Back to Journals » Infection and Drug Resistance » Volume 12
Cost-effectiveness analysis of the use of letermovir for the prophylaxis of cytomegalovirus in adult cytomegalovirus seropositive recipients undergoing allogenic hematopoietic stem cell transplantation in Italy
Authors Restelli U , Croce D, Pacelli V, Ciceri F, Girmenia C
Received 28 November 2018
Accepted for publication 25 March 2019
Published 8 May 2019 Volume 2019:12 Pages 1127—1138
DOI https://doi.org/10.2147/IDR.S196282
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sahil Khanna
Umberto Restelli,1,2 Davide Croce,1,2 Valeria Pacelli,1 Fabio Ciceri,3,4 Corrado Girmenia5
1Center for Health Economics, Social and Health Care Management, LIUC - Università Cattaneo, Castellanza, VA, Italy; 2School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; 3Hematology and BMT Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy; 4University Vita-Salute San Raffaele, Milan, Italy; 5Department of Haematology, Oncology and Dermatology, Azienda Policlinico Umberto I, Sapienza University, Rome, Italy
Background: The aim of the analysis is to assess the efficiency of the allocation of economic resources related to the use of letermovir cytomegalovirus (CMV) prophylaxis in adult seropositive recipients (R+) patients receiving an allogenic hematopoietic stem cell transplantation (HSCT), compared with a no-prophylaxis strategy, assuming preemptive antiviral administration in both groups from the perspective of the Italian National Health Service (NHS), through a cost-effectiveness analysis.
Methods: The model used is based on a decision tree which simulates on a lifetime horizon the progression of CMV infection, considering two alternatives: the use of letermovir CMV prophylaxis, followed by preemptive therapy in case of clinically significant CMV infection, or the avoided use of letermovir CMV prophylaxis, considering direct medical costs (referred to 2018) and quality-adjusted life years (QALYs), both discounted considering a 3% annual rate. Two scenarios were considered, representing the differences related to regional contexts and clinical practice of different typologies of hospitals (public or private accredited with Regional Health Services).
Results: The use of letermovir prophylaxis compared with no prophylaxis strategy would lead to an increase of QALYs and direct medical costs in the two scenarios considered, with a mean increase of 0.45 QALYs, and an increase of direct medical costs of 10,222.4 € and of 10,809.9 € in the two scenarios. The incremental cost-effectiveness ratios are equal to 22,564 €/QALY and 23,861 €/QALY. The probabilistic sensitivity analysis conducted showed a percentage of results below the threshold of 40,000 €/QALY of 67.4% and 71.3%; and below a threshold of 25,000 €/QALY equal to 50.4% and to 53.0%.
Conclusions: The use of letermovir CMV prophylaxis in adult R+ patients receiving allogenic HSCT, compared with a no-prophylaxis strategy, would be cost-effective for the Italian NHS considering the incremental cost-effectiveness thresholds of 40,000 €/QALY and of 25,000 €/QALY.
Keywords: cytomegalovirus, letermovir, allogenic hematopoietic stem cell transplantation, cost-effectiveness analysis
Background
Cytomegalovirus (CMV) infection is often asymptomatic during its latency phase and can lead to CMV disease due to immune system dysfunctions, as hematologic malignancies and hematopoietic stem cell transplantation (HSCT), solid organ transplantation, and HIV infection. Among at-risk adult patients undergone allogenic stem cells transplantation, the rate of CMV infection is between 45% and 65%.1–4
In Italy, from 2013 to 2016, a mean annual number of 1,055 allogenic HSCT were performed on adults (≥18 years) CMV-seropositive recipients (R+).5
CMV infection is associated with negative transplant outcomes due to the increased risk of coinfections (bacterial and fungal), neutropenia and poor-graft function, graft versus host disease (GVHD),6,7 all conditions translated into an increased transplant-related mortality,8 and direct transplant costs.9
The current standard of care for CMV infection is preemptive strategy. In January 2018, the orphan drug letermovir, a new anti-CMV agent, received a marketing authorization by the European Medicines Agency, being indicated for “prophylaxis of CMV reactivation and disease in adult CMV R+ of an allogeneic HSCT.”10
A phase 3 clinical trial showed that letermovir prophylaxis leads to a “significantly lower risk of clinically significant CMV infection than placebo.”11 The primary end point of the trial was “the proportion of patients with clinically significant CMV infection through week 24 after transplantation among patients without detectable CMV DNA at randomization,”11 which showed a statistically significant difference in terms of lower proportion of CMV infections in the letermovir group (37.5%) compared with the placebo group (60.6%).11
The aim of the analysis is to assess the efficiency of the allocation of economic resources related to the use of letermovir CMV prophylaxis in adult R+ patients receiving an allogenic HSCT, compared with a no-prophylaxis strategy assuming preemptive antiviral administration in both groups from the perspective of the Italian National Health Service (NHS), through a cost-effectiveness analysis.
Methods
Cost-effectiveness model structure (interventions, eligible population and time horizon)
The analysis was conducted through the adaptation to the Italian context of a cost-effectiveness model implemented by RTI Health Solutions for MSD.12 The model is based on a decision tree which simulates on a lifetime horizon the progression of CMV infection, considering two alternatives: the use of letermovir CMV prophylaxis, followed by preemptive therapy (PET) in case of clinically significant CMV infection, or the avoided use of letermovir CMV prophylaxis, as reported in Figure 1.
 | Figure 1 Structure of the decision tree.Abbreviation: CMV, cytomegalovirus. |
The decision tree considers, in a 48-week time, the probability to develop a CMV clinically significant infection (followed by the administration of PET), the probability to develop CMV disease, and, as a consequence of this, to develop complications.
Adult CMV R+ patients receiving an allogenic HSCT are assigned to the arm that considers the use of letermovir as prophylaxis for CMV (letermovir arm) or to the arm that does not consider letermovir prophylaxis (no-letermovir arm).
By day 30, patients may develop a clinically significant CMV infection. Patients who develop a clinically significant CMV infection will receive PET and may or not develop CMV disease. Those developing CMV disease may develop complications associated to the disease: bacterial or fungal infections and GVHD.
Up to 48 weeks, mortality rate is that observed in the letermovir clinical trial (as explained below, cumulative probabilities for events at 48 weeks were assumed equal to 24-week probabilities).11 For patients who are alive after 48 weeks, life expectancy is estimated applying the average adjusted annual relative risk for mortality for underlying diseases post-transplant (acute myelogenous leukemia, acute lymphoblastic leukemia, myelodysplastic syndrome, lymphoma, severe aplastic anemia, chronic myelogenous leukemia, chronic lymphocytic leukemia, myelofibrosis, plasma cell myeloma), derived from literature,13 to the Italian general population annual mortality rates for 2016.14 The relative risk at 1 year post-transplant was considered equal to the risk for year 2 post-transplant, since Wingard and colleagues (2011)13 calculated relative risk from year 2 to year 15 post-transplant. After year 15 post-transplant, relative risk for mortality was considered static, being the average risk from years 10 to 15. For diseases not considered in Wingard and colleagues calculations, the relative risk of severe aplastic anemia was considered for chronic myelogenous leukemia and chronic lymphocytic leukemia; and the risk of myelodysplastic syndrome disease was considered for myelofibrosis and plasma cell myeloma.
To adapt the model to the Italian context, two scenarios were created to represent differences related to regional contexts and clinical practice of different typologies of hospitals (public or private accredited with Regional Health Services). In details, we considered the expert opinion of an opinion leader on infectious complications in HSCT of the Department of Haematology of a public hospital located in Rome, Lazio Region (scenario 1), and of the Director of the Hematology and BMT Unit of a private hospital accredited with the Health Service of Lombardy Region, located in Milan (scenario 2). The expert opinions were provided to adapt the variables of the model to the real clinical practice of the two hospitals, in terms of PET administered, patient monitoring, management of CMV infection, CMV disease, opportunistic infections and GVHD.
The model output is the mean number of quality-adjusted life years (QALYs) per patient in each arm, the mean direct medical costs per treatment arm and the incremental cost-effectiveness ratio (ICER) of the use of letermovir prophylaxis, compared with the sole use of PET. Both costs and QALYs were discounted considering a 3% annual rate as suggested by the Italian Health Economics Association.15
Model parameters – clinical inputs
The effectiveness of letermovir prophylaxis was derived from a phase 3 clinical trial, along with the incidence of clinically relevant events, and utility values associated to the two comparative arms.11
The mean age at baseline considered in the model is 50.8 years,16 and the probabilities of occurrence for clinically significant CMV infection, CMV disease, CMV-related re-hospitalizations, opportunistic infections, GVHD and all-cause mortality for the first 48 weeks of the simulation are reported in Table 1.
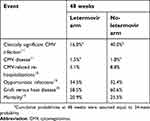 | Table 1 Clinical inputs considered in the model |
The incidence of PET adverse event (neutropenia) was considered equal to 12.5% as in Kim and colleagues, 2010.17
Model parameters – utility values
Utility values associated to each arm in each time period, were derived from letermovir phase 3 clinical trial and are reported in Table 2.18 Utility values were elicited using EQ-5D (3L) index. Due to lack of data referred to the target population, the post-trial utility value was derived from a study conducted in the UK by Castejón and colleagues (2018) referred to patients affected by acute myeloid leukemia, functionally cured.19
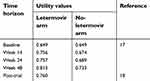 | Table 2 Utility values per week period in the two arms |
Model parameters – costs
Considering the point of view assumed in the analysis, which is that of the Italian NHS, the cost of drugs was derived from the ex-factory price as reported in the “Gazzetta Ufficiale della Repubblica Italiana,” the cost of hospitalization and outpatient activities was that reported in the tariffs’ nomenclature of the Italian NHS.20 Cost data refers to year 2018.
The costs considered in the model are reported in Table 3. A detailed description of the methodology adopted to estimate each cost is reported below, along with the costs considered per each service provided, as in Table 4.
 | Table 3 Direct medical costs considered in the analysis in each scenario |
 | Table 4 Cost per service considered |
Cost of letermovir
Considering a number of days of treatment of 69.4,16 the cost of the drug was assumed being equal to 11,242.8 €, 162 € per day.
Cost of monitoring activities
Scenario 1. The outpatient activities considered are 1 specialist visit, 1 complete blood count, biochemical examinations, CMV DNA. The activities described above are weekly in patients with standard risk and twice weekly in patients at high risk (based on the real clinical practice of the center, 50% of patients are at high risk).
Scenario 2. A weekly activity of the monitoring described above is considered.
Cost of PET
Scenario 1: PET duration was derived from data published by Solano and colleagues (2015),21 considering a mean duration of 21 days, which is coherent with the recommendations of English guidelines for the “Management of cytomegalovirus infection in haemopoietic stem cell transplantation,” that reports 14 days of treatment, followed by 7–14 days with a reduced dose.22
The drugs considered for PET were: valganciclovir (administered to 20% of patients),23 ganciclovir (administered to 50% of patients)24 and foscarnet (administered to 30% of patients).25 For ganciclovir and foscarnet, a day hospital administration was considered in 50% of patients (considering the tariff of a 1-day hospitalization for DRG 421, in which the cost of drugs is included) and the administration of drugs during a standard hospitalization with DRG 421 in 50% of patients.
Scenario 2: PET duration was considered equal to 14 days, and patients were equally distributed among valganciclovir, ganciclovir and foscarnet. Ganciclovir and foscarnet are administered at the outpatient department of the hospital.
Cost of CMV disease
Scenario 1. 50% of patients, are considered to be hospitalized. The DRGs considered derives from an analysis of all the hospitalizations with, as primary or secondary diagnoses “99685 – Bone marrow transplant complications” and “0785 – Cytomegalic disease” in Lombardy Region in 2013.25 The hospitalizations identified are related to the following DRGs: DRG 079 “respiratory infections and inflammations with complications, age >17” (2.8% of cases); DRG 403 “lymphoma and non-acute leukemia with complications” (5.6% of cases); DRG 421 “viral disease, age >17” (11.1% of cases); DRG 422 “viral disease and fever of unknown origin, age <18” (2.8% of cases); DRG 473 “acute leukemia without major surgical intervention, age >17” (11.1% of cases); DRG 481 “bone marrow transplant” (25.0% of cases); DRG 574 “major hematologic/immunologic diagnoses excluding sickle cell anemia and coagulopathy” (41.7% of cases). Among these the only DRG identified as pertinent with CMV disease and selected for the analysis was the latter, DRG 574. The outpatient activities considered are: 8 specialist visits, 8 complete blood counts, 8 biochemical examinations, 8 CMV DNA, 2 computed tomography of the thorax (1 not considered in terms of costs for hospitalized patients), 1 esophagogastroduodenoscopy (not considered in terms of costs for hospitalized patients), 1 colonoscopy with flexible endoscopy (not considered in terms of costs for hospitalized patients), 1 magnetic resonance imaging of the brain and encephalic trunk (not considered in terms of costs for hospitalized patients). Furthermore, 21-day therapy of ganciclovir and foscarnet were considered, with the posology described above.
Scenario 2. 10% of patients, are considered to be hospitalized. The DRGs considered derive from an analysis of all the hospitalizations with primary diagnosis “0785 – Cytomegalic disease” in Lombardy Region in 2013.25 The hospitalizations identified are related to the following DRGs: DRG 421 “viral disease, age >17” (98.5% of cases); DRG 578 “infectious and parasitic diseases with surgical intervention” (1.5% of cases). The weighted mean DRG tariff is 2,427 €. The outpatient activities considered are the same of Scenario 1, considering a different proportion of hospitalized patients, leading to a mean cost of 957.3 €. 21-day therapy of ganciclovir and foscarnet were considered, with the posology described above.
Cost of CMV related re-hospitalizations
For scenario 1, the cost is derived from the analysis of CMV related hospitalizations as described for CMV disease, while in scenario 2, the tariff of DRG 421 “viral disease, age >17” was considered.
Cost of opportunistic infections
Based on the real clinical practice of the center located in Milan, the following infections were considered: aspergillosis (12.5% of cases) and human herpesvirus 6 (HHV6 – 87.5% of cases). For aspergillosis, 10% of patients were considered to be hospitalized. The DRGs considered derive from an analysis of all the hospitalization with primary or secondary diagnosis “1173 – Aspergillosis” in Lombardy Region in 2013.25 The hospitalizations identified are related to the following DRGs: DRG 423 “other diagnosis related to infectious and parasitic diseases” (85.7% of cases); DRG 542 “Tracheostomy with mechanical ventilation 96+ hrs or main diagnosis except face, mouth and neck without major surgical intervention” (1.3% of cases); DRG 578 “infectious and parasitic diseases with surgical intervention” (13.0% of cases). The weighted mean DRG tariff is 6,389 €. The outpatient activities considered are: 8 specialist visits, 8 complete blood counts, 8 aspergillus antigen tests, 2 computed tomography of the thorax, 8 therapeutic drug monitoring of antifungal agents. The drug therapy considered was: the administration of intravenous liposomal Amphotericin B for 50% of patients and oral voriconazole for 50% of patients.26 The total cost estimated for aspergillosis is 2,187.84 €.
For HHV6, 10% of patients are considered to be hospitalized with a DRG 421 “viral disease, age >17.” The outpatient activities considered are: 4 specialist visits, 4 complete blood counts, 4 HHV6 DNA; 14-day therapy of ganciclovir and foscarnet were considered, with the posology described above. The total cost estimated for HHV6 is 3,270.84 €.
Cost of GVHD
Scenario 1. 100% of patients are considered to be hospitalized. The DRG considered derives from an analysis of all the hospitalizations with primary or secondary diagnoses “99685 – complications of bone marrow’s transplant” in Lombardy Region in 2013.25 The hospitalizations identified are related to the following DRGs: DRG 574 “major hematologic/immunologic diagnoses excluding sickle cell anemia and coagulopathy” (98.1% of cases) and DRG 481 “bone marrow transplant” (1.9% of cases). The tariff of DRG 574 was then considered. Considering a mean number of 28 days of hospitalization for DRG 574, as emerged from data referred to Lombardy Region in 2013,25 the outpatient activities considered are: 8 specialist visits, 8 complete blood counts, 8 biochemical examinations, 8 therapeutic drug monitoring. The drug therapy considered was: the administration of oral mycophenolate mofetil (considering the cost for only 12 days since 28 days of therapy are already covered by the hospitalization tariff),27 and oral cyclosporine, considering the cost for only 12 days since 28 days of therapy are already covered by the hospitalization tariff.24,28
Scenario 2: 15% of patients are considered to be hospitalized with DRG 574 “major hematologic/immunologic diagnoses excluding sickle cell anemia and coagulopathy” as explained above. The outpatient activities considered are: 12 specialist visits, 12 complete blood counts, 12 biochemical examinations, 12 therapeutic drug monitoring. The drug therapy considered was the administration of oral mycophenolate mofetil and oral cyclosporine.
Cost of neutropenia – PET related adverse event
15% of patients are considered to be hospitalized with DRG 574 “major hematologic/immunologic diagnoses excluding sickle cell anemia and coagulopathy.” The outpatient activities considered are: 4 specialist visits, 4 complete blood counts, 4 biochemical examinations, 4 CMV DNA. The drug therapy considered was the administration of subcutaneous granulocyte colony stimulating factor and oral levofloxacine in 60% of patients.29,30
Sensitivity analysis
A probabilistic sensitivity analysis was conducted, modifying each variable using beta distributions for clinical parameters and gamma distributions for economic parameters. 1,000 iterations were simulated to perform the analysis.
Results
The use of letermovir prophylaxis in adult CMV R+ patients receiving an allogenic HSCT compared with no prophylaxis strategy would lead to an increase of QALYs and direct medical costs in the two scenarios considered. In details, a mean increase of 0.45 QALYs (0.51 life years) is observed, with an increase of direct medical costs of 10,222.4 € in scenario 1 and of 10,809.9 € in scenario 2.
The ICER of the use of letermovir prophylaxis two scenarios is reported in Table 5.
 | Table 5 Cost-effectiveness analyses results |
The incremental cost per QALY in scenario 1 (which considers the clinical practice of a public hospital located in Lazio Region) would be equal to 22,564 €, while in scenario 2 (which considers the clinical practice of a private hospital accredited by the Regional Health Service of Lombardy Region) would be equal to 23,861 €.
The results per each scenario in terms of clinical outcomes and costs are reported in Table 6.
 | Table 6 Clinical outcomes and costs of each scenario in each arm |
Considering a hypothetic number of 1,000 patients, in line with the mean number of CMV R+ patients who have undergone HSCT between 2013 and 2016 in Italy, the use of letermovir prophylaxis would lead to a reduction of the incidence of clinically significant CMV infections (−240), of CMV disease cases (−4.6), of CMV related re-hospitalizations (−57.5), of GVHD (−21.3), PET related neutropenia (−30.0); and to an increase of opportunistic infections (+21.1). Furthermore, a reduction of −4.6% of CMV-related deaths through 48 weeks due to the use of letermovir should be expected, as emerged within letermovir phase 3 clinical trial.15
In terms of costs, the main cost component in the letermovir arm is the cost of prophylaxis, representing >72.5% of costs in both scenarios. Other relevant cost components are GVHD (16.32% in Scenario 1 and 7.06% in Scenario 2), and opportunistic infections (6.98% in Sceanrio 1 and 7.96% in Scenario 2).
Sensitivity analysis
The results of the sensitivity analysis are reported in Figures 2 and 3.
 | Figure 2 Probabilistic sensitivity analysis results – Scenario 1. |
 | Figure 3 Probabilistic sensitivity analysis results – Scenario 2. |
The two figures show the distribution of the results of the base case and sensitivity analyses in the cost-effectiveness plane, in which the incremental costs and the incremental effectiveness of the use of letermovir CMV prophylaxis compared with a no-prophylaxis strategy, assuming preemptive antiviral administration are presented. Most results are positioned in the north-east quadrant, in which both utility (effectiveness) and costs are higher than the comparator. The two dashed lines show the cost-effectiveness thresholds considered in the analysis, in all the analysis in which the result is located below each threshold, the use of letermovir should be considered cost-effective compared with the no prophylaxis strategy. The results located in the north-west quadrant show a decrease in effectiveness and an increase of costs. In all the analyses in which the result is located in this quadrant, the use of letermovir prophylaxis should be considered dominated compared with the no prophylaxis strategy.
The percentage of results below the ICER threshold of 40,000 € considered in the analysis is 67.4% in scenario 1 and 71.3% in scenario 2. More than 99% of the simulations in both scenarios show an increase of costs and QALYs related to the use of letermovir prophylaxis, while 0.96% of simulations in scenario 1 and 0.79% of simulations in scenario 2 show an increase in terms of costs and a decrease of QALYs.
Considering an ICER threshold of 25,000 €/QALY, the percentage of results below it is equal to 50.4% in Scenario 1 and to 53.0% in Scenario 2.
Discussion
The results of the analysis show that the use of letermovir would lead to an increase of costs and QALYs compared with a scenario in which no CMV prophylaxis is considered, with a cost-effectiveness ratio below 25,000 €/QALY. The results are particularly relevant, considering the fact that for orphan technologies there is not accordance with literature, whether to consider standard ICER’s thresholds or if higher threshold values should be taken into consideration.31,32 In England, for instance, in 2016 the National Institute for Health and Care Excellence launched a public consultation to discuss whether to consider an ICER threshold of 100,000 £/QALY for highly specialized technologies, instead of the 20,000–30,000 £/QALY thresholds adopted for health technologies.33
A total of 240 avoided clinically significant CMV infections, 4.6 avoided CMV disease cases, 57.5 avoided CMV related re-hospitalizations, 21.3 avoided GVHD, 30 avoided cases of PET related neutropenia, and 21.1 further opportunistic infections referred to a hypothetic number of 1,000 patients are likely to be expected in the Italian national context, considering the 1,055 mean annual number of CMV R+ patients who have undergone HSCT.
To the best of our knowledge, no health economic analyses have been published so far concerning the use of letermovir for CMV prophylaxis. Four cost-effectiveness analyses referred to the English, Scottish, Portuguese and US contexts have been presented as posters at international congresses.12,34–36 The results of these analyses suggest that the use of letermovir as prophylaxis against CMV-reactivation in seropositive patients who undergone allogenic hematopoietic stem cells transplant would be cost-effective compared to the sole use of PET, considering a threshold value of 20,000 £/QALY in England and Scotland and of 50,000 $/QALY in the US. Furthermore, Ferreira and colleagues state that “letermovir prophylaxis is a cost-effective strategy at commonly accepted ICERs for orphan drugs in Portugal.”
The two scenarios considered in the analysis allow to consider different settings of care, as public hospitals and private hospitals accredited by the Italian NHS. The results in the two scenarios are similar, leading to the same conclusions. This strength may also be a limit of the analysis, since direct medical costs are related to the clinical practice of the two centers considered.
Due to lack of data referred to the proportion of patients reporting clinically significant CMV infection at week 48, the proportion assessed at week 24 in the clinical trial (primary end point) was considered.11 The same approach was adopted for CMV disease, since data referred to week 48 is not available.
The analysis did not take into consideration the cost of adverse events related to the use of letermovir. This choice was driven by the absence of a statistically significant difference in terms of incidence of adverse events within the phase 3 clinical trial between letermovir arm and placebo arm for adverse events occurred in >10% of patients through week 16 after transplantation.11 Furthermore, side effects related to drug interactions with immunosuppressive agents and azoles were not considered in the analysis.37,38
The cost related to GVHD does not take into consideration the use of steroids; however, this cost category is minimal and would not modify the final results. Due to the point of view assumed, the Italian NHS, the cost of events like GVHD, clinically significant CMV infections and CMV disease might have been underestimated. In fact, we considered tariffs related to DRGs and outpatient activities, which are costs for the payer,39 however, the tariffs are not always equal to the costs incurred by the provider,40 and, at the end of the fiscal year, the NHS might have to provide further economic resources to providers to cover the higher expenditures incurred. The approach we used is consistent with other published studies that considered direct medical costs assuming the perspective of the Italian NHS.41–43 The hypothetical lower costs considered, lead to a conservative analysis, since CMV related complications have a higher impact in the arm that does not consider the use of letermovir.
Conclusion
The use of letermovir CMV prophylaxis in adult R+ patients receiving an allogenic HSCT, compared with a no-prophylaxis strategy, would be cost-effective for the Italian NHS considering the ICER thresholds of 40,000 €/QALY and of 25,000 €/QALY identified by the Italian Health Economics Association, increasing both QALYs and direct medical costs.
Acknowledgment
The analysis was performed thanks to an unconditional grant from MSD.
Disclosure
DC declares advisory board fees from Mundipharma, Takeda and Abbvie. CG has received honoraria from Gilead Sciences, Astellas Pharma, Basilea, MSD, Pfizer Pharmaceuticals, and Celgene. UR report grants from MSD, during the conduct of the study; personal fees from Astrazeneca, personal fees from Bayer, personal fees from Italfarmaco, outside the submitted work. The remaining authors report no conflicts of interest in this work.
References
1. Rowe RG, Guo D, Lee M, Margossian S, London WB, Lehmann L. Cytomegalovirus infection in pediatric hematopoietic stem cell transplantation: risk factors for primary infection and cases of recurrent and late infection at a single center. Biol Blood Marrow Transplant. 2016;22(7):1275–1283. doi:10.1016/j.bbmt.2016.04.004
2. George B, Pati N, Gilroy N, et al. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl Infect Dis. 2010;12(4):322–329. doi:10.1111/j.1399-3062.2010.00504.x
3. Miller W, Flynn P, McCullough J, et al. Cytomegalovirus infection after bone marrow transplantation: an association with acute graft-v-host disease. Blood. 1986;67(4):1162–1167.
4. Ljungman P, Perez-Bercoff L, Jonsson J, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 2006;91(1):78–83.
5.
6. Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis. 2002;185(3):273–282. doi:10.1086/338624
7. Cantoni N, Hirsch HH, Khanna N, et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(9):1309–1314. doi:10.1016/j.bbmt.2010.03.020
8. Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427–2438. doi:10.1182/blood-2015-11-679639
9. Robin C, Hémery F, Dindorf C, et al. Economic burden of preemptive treatment of CMV infection after allogeneic stem cell transplantation: a retrospective study of 208 consecutive patients. BMC Infect Dis. 2017;17(1):747. doi:10.1186/s12879-017-2757-2
10.
11. Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377(25):2433–2444. doi:10.1056/NEJMoa1706640
12. Schelfhout J, Jiang Y, Miles L, Merchant S, Graham J. Cost effectiveness of letermovir in prevention of clinically significant CMV infection in CMV seropositive allogeneic hematopoietic stem cell transplant recipients [BMT abstract 557]. Biol Blood Marrow Transplant. 2018;24(3 suppl1):S384. doi:10.1016/j.bbmt.2017.12.473
13. Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–2239. doi:10.1200/JCO.2010.33.7212
14.
15.
16.
17. Kim ST, Lee MH, Kim SY, et al. A randomized trial of preemptive therapy for prevention of cytomegalovirus disease after allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2010;91(5):886–891. doi:10.1007/s12185-010-0580-2
18.
19. Castejón N, Cappelleri JC, Cuervo J, et al. Social preferences for health states associated with acute myeloid leukemia for patients undergoing treatment in the United Kingdom. Health Qual Life Outcomes. 2018;16(1):66. doi:10.1186/s12955-018-0897-8
20.
21. Solano C, de la Cámara R, Vázquez L, López J, Giménez E, Navarro D. Cytomegalovirus infection management in allogeneic stem cell transplant recipients: a national survey in Spain. J Clin Microbiol. 2015;53(8):2741–2744. doi:10.1128/JCM.01057-15
22. Emery V, Zuckerman M, Jackson G, et al.;
23.
24.
25. ALEE-AO: epidemiologic and economic atlas of hospital activities in Lombardy. Online accessible database.
26.
27.
28.
29.
30.
31. Juth N. For the sake of justice: should we prioritize rare diseases? Health Care Anal. 2017;25(1):1–20. doi:10.1007/s10728-014-0284-5
32. Sandman L, Gustavsson E. The (ir)relevance of group size in health care priority setting: a reply to Juth. Health Care Anal. 2017;25(1):21–33. doi:10.1007/s10728-016-0333-3
33.
34. Brown C, Mayes A, Schelfhout J, Jiang Y, Glover M, Taymor E. PSY93 – cost effectiveness of letermovir as prophylaxis of clinically significant cytomegalovirus reactivation and disease in adult CMV-seropositive allogeneic haematopoietic stem cell transplant: an English payer perspective. Value Health. 2018;21(suppl. 3):S451–S452. doi:10.1016/j.jval.2018.09.2668
35. Brown C, Schelfhout J, Taymor E, Jiang Y, Glover M. P544 – cost effectiveness of letermovir as prophylaxis of clinically significant cytomegalovirus reactivation and disease in adult CMV-seropositive allogeneic haematopoietic stem cell transplant: a Scottish payer perspective. Bone Marrow Transplant. 2018. Available from:
36. Ferreira J, Pereira R, Schelfhout J, Jiang Y. PIN31 – economic evaluation of letermovir as prophylaxis of clinically significant cytomegalovirus reactivation and disease on CMV-seropositive receptors of allogeneic haematopoietic stem cell transplant in the Portuguese setting. Value Health. 2018;21(suppl. 3):S225–S226. doi:10.1016/j.jval.2018.09.1350
37. Kropeit D, von Richter O, Stobernack H-P, Rübsamen-Schaeff H, Zimmermann H. Pharmacokinetics and safety of letermovir coadministered with cyclosporine A or tacrolimus in healthy subjects. Clin Pharmacol Drug Dev. 2018;7(1):9–21. doi:10.1002/cpdd.388
38. Marshall WL, McCrea JB, Macha S, et al. Pharmacokinetics and tolerability of letermovir coadministered with azole antifungals (posaconazole or voriconazole) in healthy subjects. J Clin Pharmacol. 2018;58(7):897–904. doi:10.1002/jcph.1094
39. Fattore G. Measuring Public Value: A Cost-Benefit Analysis of in Vitro Fertilisation in Italy. Milan: Egea; 2009:1–274.
40. Fattore G, Torbica A. Inpatient reimbursement system in Italy: how do tariffs relate to costs? Health Care Manage Sci. 2006;9:251–258. doi:10.1007/s10729-006-9092-2
41. Rognoni C, Ciani O, Sommariva S, Tarricone R. Real-world data for the evaluation of transarterial radioembolization versus sorafenib in hepatocellular carcinoma: a cost-effectiveness analysis. Value Health. 2017;20(3):336–344. doi:10.1016/j.jval.2016.09.2397
42. Restelli U, Alberti A, Lazzarin A, Bonfanti M, Nappi C, Croce D. Cost-effectiveness analysis of the use of daclatasvir + sofosbuvir + ribavirin (16 weeks and 12 weeks) vs sofosbuvir + ribavirin (16 weeks and 24 weeks) for the treatment of cirrhotic patients affected with hepatitis C virus genotype 3 in Italy. Eur J Health Econ. 2018;19(1):37–44. doi:10.1007/s10198-016-0865-3
43. Scalone L, Borghetti F, Brunori G, et al. Cost-benefit analysis of supplemented very low-protein diet versus dialysis in elderly CKD5 patients. Nephrol Dial Transplant. 2010;25(3):907–913. doi:10.1093/ndt/gfp572
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
