ~£50,000 (in US dollars, ~$72,900) per case.6
Approximately 60%–70% of IFIs in patients who have received alloHSCT are attributable to yeasts, invasive aspergillosis, or invasive candidiasis infection.7 Invasive aspergillosis is the most common IFI in this setting, with estimates of the proportion of IFIs due to invasive aspergillosis typically in the range of 43%–59%.2,3,8 The 1-year incidence of invasive aspergillosis following alloHSCT is ~10%–14%.9–11 Mortality from invasive aspergillosis following alloHSCT can be as high as 18% after 12 weeks in patients receiving antifungal treatment for probable/proven aspergillosis,12 and survival rates of ~30% at 6 months and 20% after 1 year have been reported.11
There is a paucity of data on IFI incidence and triazole prophylaxis in alloHSCT recipients in Latin America. Two reviews of Latin American data13,14 suggest that the incidence of IFIs (and specifically candidiasis and aspergillosis infections) is higher across Latin America than in the USA or Europe.3 In a single-center Chilean survey of IFIs in patients with hemato-oncological complications and alloHSCT recipients, the majority (63.4%) of cases were attributable to aspergillosis infection.15 A prospective multi-center cohort study in 8 Brazilian centers suggested that the 1-year incidence of IFI after alloHSCT was 11.3%.16
Several antifungal therapies are available for the prophylaxis of IFI in patients receiving alloHSCT, including the polyene amphotericin B (AmB), the generic oral triazoles fluconazole and itraconazole, and the newer generation oral triazoles posaconazole and voriconazole. In Mexico, the preferred prophylaxis options are fluconazole, AmB, and voriconazole. Fluconazole and AmB have demonstrated prophylactic efficacy in this clinical context and are available as generic formulations. Resistance to triazoles can be an issue, as observed in a recent trial in the Netherlands,17 and in such cases, liposomal AmB is the preferred treatment option. However, AmB is associated with increased toxicity, and fluconazole does not protect against invasive aspergillosis.18 A recent meta-analysis reported that mold-active prophylaxis significantly reduced IFI-related mortality in patients receiving chemotherapy or alloHSCT,19 and early antifungal prophylaxis with broad-spectrum (ie, targeting both molds and yeasts) triazoles following alloHSCT reduces IFI-related morbidity and mortality in this high-risk patient population.1,20 Accordingly, mold-active, newer generation triazoles have been developed, and voriconazole has been demonstrated to have comparable prophylactic efficacy to itraconazole and fluconazole,21,22 and improved tolerability compared with itraconazole.21
A mixed treatment comparison (MTC) assessing oral triazoles for prophylaxis of IFIs in patients receiving alloHSCT reported that voriconazole was associated with fewer IFIs than fluconazole during 180 days of follow-up.23 As there are currently no economic evaluations of prophylaxis of IFI in patients receiving alloHSCT in Latin American countries, we used a cost-effectiveness model to estimate clinical and economic outcomes of prophylaxis therapies for IFIs in patients who had undergone alloHSCT, from the perspective of institutional payers in Mexico.
Methods
Model structure
A decision-analytic model with a hypothetical cohort of 1,000 patients was used to estimate costs and outcomes for patients receiving prophylaxis for IFIs following alloHSCT (Figure 1). Microsoft Excel software was used to compute costs and outcomes.
The model was designed from the perspective of institutional payers in charge of social security services in Mexico based on a similar decision-tree model used in an economic assessment of IFI prophylaxis in alloHSCT recipients in Spain, and verified by local clinical experts.24 A decision-tree model was chosen based on the short time horizon of 6 months, the low number of disease states, and the low likelihood of disease recurrence.25 The Instituto Mexicano del Seguro Social (IMSS) is the largest health care insurance system in Mexico, and publishes publicly available details of resource use and costs, which were used as sources for this analysis.
Locally approved prophylaxis agents routinely used in Mexico (based on local expert clinical opinion) considered in this study were voriconazole, fluconazole, and conventional AmB. At the time of the analysis, itraconazole was not widely used in Mexican clinical practice and posaconazole was not approved as prophylaxis in this setting; these treatments were, therefore, not included in the model. Prophylaxis was assumed to begin on the day of transplant (Day 0) and all IFI events were assumed to occur in the short term, that is, within 180 days of alloHSCT; co-infection was assumed not to occur. The model accounted for event rates of IFIs during each prophylactic treatment, assuming that those IFIs would be due to invasive aspergillosis, invasive candidiasis, or other IFIs, and that the outcome for patients during follow-up was IFI-related death, death from other causes, or survival.
The model accounted for use of other licensed antifungal therapies (OLATs), either as alternative antifungal prophylaxis or empirical therapy of suspected IFI. It was assumed that the duration of OLATs was 18 days (based on local expert clinical opinion of the investigators [RM-O, MA-I, ER-N, and JR-S]) and that patients were treated only once during the 180-day post-alloHSCT period. The model did not specifically incorporate prophylaxis discontinuation rates but instead assigned mean duration of prophylaxis when calculating costs.
Clinical inputs
Key clinical inputs and sources were obtained by a targeted literature search and are summarized in Table 1.18,23,26–30 For the base-case analysis, event rates of IFI and OLAT were obtained from an MTC (for voriconazole and fluconazole)23 and published literature (for AmB).18 Inputs for duration of prophylaxis and mortality were taken from published literature.18,23,28,29 All inputs were reviewed by local clinical experts.
  | Table 1 Costs and clinical inputs for cost-effectiveness model of antifungal prophylaxis following alloHSCT in Mexico (base-case) Notes: aData presented are based on the cited source material and expert clinical opinion. bDuration of prophylaxis for AmB is assumed to be the same as the duration of prophylaxis for voriconazole. cRates for voriconazole and fluconazole calculated from a mixed treatment comparison (Bow et al23); rates for AmB calculated by applying a risk ratio (calculated from AmB vs fluconazole data from Wolff et al18) to the mixed treatment comparison base-rate for fluconazole. dCost of treating “other IFI” is assumed to be the same as cost of treating invasive aspergillosis. Abbreviations: alloHSCT, allogeneic hematopoietic stem cell transplantation; AmB, amphotericin B; IA, invasive aspergillosis; IC, invasive candidiasis; IFI, invasive fungal infection; MXN, Mexican pesos; OLATs, other licensed antifungal therapies. |
The distribution of the type of OLAT was based on local expert clinical opinion: the distribution of therapies in patients receiving OLAT during voriconazole therapy was 20% liposomal AmB, 70% AmB, and 10% itraconazole; for patients requiring OLAT during fluconazole therapy, 10% liposomal AmB, 50% AmB, and 40% voriconazole; and for patients requiring OLAT during AmB therapy, 50% liposomal AmB and 50% voriconazole.
Cost inputs
Key cost inputs are also presented in Table 1; all costs are presented in Mexican pesos (MXN) and converted to US dollars ($) (using an exchange rate of 1 MXN = $0.0559152, March 3, 2016). No discounting was used due to the relatively short time horizon. Costs for all therapies (including OLATs) were obtained from published literature and verified by local expert clinical opinion. Monitoring and management costs were taken from IMSS.27 Drug costs were obtained from Instituto de Investigación e Innovación Farmacéutica.26
Total costs of prophylaxis were derived by multiplying the duration of antifungal therapy by the unit cost per day; costs of OLATs were calculated based on an average patient weight of 75 kg. The cost of treating a breakthrough IFI was MXN 266,531 ($14,903) for invasive aspergillosis and MXN 455,729 ($25,482) for invasive candidiasis27; costs for any other IFIs were assumed to be the same as for invasive aspergillosis. Frequency of monitoring was based on local expert clinical opinion; an enzyme immunoassay or sandwich enzyme-linked immunosorbent assay for galactomannan testing was assumed to be conducted twice weekly at a cost of MXN 490 ($27) per test, and a computed tomography scan was assumed to be conducted once every 4 weeks at a cost of MXN 2,787 ($156) per test, amounting to a total monitoring cost of MXN 43,116 ($2,411) per patient over the 180-day post-alloHSCT follow-up period.
Determination of cost-effectiveness
Cost-effectiveness was assessed using incremental cost-effectiveness ratios (ICERs; ie, the ratio of the difference in costs to the difference in clinical outcomes for one treatment compared with another). The resulting ICER was a numerical value if a treatment provided additional improved clinical outcomes at an increased cost compared with another treatment. However, if a treatment was expected to provide fewer health benefits at an increased cost, the ICER was not calculated, and the drug was considered “dominated” by the comparator treatment.
Sensitivity analyses
A one-way sensitivity analysis was performed, assessing the impact on results when each model parameter was independently varied. A probabilistic sensitivity analysis involving 1,000 model replications was used to measure the overall uncertainty associated with model results (as a function of uncertainty about each parameter at the same time); this evaluated the impact of this uncertainty on the estimated cost-effectiveness of the comparator agents involved.
Results
Costs and outcomes based on the hypothetical cohort of 1,000 patients are summarized in Table 2. Total costs per patient were lower with fluconazole (MXN 72,944 [$4,079]) than with voriconazole (MXN 101,413 [$5,671]) or AmB (MXN 110,529 [$6,180]). Costs per patient for treatment of breakthrough IFIs were considerably lower for voriconazole (MXN 11,718 [$655]) than for fluconazole (MXN 25,147 [$1,406]) or AmB (MXN 30,872 [$1,726]); costs of prophylaxis antifungal therapy were higher for voriconazole (MXN 41,903 [$2,343]) than either fluconazole (MXN 257 [$14]) or AmB (MXN 21,713 [$1,214]). Total monitoring costs were MXN 43,116 ($2,411) per patient over the 180-day post-alloHSCT follow-up period, regardless of prophylactic agent.
  | Table 2 Model predictions of costs/clinical outcomes/utilities over 180 days of antifungal prophylaxis following alloHSCT in Mexico Notes: aUtility values used to compute QALYs are shown in Table S1. Dominated: the comparator treatment was both less costly and associated with improved clinical outcomes. Abbreviations: alloHSCT, allogeneic hematopoietic stem cell transplantation; AmB, amphotericin B; IA, invasive aspergillosis; IC, invasive candidiasis; ICER, incremental cost-effectiveness ratio; IFI, invasive fungal infection; MXN, Mexican pesos; OLATs, other licensed antifungal therapies; QALY, quality-adjusted life-year. |
Voriconazole was associated with the lowest number of total breakthrough IFIs, IFIs due to invasive aspergillosis and invasive candidiasis, IFI-related deaths, invasive aspergillosis-related deaths, invasive candidiasis-related deaths, and total number of deaths. The number of patients needed to treat to avoid an additional IFI, relative to AmB (the treatment with the highest number of IFIs), was 18 for voriconazole and 120 for fluconazole. The number of additional patients needed to treat to avoid a death, relative to fluconazole (the treatment with the highest all-cause mortality), was 37 for voriconazole and 642 for AmB.
Life-years gained and quality-adjusted life-years (QALYs) gained were highest for voriconazole, followed by fluconazole and then AmB. In terms of ICER calculations, voriconazole “dominated” AmB (ie, the former had better clinical outcomes with a lower cost of MXN 9,116 [$510]) and provided improved clinical outcomes compared with fluconazole, with a cost per IFI avoided of MXN 599,359 ($33,513), costs per death avoided of MXN 1,058,090 ($59,163), costs per life-year gained of MXN 103,808 ($5,804), and costs per QALY gained of MXN 144,057 ($8,055); a deterministic cost-effectiveness plane for incremental cost per IFI avoided is provided in Figure S1. In the one-way sensitivity analysis, the incremental cost-effectiveness per IFI avoided for voriconazole compared with AmB was most sensitive to variation in drug and monitoring costs, and to the duration of prophylaxis (Figure 2).
Cost-effectiveness acceptability curves for an additional life-year gained with prophylaxis (Figure 3) suggest that voriconazole has a higher probability of being cost-effective than AmB or fluconazole.
  | Figure 3 Cost-effectiveness acceptability curves for an additional life-year gained with prophylaxis. Abbreviations: AmB, amphotericin B. |
Discussion
In this study, we performed a cost-effectiveness analysis of prophylaxis therapies for the prevention of IFIs in patients who have recently undergone alloHSCT in Mexico. Voriconazole had the best clinical outcome profile of the assessed prophylactic treatments (ie, was associated with the fewest IFIs and IFI-related deaths); treatment costs with voriconazole were higher, but were offset to some degree by reduced IFI treatment costs. Fluconazole and AmB, available in generic form, were associated with lower overall treatment costs per patient than voriconazole; however, the number of IFIs was higher with these treatments than with voriconazole.
Voriconazole was cost-effective relative to fluconazole when considering a local willingness-to-pay threshold of ~1 gross domestic product per capita per life year gained (around MXN 184,665 [$10,326]).31 Although upfront treatment costs were higher with voriconazole than fluconazole, breakthrough events and consequently ongoing resource use was lower. Adherence to antifungal prophylaxis is also crucial, as tolerability-related discontinuation is associated with increased resource use and costs; voriconazole has been shown to have better medication persistence than itraconazole, with fewer discontinuations, less switching to OLATs, and less resource costs.32 Extensive sensitivity analyses (Figure S2) reinforced the main findings of this study.
This study assessed only those treatments currently approved and routinely used for prophylaxis of IFIs in Mexican clinical practice. As itraconazole and posaconazole were not available through IMSS at the time of the analysis, neither were included in the model; however, itraconazole has recently been added to the list of IMSS-approved therapies. It may be prudent to update this cost-effectiveness analysis in the future when equivalent data for itraconazole are available. Additionally, with the advent of alternative therapies, fluconazole is currently used less than in previous years as therapy during general febrile neutropenia, but remains an important agent for prophylaxis of IFI following alloHSCT. The model was developed using data from the IMSS, the largest social security provider in Mexico, which covers over 50% of the working population. The Mexican health care system is a multi-payer system, and the IMSS is supplemented by other institutions, such as the Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (which covers ~9% of the population who are government employees), and other industry-specific decentralized institutional providers. Access to health care for those falling outside of institutional social security is provided by the Seguro Popular, a financial scheme supporting access for people with low incomes. These results represent the perspective of the single largest social security payer and, therefore, best represent routine clinical practice at public institutions in Mexico.
The present findings were in broad agreement with cost-effectiveness analyses conducted in other countries. A study of prophylactic triazole use in Spain predicted that voriconazole would be associated with higher costs than itraconazole; however, the analysis suggested that this would be partially offset by fewer IFIs and improved mortality.24 Another economic evaluation of antifungal prophylaxis in the Netherlands suggested that using voriconazole as primary prophylaxis, followed by caspofungin, was more likely to provide cost-effectiveness than using liposomal AmB as primary prophylaxis.33
Cost-effectiveness analyses, such as the presented study, have become a useful tool for evaluating costs and outcomes for clinical practice. The study presented multiple strengths, including model input on local resource use and costs, and was validated by local experts. However, there were several inherent limitations in our study. First, the model used data from an MTC analysis, as no studies were identified that directly compared all treatments. AmB was not included in this MTC, so results from a clinical trial18 were used to estimate the relative risks of IFI and OLATs with AmB compared with fluconazole. These relative risks were then applied to the base-rate for fluconazole from the MTC to generate adjusted rates for AmB. Second, we considered only prophylaxis, rather than empirical treatment of suspected neutropenia or diagnosis-based treatment. Additionally, we considered a hypothetical cohort of homogeneous patients, and as such did not account for individual risk factors or contraindications. This study considered only additional resource use arising from IFIs, and did not consider concomitant therapies given to alloHSCT recipients, such as antivirals or antimicrobials, which may vary by treatment center. Also, our analysis did not include posaconazole, as at the time of the analysis, this was not licensed locally by the Mexican National Health Council for treatment of IFIs in patients who have received alloHSCT, and was not listed by the IMSS. Finally, this analysis was conducted in 2016, when AmB deoxycholate was on the market and a relevant comparator for the model. There are other representations of amphotericin on the market that do not contain deoxycholate, such as liposomal amphotericin (Ambisome), which is ~10 times more expensive than AmB. Not including liposomal amphotericin as a comparator can be considered a limitation, but considering the substantial price difference, the current results reflect a conservative scenario, as we would expect voriconazole to have even better cost-effectiveness if compared against higher priced liposomal amphotericin.
Conclusion
In conclusion, results of the model suggested that voriconazole as prophylaxis for the prevention of IFIs in alloHSCT recipients was associated with more favorable clinical outcomes than AmB or fluconazole. Overall prophylaxis costs with voriconazole were lower than with AmB but higher than with fluconazole; these were partially offset by reduced IFI treatment costs. Overall, voriconazole can be considered a cost-effective option for prophylaxis of IFI in Mexico.
Data sharing statement
The datasets analyzed during the current study are available from the corresponding author ([email protected]) on reasonable request.
Acknowledgments
The model was originally developed by Sonja Sorensen of Evidera and funded by Pfizer. The authors would like to thank Alberto Batista of Pharmerit International for assistance in developing the model. Medical writing support, under the direction of the authors, was provided by Martin Bell, PhD, of CMC CONNECT, a division of Complete Medical Communications Ltd, Glasgow, UK, funded by Pfizer, in accordance with Good Publication Practice (GPP3) guidelines. This study was sponsored by Pfizer.
Author contributions
DAP and JMS adapted the model locally, gathered data and ran the analysis, based on feedback from all authors. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
ERN has received lecture and consulting fees from Pfizer. DAP and JMS are employees of Pharmerit International. Pharmerit International received research funding from Pfizer to conduct this research. MDF and CFM are employees of Pfizer Inc, and CC is an employee of Pfizer International Operations. The authors report no other conflicts of interest.
References
Maertens J, Marchetti O, Herbrecht R, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3--2009 update. Bone Marrow Transplant. 2011;46(5):709–718. | ||
Corzo-León DE, Satlin MJ, Soave R, et al. Epidemiology and outcomes of invasive fungal infections in allogeneic haematopoietic stem cell transplant recipients in the era of antifungal prophylaxis: a single-centre study with focus on emerging pathogens. Mycoses. 2015;58(6):325–336. | ||
Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–1100. | ||
des Champs-Bro B, Leroy-Cotteau A, Mazingue F, et al. Invasive fungal infections: epidemiology and analysis of antifungal prescriptions in onco-haematology. J Clin Pharm Ther. 2011;36(2):152–160. | ||
Karthaus M. Prophylaxis and treatment of invasive aspergillosis with voriconazole, posaconazole and caspofungin: review of the literature. Eur J Med Res. 2011;16(4):145–152. | ||
Ceesay MM, Sadique Z, Harris R, Ehrlich A, Adams EJ, Pagliuca A. Prospective evaluation of the cost of diagnosis and treatment of invasive fungal disease in a cohort of adult haematology patients in the UK. J Antimicrob Chemother. 2015;70(4):1175–1181. | ||
Hicheri Y, Cook G, Cordonnier C. Antifungal prophylaxis in haematology patients: the role of voriconazole. Clin Microbiol Infect. 2012;18(Suppl 2):1–15. | ||
Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48(3):265–273. | ||
Fukuda T, Boeckh M, Carter RA, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102(3):827–833. | ||
Garcia-Vidal C, Upton A, Kirby KA, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47(8):1041–1050. | ||
Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100(13):4358–4366. | ||
Herbrecht R, Caillot D, Cordonnier C, et al. Indications and outcomes of antifungal therapy in French patients with haematological conditions or recipients of haematopoietic stem cell transplantation. J Antimicrob Chemother. 2012;67(11):2731–2738. | ||
Nucci M, Queiroz-Telles F, Tobón AM, Restrepo A, Colombo AL. Epidemiology of opportunistic fungal infections in Latin America. Clin Infect Dis. 2010;51(5):561–570. | ||
Sifuentes-Osornio J, Corzo-León DE, Ponce-de-León LA. Epidemiology of invasive fungal infections in Latin America. Curr Fungal Infect Rep. 2012;6(1):23–34. | ||
Rabagliati BR, Fuentes LG, Guzmán DAM, et al. Invasive fungal disease in hemato-oncological and hematopoietic stem cell transplantation patients from Hospital Clinico Universidad Católica, Santiago-Chile using revised EORTC/MSG diagnostic criteria. Rev Chilena Infectol. 2009;26(3):212–219. | ||
Nucci M, Garnica M, Gloria AB, et al. Invasive fungal diseases in haematopoietic cell transplant recipients and in patients with acute myeloid leukaemia or myelodysplasia in Brazil. Clin Microbiol Infect. 2013;19(8):745–751. | ||
Lestrade PP, Meis JF, Arends JP, et al. Diagnosis and management of aspergillosis in the Netherlands: a national survey. Mycoses. 2016;59(2):101–107. | ||
Wolff SN, Fay J, Stevens D, et al. Fluconazole vs low-dose amphotericin B for the prevention of fungal infections in patients undergoing bone marrow transplantation: a study of the North American Marrow Transplant Group. Bone Marrow Transplant. 2000;25(8):853–859. | ||
Ethier MC, Science M, Beyene J, Briel M, Lehrnbecher T, Sung L. Mould-active compared with fluconazole prophylaxis to prevent invasive fungal diseases in cancer patients receiving chemotherapy or haematopoietic stem-cell transplantation: a systematic review and meta-analysis of randomised controlled trials. Br J Cancer. 2012;106(10):1626–1637. | ||
Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56–93. | ||
Marks DI, Pagliuca A, Kibbler CC, et al. Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic haematopoietic stem-cell transplantation. Br J Haematol. 2011;155(3):318–327. | ||
Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116(24):5111–5118. | ||
Bow EJ, Vanness DJ, Slavin M, et al. Systematic review and mixed treatment comparison meta-analysis of randomized clinical trials of primary oral antifungal prophylaxis in allogeneic hematopoietic cell transplant recipients. BMC Infect Dis. 2015;15:128. | ||
Solano C, Slavin M, Shaul AJ, et al. Economic evaluation of azoles as primary prophylaxis for the prevention of invasive fungal infections in Spanish patients undergoing allogeneic haematopoietic stem cell transplant. Mycoses. 2017;60(2):79–88. | ||
Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ. 2006;15(12):1295–1310. | ||
Instituto de Investigación e Innovación Farmacéutica ACI. Costs of drug treatments; 2015. Available from: http://www.investigacion-farmaceutica.org/. Accessed July 28, 2016. | ||
Social IMdS. Monitoring and management costs; 2015. Available from: http://compras.imss.gob.mx/?P=imsscompro. Accessed July 28, 2016. | ||
Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study--Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis. 2007;45(9):1161–1170. | ||
Martín-Peña A, Aguilar-Guisado M, Espigado I, Parody R, Miguel Cisneros J. Prospective study of infectious complications in allogeneic hematopoietic stem cell transplant recipients. Clin Transplant. 2011;25(3):468–474. | ||
[Book of the Diagnostic Related Costs]. Instituto Mexicano del Seguro Social, Producto Hospitalario; 2008. Available from: http://compras.imss.gob.mx/. Accessed July 31, 2015. Spanish. | ||
Consejo de Salubridad General. Guía para la Conducción de Estudios de Evaluación Económica para la Actualización del Cuadro Básico y Catálogo de Insumos del Sector Salud en México [Conduction of economic evaluation studies for the update of the basic table and catalog of supplies of the health sector in Mexico]; 2015. Available from: http://www.canifarma.org.mx/descargables/Docs_interes_paps/Gu%C3%ADa%20de%20Conducci%C3%B3n%20de%20Estudios%20de%20Evaluaci%C3%B3n%20Econ%C3%B3mica%202015%20-%20CSG.pdf. Accessed October 26, 2016. Spanish. | ||
Gao X, Marks DI, Schlamm HT, Ji X, Stephens JM, Tarallo M. Association between drug tolerability and medical resource use in prophylaxis of invasive fungal infections after allogeneic hematopoietic stem cell transplant. J Med Econ. 2013;16(8):1061–1070. | ||
Ament AJ, Hübben MW, Verweij PE, et al. Economic evaluation of targeted treatments of invasive aspergillosis in adult haematopoietic stem cell transplant recipients in the Netherlands: a modelling approach. J Antimicrob Chemother. 2007;60(2):385–393. |
Supplementary materials
  | Figure S1 Cost-effectiveness plane of incremental cost vs incremental IFIs avoided, based on deterministic results. Abbreviations: AmB, amphotericin B; IFI, invasive fungal infection. |
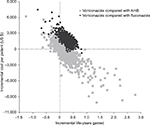  | Figure S2 Cost-effectiveness plane of incremental cost vs incremental life years gained, based on probabilistic results. Abbreviation: AmB, amphotericin B. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.