Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Cost-Effectiveness Analysis of the TCM “Yupingfeng Granules” in the Treatment of Acute Exacerbations of COPD Based on a Randomized Clinical Trial
Authors Hu M , Ding P, Ma J, Yang N, Zheng J , Zhou N
Received 31 May 2022
Accepted for publication 10 September 2022
Published 23 September 2022 Volume 2022:17 Pages 2369—2379
DOI https://doi.org/10.2147/COPD.S374782
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Ming Hu,1,* Pan Ding,1,* Jinfang Ma,2 Nan Yang,1 Jinping Zheng,2 Naitong Zhou1
1West China School of Pharmacy Sichuan University, Chengdu, People’s Republic of China; 2State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Naitong Zhou, West China School of Pharmacy Sichuan University, No. 17, 3rd Section, Renmin South Road, Chengdu, 610041, People’s Republic of China, Tel +86-13881962880, Fax +86-28-85501387, Email [email protected] Jinping Zheng, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, 151 Yanjiang Road, Guangzhou, 510120, People’s Republic of China, Tel +86 20 8306 2869, Fax +86 20 8306 2729, Email [email protected]
Introduction: Traditional Chinese medicine (TCM) is becoming increasingly important as it provides further options for treating many diseases worldwide. The TCM “Yupingfeng” has been used in China for over 800 years, and its clinical efficacy and safety for COPD treatment have been proven in previous studies. The objective of this study was to compare the long-term cost-effectiveness of Yupingfeng granules and the current conventional treatment for COPD patients in China.
Methods: A Markov model was constructed from the perspective of the Chinese healthcare system using TreeAge Pro 2011. The model cycle length was 12 months, and the cycle time was set to 10 years. Data from a randomized controlled trial were used to generate the number of acute exacerbations, COPD assessment test (CAT) score and actual medication used. The state transition probabilities, costs and quality-adjusted life years (QALYs) were derived from available sources. A threshold of 72,447 yuan per QALY gained was used as a cost-effectiveness criterion. One-way and probabilistic sensitivity analyses were conducted to verify the model. In addition, the cost-effectiveness of a 35-year cycle was evaluated as a scenario analysis.
Results: In the basic-case analysis, the ICER of adding Yupingfeng granules to the current conventional treatment drugs was ¥ 2123.04 per QALY, which was less than the threshold (one-time per capita GDP).Sensitivity analyses showed the results to be robust. Probabilistic sensitivity analysis showed that the probability of the ICER being less than the one-time per capita GDP threshold was 100%. In the scenario analysis, the incremental cost-effectiveness was ¥ 12,051.27 per QALY which was also under the one-time per capita GDP.
Conclusion: By reducing the number of acute exacerbations of COPD, thereby correspondingly reducing the follow-up treatment cost, Yupingfeng granules combined with conventional treatment were found to provide a cost-effective therapeutic strategy for COPD.
Keywords: Yupingfeng granule, traditional Chinese medicine, COPD, Markov model, cost-effectiveness analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases. Chronic obstructive pulmonary disease is currently the fourth leading cause of death worldwide and, among diseases, is estimated to cause the largest economic burden in China.1 COPD kills more than 3 million people worldwide every year.2 Epidemiological studies have shown that the prevalence of COPD among people 40 years and older in the population is significantly higher than that in the younger population.3 Patients with acute exacerbations of COPD generally experience aggravated symptoms such as cough, wheezing, and shortness of breath. Some patients also have symptoms such as fever. If the condition continues to progress, heart failure and organ dysfunction may develop secondary to COPD.4 A major factor that affects COPD-related mortality is the acute exacerbation of COPD (AECOPD).5 Consequently, effective prevention and control of acute attacks play an important role in the management of COPD.
Traditional Chinese medicine (TCM) is becoming increasingly important, providing further options for the treatment of many chronic diseases, including COPD, worldwide. The TCM “Yupingfeng” is a classic traditional Chinese medicine prescription and has been used in China for over 800 years. The main ingredients of Yupingfeng are Astragalus, Atractylodes, and Fangfeng.6 Its main functions are to invigorate qi, consolidate exterior and stop sweating, for patients who have exterior asthenia instability, spontaneous sweating, aversion to wind, pale complexion or deficiency susceptible to wind evil.7,8 At present, several randomized controlled trials (RCTs) have proven the positive effect of Yupingfeng in the treatment of cough variant asthma;9 additionally, some studies have shown that Yupingfeng granules combined with Western medicine treatment can reduce the rate of acute asthma attacks.10
In 2018, an RCT conducted by Ma and others6 showed that Yupingfeng granules provided an additional option for COPD treatment; the addition of Yupingfeng treatment can effectively prevent acute exacerbations of COPD. At the same time, Yupingfeng granules have been proven to have a good safety profile. However, additional treatments were often accompanied by an additional economic burden. Until now, there has been a little evidence that traditional Chinese medicine treatment of COPD is cost-effective in China. Therefore, our study aimed to assess the cost-effectiveness of adding Yupingfeng granules to the current drug treatments for COPD patients in China.
Materials and Methods
Intervention
Some of the data in this study came from a Phase III randomized clinical trial.6 The RCT followed a double-blind, double-simulation, parallel-group, multicentre study design. In the RCT, eligible patients were randomly assigned to one of two groups, the Yupingfeng group or the placebo group. The patients in the Yupingfeng group took Yupingfeng granules (5 g, three times daily) orally, while the control group took the well-designed placebo orally (5 g, three times daily).
If patients took short-acting or long-acting bronchodilators (including β2 agonists, anticholinergics, and theophylline), inhaled glucocorticoids, mucolytics, or antitussives before the study, they could continue to use them during the study, and the dose and type remained the same throughout. For those who took theophylline routinely before the study, their medication was uniformly replaced with theophylline sustained-release tablets. The patients who did not take medicine before entering the study were all given theophylline sustained-release tablets, and other medicines were not taken. When AECOPD occurred in the trial, the patients were treated according to the conventional treatment for AECOPD.6
Model Description and Structure
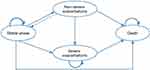
A multistate Markov model was constructed from the perspective of the Chinese health care system using Treeage Pro 2011 (Figure 1). The Markov model was used to calculate the cumulative cost and cumulative effect of the two drug treatment programs and then compare the long-term cost-effectiveness of the two programs. The Markov model included four disease states: stable phase, nonsevere exacerbations, severe exacerbations, and death. The transition to different states was determined by the number of acute exacerbations during the RCT and from related research reports. We defined disease severity, nonsevere exacerbations and severe exacerbations according to the need for extra medical resources. With reference to the COPD GOLD report (2021 GLOBAL STRATEGY FOR PREVENTION, DIAGNOSIS AND MANAGEMENT OF COPD), the ABCD grouping is based on the symptoms and the history of acute exacerbations. This study defined the number of acute exacerbations from 0 to 1 per year without hospitalization as a nonsevere exacerbation, and more than 1 or 2 acute exacerbations was defined as a severe exacerbation. Severe exacerbation leads to hospitalization and the need to pay additional hospital treatment costs. The model’s operating time limit was a difference, subtracting the average age in the trial population(67.8 years old) from the average life expectancy in China in 2019(77.3 years old). The baseline characteristics of patients in the model were obtained from an RCT of Yupingfeng granules in patients with COPD.6 Therefore, the model cycle length was 12 months, and the cycle time was set to 10 years.
Data Sources
Health Care Costs
Costs are measured according to the research perspective. Considering that the research perspective of this study is the Chinese health care system perspective, only direct medical costs were included in the model, including drug acquisition, general practitioner treatment, laboratory inspection costs, and extra cost of treatment during exacerbation.
The costs involved, their sources, and interval settings are shown in Table 1. The cost item refers to previous pharmacoeconomics research on COPD.11 The drug cost refers to the real-world patient’s medication situation, and the median price of the drug’s bid price was selected for calculation.12 The general practitioner’s diagnosis cost refers to the price of medical services in Beijing. The rest of the prices are from published literature (Appendix I).
 |
Table 1 Markov Model Cost Parameters and Sources |
Transition Probabilities
The transition probability was estimated as the probability of patients switching from one treatment to another. Based on a multicentre RCT study conducted in China, the transition probability of COPD patients was measured from the stable phase to the nonsevere exacerbation phase, severe exacerbation phase, or death state. Other transition probabilities were derived from publicly available sources. The natural population mortality rate was from the “China Population and Employment Statistics Yearbook 2019”: National Death Population Status by Age and Sex (2019) (Table 2).
 |
Table 2 Markov Transition Probability Parameters and Sources |
Quality-Adjusted Life Years
A quality-adjusted life year (QALY) is an index that represents effects in this model and was calculated using life years multiplied by health preference utility. Mapping algorithms can be used to predict EQ-5D-3L utilities from the COPD Assessment Test (CAT), which was measured in the RCT. The mapping formula was used to calculate the health utility value of each state. The mapping model was developed using the EQ-5D-3L utilities as the dependent variables and the scores of each of the eight CAT items as the explanatory variables, which were derived from Korean data because no Chinese population-based mapping model was available. The mapping formula was as follows (Table 3):16
 |
Table 3 Utility Parameters and Sources |
EQ-5D-3LUtility=1.0661−0.0103Q3−0.0120Q4−0.0168Q5−0.0255Q6−0.0125Q8
Willingness to Pay (Threshold Value)
With reference to the “China Guidelines for Pharmacoeconomic Evaluations (2020)”17 and the World Health Organization (WHO) guidelines, (1) if the incremental cost-effectiveness ratio (ICER) was < GDP per capita, then the incremental cost is worthwhile; (2) if the ICER fell between the GDP per capita and three times the GDP per capita, then the incremental cost is acceptable; and (3) if the ICER was > three times the GDP per capita, then the incremental cost is not worthwhile. This study set one-time per capita GDP as the threshold value for willingness-to-pay (WTP). The 2020 per capita GDP was ¥72,447 in China according to official data from the National Bureau of Statistics.18
Discounting
The baseline discount rate was 5% according to the China Guidelines for Pharmacoeconomic Evaluations (2020).17 The lower and upper limits of the sensitivity analysis were set to 0% and 8%, respectively.
Sensitivity Analysis and Scenario Analyses
One-Way Sensitivity Analysis
In the one-way sensitivity analysis, the varied range of the input value in the model was ± 10% of the basic value. With reference to most of the previously published studies, the discount rate’s variation range was set to 0–8%. One-way sensitivity analysis was used to identify the key factors that affected the results. A one-way analysis tornado diagram was used to summarize the results.
Probabilistic Sensitivity Analysis
Probabilistic sensitivity analysis (PSA) applied Monte Carlo simulation. The results of 1000 simulations of simultaneous changes in the study parameters within a certain variation range are shown. The simulation analysed the effect of simultaneous changes in different parameters on the results and the effect of changes in thresholds on the final decision. The Monte Carlo simulation scatter plots and cost-effectiveness acceptability curves clearly showed the result.
Scenario Analysis
According to an epidemiological study of COPD,19 COPD is prevalent among individuals 40 years old or older in China. A meta-analysis from China of the prevalence of COPD among adults aged 40 years and older also supported this conclusion.20 A study showed that the loss of life due to COPD is 2.13 years,21 and the average life expectancy in China was 77.3 years old.22 Therefore, the model cycle time limit was set to 35 years in the scenario analysis to further explore the applicability of the analysis results in practical situations.
Results
Base-Case and Scenario Analyses
The results of the base-case and scenario analyses are shown in Table 4. In the base-case analysis, lifetime costs per patient and QALYs in the Yupingfeng group were ¥104,346.93 and 5.81 compared with ¥103,355.81 and 5.34, respectively, in the control group. The strategy of adding Yupingfeng would incur an additional cost of ¥991.12 and gain 0.47 extra QALYs. The use of Yupingfeng combined with western medicine for COPD was cost-effective, with an incremental cost-effectiveness ratio of ¥2123.04 per quality- adjusted life year gained. The ICER was less than the set threshold of ¥72,447/QALY. Scenario analyses showed that adding Yupingfeng to routine treatment was also cost-effective, with an incremental cost-effectiveness ratio of ¥12,051.27 per quality- adjusted life year gained.
 |
Table 4 Cost-Effectiveness of Yupingfeng Group Compared with Placebo Group |
One-Way Sensitivity Analysis
Results of the one-way sensitivity analysis are shown in Figure 2. The top five variables that influenced the results of the cost-effectiveness analysis were: the medicine cost of the Yupingfeng group, the cost of conventional treatment medicine, the transition probability of the stable phase-severe exacerbation state in the Yupingfeng group, the transition probability of the stable phase-stable phase in the placebo group, and the average hospitalization expenses every time. However, within the upper and lower limits, the cost-effectiveness ratio was less than the WTP threshold. One-way sensitivity analyses showed that varying any parameters over a plausible range did not substantially change the results.
Probabilistic Sensitivity Analysis
The ICER scatter plot shows the 1000 simulation results, as shown in Figure 3. The slope of each point in the graph relative to the origin represents the simulated ICER value at that point. The slash in the figure represents the per capita GDP of China (¥72,447). As seen from the figure, 100% of the scatter points are located at the lower right side of the line, which means that all simulated points are less than the set threshold. The results of the cost-effectiveness acceptability curve are shown in Figure 4. The probabilistic sensitivity analysis showed that at willingness-to-pay thresholds greater than ¥11,250 roughly per quality-adjusted life year gained, a strategy of adding Yupingfeng granule is favored. At the ¥72,447 per quality-adjusted life year gained threshold, there is a 100% likelihood that a strategy employing Yupingfeng granule for COPD is favorable.
 |
Figure 3 Monte Carlo simulation pseudoscatter plot. |
 |
Figure 4 Cost-effectiveness acceptable curve plotting. |
Discussion
COPD is a common respiratory disease. The pathogenesis of COPD is closely related to airway inflammation, airway mucus hypersecretion, oxidative/antioxidant imbalance, ciliary dysfunction, and immune imbalance, among which inflammatory factors play an important role. Studies have shown that the peripheral blood T lymphocyte subsets in patients with stable COPD are disordered and that the cellular immune function of the body is significantly reduced, mainly manifesting as abnormally reduced levels of CD4+ and CD4+/CD8+ and abnormally increased levels of CD8+. This may be one of the reasons why patients with stable COPD are prone to repeated exacerbations.23
The TCM “Yupingfeng” is a well-known prescription that is mainly used for immune regulation and anti-inflammation.24 Increasing the numbers of CD4+ and CD8+ T-cell subsets in the blood and increasing the numbers of CD4+/CD8+ cells can regulate immune disorders.25–27 Yupingfeng granules can improve immunity, improve symptoms and anti-allergy and prevent recurrence,28,29 which may explain the pharmacological reasons why they can be used to treat COPD. The RCT conducted by Ma and others6 also confirmed that Yupingfeng is more effective for the stable period of COPD because it improves the body’s immunity and reduces the number of acute attacks; moreover, it was found to be safer because of its mild mechanism of action. In recent years, traditional Chinese medicine has played an increasingly important role in the treatment of diseases because of its unique role and high levels of safety. For example, during the COVID-19 outbreaks, TCMs were proven to be effective30,31 and are listed in China’s anti-COVID-19 pneumonia guidelines.32–34 The economic aspect of TCMs is increasingly taken into consideration by medical decision-makers.
A study35 by Jiansheng Li et al compared the effectiveness and economic evaluation of three treatments of COPD: conventional Western medicine, TCMs (Bu-Fei granule, Bu-Fei Jian-Pi granule, Bu-Fei Yi-Shen granule, and Yi-Qi Zi-Shen granule), and a combination of both conventional Western medicine and TCM treatments. It found a combination of conventional medicine and TCM treatment is the most suitable for COPD patients, with better efficacy and economy. However, this study did not report the economic evaluation results with sufficient data. Two studies reported the economic evaluation results of TCM Yupingfeng. In the treatment of upper respiratory tract infection in children, Yupingfeng granule combined with conventional treatment has higher efficacy than conventional treatment, and healthcare spending is reduced.36 In the treatment of postchemotherapy hyperhidrosis, adding the Yupingfeng to the routine therapy makes the effect faster without increasing the cost.37
Similarly, the results in this study showed that regardless of whether the cycle time was 10 years or 35 years, the ICER of the Yupingfeng group compared with that of the placebo group was lower than one-time the per capita GDP of China in the long term. Adding Yupingfeng Granules had more economical advantages in the long term because of the significant clinical benefit and improved quality of life. Therefore, combining traditional Chinese medicines with chemical medicines is a more cost-effective strategy in the treatment of COPD. This can be used as evidence for clinical decision-makers to improve treatments.
In this study, the models were divided according to the number of acute exacerbations during the RCT, with reference to the ABCD group in the COPD GOLD report (2021 GLOBAL STRATEGY FOR PREVENTION, DIAGNOSIS AND MANAGEMENT OF COPD). Due to the particularity of TCM, as well as to ensure experimental rigor, the 1% forced expiratory volume value was not used in this study. Although the 1% distinction has usually been used to classify disease progression into severity in COPD studies, it was not necessarily applicable in studies of TCM considering the difference in the effects between TCM and chemical medicines. Instead, the model was divided into four disease states: stable phase, nonsevere exacerbations, severe exacerbations, and death. Nonsevere exacerbations and severe exacerbations correspond to different degrees of consumption of additional resources.
A mapping formula was used to convert the CAT score into a utility value in this study. Instead of taking the commonly used EQ-5D-3L or EQ-5D-5L utility scale, we used the CAT scale, which is an admittedly special-purpose assessment scale for COPD. Data availability was considered, and we believed that scales applicable to specific diseases were more accurate. In addition, the model including the selected CAT items provided more accurate estimates than the total CAT score model.
However, this study also had certain limitations due to data and methodological deficiencies. First, the Markov model was created on the condition that certain assumptions ideally hold. For instance, it was assumed that the remission rate was the same from the nonsevere exacerbation phase to the stable phase between the Yupingfeng group and the control group. Moreover, mortality during nonsevere exacerbations was assumed to be natural mortality. Therefore, these assumptions affected the extrapolation of the results to some extent. Second, due to the unavailability of some cost data, the sources of cost parameters were inconsistent, which may affect the results to a certain extent. Third, this study was carried out as a clinical trial, and the follow-up time was relatively short. Finally, the mapping formula used utility calculations from Korean research, which may be potentially different from those from the Chinese population in some respects.
Conclusion
By reducing the number of acute exacerbations of COPD, thereby correspondingly reducing the follow-up treatment cost, Yupingfeng granules combined with the conventional treatment provided a cost-effective therapeutic strategy for COPD.
Trial Registration and Ethics
This trial which the study based on was registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn; registration number: ChiCTR-IPR-15007023). The trial was approved by the local medical ethics committee of the First Affiliated Hospital of Guangzhou Medical University, Beijing Friendship Hospital Affiliated with Capital Medical University, Zhongshan Hospital Affiliated with Fudan University, the Affiliated Hospital of Guizhou Medical University, the Liwan Hospital of the Third Affiliated Hospital of Guangzhou Medical University, Nanshan Peoples’ Hospital, the First People’s Hospital of Huizhou, and the China Resource and Wisco General Hospital complied with the Declaration of Helsinki and the Good Clinical Practice regulations and was conducted strictly according to the requirements of the trial protocol. (Approved No. of the ethics committee: Medical Science Review 2014 No. 43).
Acknowledgments
All named authors take responsibility for the integrity of the work as a whole and have given their approval for this version to be published. The abstract has been presented as a poster for the ISPOR 2022 conference. https://www.ispor.org/heor-resources/presentations-database/presentation/intl2022-3463/116824. Ming Hu and Pan Ding are co-first authors for this study.
Author Contributions
MH and PD conceived the overall study, set up the Markov model, collected data, and drafted the manuscript. JM and NY participated in data collection. JZ designed and conducted the RCT, and participated in revising the text. NZ designed the research framework. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi:10.1164/rccm.201701-0218PP
2. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389:1931–1940. doi:10.1016/S0140-6736(17)31222-9
3. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391:1706–1717. doi:10.1016/S0140-6736(18)30841-9
4. Chronic Obstructive Pulmonary Disease Group of Respiratory Medicine Branch of Chinese Medical Association, Chronic Obstructive Pulmonary Disease Working Committee of Respiratory Physician Branch of Chinese Medical Doctor Association. Guidelines for diagnosis and treatment of chronic obstructive pulmonary disease (2021 revised edition). J Tuberc Respir. 2021;44(03):170–205.
5. Hillas G, Perlikos F, Tzanakis N. Acute exacerbation of COPD: is it the “stroke of the lungs”? Int J Chron Obstruct Pulmon Dis. 2016;11:1579–1586. doi:10.2147/COPD.S106160
6. Ma J, Zheng J, Zhong N, et al. Effects of YuPingFeng granules on acute exacerbations of COPD: a randomized, placebo-controlled study. Int J Chron Obstruct Pulmon Dis. 2018;13:3107–3114. doi:10.2147/COPD.S170555
7. Zhu H, Zhang H, Chen H. Effect of Yupingfeng granule on recurrent upper respiratory tract infection in children and its regulation on humoral immune function. Chin J Trad Chin Med. 2022;40(05):219–222.
8. Guo W, He Y, Zhang X, Wen J, Liu Y. Modified Yupingfeng powder in adjuvant therapy of allergic rhinitis: asystematic review. China Pharm. 2017;28(21):2947–2950.
9. Lin J, Sun Z. Guidelines for the clinical application of Chinese patent medicine in the treatment of adult bronchial asthma (2021). China J Integr Med. 2022;20:1–11. doi:10.1016/j.joim.2021.11.008
10. Liu H, Tian T, Wu Y, Tian L. Research progress of Yupingfeng Powder in the treatment of respiratory allergic diseases. J Tradit Chin Med Eye Otolaryngol. 2020;10:222–225.
11. Fan C. Cost-effectiveness analysis of indacaterol and tiotropium bromide in the treatment of COPD under the cost environment of China. Drug Eval. 2016;13:34–39.
12. Yaozhi. Drug winning database, Yupingfeng (April 29, 2020–December 16, 2020). Available from: https://www.yaozh.com.
13. Zhou Y, Wang X, Zeng X, et al. Positive benefits of theophylline in a randomized, double-blind, parallel-group, placebo-controlled study of low-dose, slow-release theophylline in the treatment of COPD for 1 year. Respirology. 2006;11:603–610. doi:10.1111/j.1440-1843.2006.00897.x
14. Zheng J, Zhong N, Wang C, et al. The efficacy and safety of once-daily fluticasone furoate/umeclidinium/vilanterol versus twice-daily budesonide/formoterol in a subgroup of patients from China with symptomatic COPD at risk of exacerbations (FULFIL trial). COPD. 2018;15:334–340. doi:10.1080/15412555.2018.1481022
15. Buxton KL, Roberts CM, Buckingham RJ, Pursey N, Stone RA. Palliative care service provision for chronic obstructive pulmonary disease patients: results from the 2008 national chronic obstructive pulmonary disease audit. In: Winter Meeting of the British-Thoracic-Society. London: Thorax; 2008:63.
16. Lim J, Choi SE, Bae E, Kang D, Lim EA, Shin GS. Mapping analysis to estimate EQ-5D utility values using the COPD assessment test in Korea. Health Qual Life Outcomes. 2019;17:97. doi:10.1186/s12955-019-1148-3
17. Liu G. China Guidelines for Pharmacoeconomic Evaluations. Beijing: Science Press; 2020.
18. National Bureau of Statistics. The People’s Republic of China 2020 National economic and social development statistical bulletin; 2021. Available from: http://www.stats.gov.cn/tjsj/zxfb/202102/t20210227_1814154.html.
19. Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176:753–760. doi:10.1164/rccm.200612-1749OC
20. Rong J, Ge Y, Chen G, Wang L, Ding H. Meta-analysis of the prevalence of chronic obstructive pulmonary disease in Chinese adults aged 40 and over from 2010 to 2019. Mod Prev Med. 2020;47:2305–9+36.
21. Zeng J, Deng Y, Ji K, Xu X, Cheng S. Study on the change trend of life expectancy and death spectrum of residents in Sichuan Province from 1989 to 2018. China Chronic Dis Prev Control. 2019;27:570–574.
22. People’s Daily. National health supports all-round well-off; 2021. Available from: http://www.nhc.gov.cn/wjw/mtbd/202106/4842f687b131495ca9b2568caf0218fb.shtml.
23. Zhang ZL, Li SN, Chen H, Luo T, Chen YL. Expression and influence of chemokine receptor 3 and lymphocyte subsets in different stages of chronic obstructive pulmonary disease. Hainan Med. 2017;28:3208–3210.
24. Jue S, Jun L, Shirui Z, et al. Anti-inflammatory and immunoregulatory effects of yupingfeng powder on chronic bronchitis rats. Chin J Integr Med . 2013;2013(5):7.
25. Huang A. Clinical efficacy and safety evaluation of budesonide combined with Yupingfeng granules in the treatment of bronchial asthma in children. J Mathematical Med 2018 [Chinese]. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2018&filename=SLYY201803035&uniplatform=NZKPT&v=NefN0VZaSCizXhNkMdbY22qFfbJelw-ixZaxN1FNARExsjxDOp_qrN0-7j7WCdwp.
26. Fan W, Zheng P, Wang Y, Hao P, Liu J, Zhao X. Analysis of immunostimulatory activity of polysaccharide extracted from Yu-Ping-Feng in vitro and in vivo. Biomed Pharmacother. 2017;93:146–155. doi:10.1016/j.biopha.2017.05.138
27. Li Y, Zheng B, Tian H, et al. Yupingfeng Powder relieves the immune suppression induced by dexamethasone in mice. J Ethnopharmacol. 2017;200:117–123. doi:10.1016/j.jep.2017.01.054
28. Gao J, Li J, Shao X, et al. Antiinflammatory and immunoregulatory effects of total glucosides of Yupingfeng powder. Chin Med J. 2009;122(14):1636–1641.
29. Yang Z, Li L, Zhang X, et al. Effect of western medicine combined with Yupingfeng powder on airway inflammation and immune regulation in 58 cases of allergic rhinitis asthma syndrome. Chin Ethnic Folk Med. 2021;30(18):109–111, 118.
30. Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. 2020;156:104761. doi:10.1016/j.phrs.2020.104761
31. Hu K, Guan WJ, Bi Y, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85:153242. doi:10.1016/j.phymed.2020.153242
32. Liu C, Liu T, Zhang J. On the cognitive process of new coronavirus pneumonia from the update of the new coronavirus pneumonia diagnosis and treatment guidelines. J Xi’an Jiaotong Univ. 2021;42:333–338.
33. Jin Y, Zhan Q, Peng Z, et al. Evidence-based clinical practice guidelines for drug prevention, diagnosis, treatment and discharge management of novel coronavirus pneumonia (updated version). PLA Med J. 2020;45(10):1003–1031.
34. Xiao M, Tian J, Zhou Y, et al. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol Res. 2020;161:105126. doi:10.1016/j.phrs.2020.105126
35. Li JS, Xie Y, Li SY, Yu XQ. Comparison of conventional medicine, TCM treatment, and combination of both conventional medicine and TCM treatment for patients with chronic obstructive pulmonary disease: study protocol of a randomized comparative effectiveness research trial. Trials. 2014;15:153. doi:10.1186/1745-6215-15-153
36. Xuan J, Lu Y, Liu B. Cost-effectiveness analysis of Yupingfeng granules in the treatment of children with recurrent respiratory tract infections. China Pharmacoecon. 2017;12(07):5–9.
37. Zeng B, Cai G, He X. Clinical observation and pharmacoeconomic analysis of Danggui Liuhuang Decoction and Yupingfeng Powder in the treatment of post-chemotherapy hyperhidrosis. China Med Innov. 2008;5(34):1–2.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.