Back to Journals » Clinical Interventions in Aging » Volume 12
Cost-effectiveness analysis of oral versus intravenous drip infusion of levofloxacin in the treatment of acute lower respiratory tract infection in Chinese elderly patients
Received 8 November 2016
Accepted for publication 18 February 2017
Published 12 April 2017 Volume 2017:12 Pages 673—678
DOI https://doi.org/10.2147/CIA.S127009
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Libin Zhang, Ping Hu
Department of Pharmaceutics, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Yangpu, Shanghai, People’s Republic of China
Aim: Pharmacoeconomic cost-effectiveness analysis of two different dosage regimens of levofloxacin in the treatment of acute lower respiratory tract infection in elderly patients.
Methods: A total of 108 elderly patients with acute lower respiratory tract infection who visited by our hospital between September 2013 and September 2014 were randomly divided into Group A and Group B, with 54 patients in each group. In Group A, levofloxacin injection was given for continuous intravenous infusion treatment, whereas in Group B, levofloxacin injection and levofloxacin capsule were given as sequential therapy (ST). The period of treatment for both the groups was 10 days, and minimum cost analysis was used to analyze the treatment.
Results: Groups A and B had cure rates of 61.1% and 59.3% (P>0.05), effective rates of 88.9% and 83.3% (P>0.05), bacterial clearance rates of 96.3% and 92.6% (P>0.05), and incidence rates of adverse reactions of 7.4% and 3.7% (P>0.05), respectively. Treatment costs of Groups A and B were 1,588 RMB and 1,150 RMB, respectively, whereas the cost-effectiveness of the two groups was at 17.86 and 13.81, respectively (P<0.05).
Conclusion: Levofloxacin ST had relatively higher cost-effectiveness ratio for the treatment of acute lower respiratory tract infection in elderly patients, especially Chinese.
Keywords: Pharmacoeconomic, cost-effectiveness, dosage, elderly patient, treatment cost, Chinese
Introduction
Respiratory tract infection includes upper respiratory tract infection and lower respiratory tract infection. The former, one of the most common infectious diseases, was referred to acute inflammation from nasal cavity to the throat, and the latter, also a commonly seen infectious disease, could be cured only in the condition that an effective antibiotic is selected based on the identification of the pathogen that has caused the infection. Acute lower respiratory infections (ALRIs) continue to be an important cause of acute illnesses and mortality worldwide (especially in elderly people).1,2 The main reasons that cause acute lower respiratory tract infection in the elderly include3: 1) decreased immunity due to aging of the body, 2) weakened natural ventilation function and airway scavenging ability due to lower central cough reflex, 3) aspiration pneumonia, 4) mental factors, 5) changes in the climatic environment, and 6) bacterial infections.
The Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) estimates that there were 2.8 million deaths because of lower respiratory infections globally in 2010 (5.3% of the total deaths).2 The main etiological agents responsible for ALRI include bacteria (Streptococcus pneumoniae, Haemophilus influenzae type b, Staphylococcus aureus, etc), viruses, and fungi. Respiratory syncytial virus, human influenza viruses, human parainfluenza viruses type 1, 2, and 3 (PIV-1, PIV2, and PIV-3), human rhinoviruses, adenoviruses, human metapneumovirus, human coronavirus, and human bocavirus have been identified among patients with ALRI.4–7
Over the past decade, the development of successive generations of fluoroquinolones such as levofloxacin, grepafloxacin, sitafloxacin, sparfloxacin, trovafloxacin, moxifloxacin, gatifloxacin, and gemifloxacin has been prompted primarily by the lesser in vitro potency of the original second-generation agents, such as ciprofloxacin, against S. pneumoniae.8–11
However, in China, levofloxacin is still the classical drug for ALRI treatment. With the development of pharmacoeconomics and continuously enhanced self-care awareness, antimicrobial therapy – sequential therapy (ST) has started to be applied for clinical use.12,13 This study used levofloxacin injection and levofloxacin capsule as the treatment method, analyzed the clinical and economic effects of “intravenous to oral” ST for the treatment of lower respiratory tract bacterial infection.14–16 This treatment method was compared with continuous intravenous infusion treatment to provide statistical reference for clinical use.
Methods
Patient selection and clinical evaluation
The inclusion criteria were 1) hospitalized patients at the age of ≥60 years, 2) patients diagnosed with lower respiratory tract bacterial infection according to clinical symptoms and through chest X-ray examination, 3) phlegm bacteriological culture for pathogen growth, and 4) patients who underwent no other antibiotic treatment recently. Exclusion criteria were 1) sensitivity to levofloxacin, 2) functional abnormality in liver and kidney, 3) mental or neurological diseases history, and 4) patients with complex infection treated by combination therapy.
Between September 2013 and September 2014, a total of 108 hospitalized patients satisfied the aforementioned criteria and agreed to participate in clinical trials. The patients included 52 male and 56 female patients aged between 58 and 76 years; 15 had bronchiectasis with infection, 16 had bacterial pneumonia, 8 had tuberculosis with infection, and 69 had acute attack of chronic obstructive pulmonary disease. The patients were randomly divided into Group A and Group B, each containing 54 patients. No significant difference was found in the two groups through x2 examination (P>0.05). As seen in Table 1, comparability of the data existed.
  | Table 1 Clinical information of the two groups (n=54 in each group) |
Treatment method
Group A had 200 g intravenous infusion of levofloxacin, bid. Upon the improvement of patients’ condition, intravenous infusion was continued. The total treatment period was 10 days. Group B had ST, which first involved 200 g intravenous infusion of levofloxacin, bid, for 5 days until patients’ condition improved and then oral intake of 200 mg levofloxacin capsule, bid, for 5 days.17–19 The total treatment period for both the groups was 10 days.
Clinical observation and auxiliary examination
Sputum bacteria culture and sensitivity test20 were performed before and after the treatment, so did the hematuria routine, liver and kidney function test, and chest X-ray examination. Patients’ clinical symptoms, signs and adverse reactions, hemogram and other parameters were recorded daily after treatment.
Efficacy evaluation criteria
“Guideline for clinical use of antimicrobial drugs” published by the Ministry of Health was applied for efficacy evaluation by 4 levels: recovery, significantly improved, improved, and no effect. “Recovery” and “significantly improved” were used for the calculation of efficacy rate. Bacteriological efficacy was evaluated by the clearance or nonclearance of bacterial strain after the treatment period.
Calculation of cost
Treatment costs of lower respiratory tract bacterial infection included both direct and indirect costs. Direct costs were divided into direct medical expenses and direct nonmedical expenses. Direct medical expense included the medicine cost (mainly the cost of medicine, intravenous infusion, and other materials), administration costs (including intravenous infusion and nursing cost), examination cost, and hospitalization cost. Nonmedical expenses, which included transportation and accommodation costs, were not included in this article. Indirect costs mainly included patients’ salary loss because of hospitalization or medical treatment. Since the period of hospitalization for patients with lower respiratory tract bacterial infection was relatively short, this article only included direct costs. The calculation of total medicine costs was based on the medicine price in our hospital in September 2013. Among them, the cost of l00 mg/piece levofloxacin injection was 26.9 RMB per piece and 100 mg/piece Levofloxacin capsule, which had 12 pieces in each box, was 18.0 RMB·per box. Therefore, the medicine costs of each group were: CA =26.9×2×10=538 RMB; CB =26.9×2×5+1.5×2×5=284 RMB.
Ethical considerations
Ethical approval was obtained from the Clinical Research Ethics Committee of the Shanghai Pulmonary Hospital. Permission to conduct the study in the selected center was also obtained. The subjects participated voluntarily in the study and were informed of its purposes. A written informed consent was obtained from every eligible subject.
Results
Comparison of costs
In this research, apart from medicine expenses for the 2 groups of patients, other costs included hospitalization cost, examination cost, administration cost, and so on. Thus, this study calculated the total expenses for the 2 groups of patients as shown in Table 2.
  | Table 2 Expenses for the two groups of patients (n=54 in each group) |
According to Table 2, the average cost of Group A and B was 1,588 RMB and 1,150 RMB, respectively, with statistical differences (P<0.05). As for medicine costs only, Group A had 254 RMB more than Group B.
Clinical efficacy ratio
According to Table 3, most patients in Group A could benefit from the treatment, with a cure rate of up to 61.1% and an effective rate of 88.9% effective rate = (Number of recovered patients + significant patients)/(total number of patients ×100). In Group B, the cure rate and effective rate were 59.3% and 83.3%, respectively, with no statistically significant differences between the 2 results (P>0.05). However, compared to the group treated with continuous intravenous infusion, clinical results show that Group B had more patients with “improved” and “no effect” results but no statistically significant differences (9.3% vs 13.0%, 1.9% vs 3.7%, P>0.05).
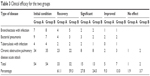  | Table 3 Clinical efficacy for the two groups |
Bacteriological efficacy
According to Table 4, the positive rates of 2 bacteriological cultures were both 100%. After 1 treatment period, most of the bacteria could be removed from the 2 groups. Group A had a bacterial strain clearance rate of 96.3%. Apart from several S. aureus and Pseudomonas aeruginosa, all others could be cleared. Group B had similar clearance situation with that of Group A. The difference was that in Group B, 1 Serratia patient was not cleared. The overall bacterial strain clearance rate was 92.6% with no statistically significant differences (P>0.05).
  | Table 4 Results of bacteriological examination of the two groups after treatment |
Minimum cost evaluation
The purpose of cost-effectiveness analysis was to determine the treatment plan with the least cost for certain treatment effect, which was represented by the cost per unit of performance. Since the 2 groups had no significant difference in results, incremental cost-effectiveness analysis was not conducted. Cost-effectiveness analysis was carried out as shown in Table 5.
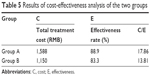  | Table 5 Results of cost-effectiveness analysis of the two groups |
Sensitivity analysis
Often, it was difficult to measure the variables in pharmacoeconomic research accurately, and many factors that were difficult to control also had influence on the analysis results. Therefore, assumed or estimated data would be applied. Sensitivity analysis was to verify the influence of different assumptions or estimations on the analysis of the results. It was assumed that the treatment cost would increase by 10% and the medicine cost would decrease by 10%. Sensitivity analysis produced almost the same results, which were shown in Table 6.
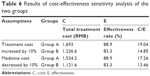  | Table 6 Results of cost-effectiveness sensitivity analysis of the two groups |
Adverse drug reactions
During this research, no obvious adverse reaction was found in any of the 2 groups. Among them, Group A had 4 cases of adverse reactions, 1 with nausea, 1 with epigastric discomfort, and 2 with central nervous system symptoms including dizziness and insomnia. The adverse reaction incidence rate was at 7.4%, but all the reactions could be tolerated with no influence on clinical treatment. Group B had 2 patients with adverse reactions, both showed gastrointestinal symptoms including epigastric discomfort and nausea. The adverse reaction incidence rate was 3.7%. After examination, no obvious adverse reactions in liver, kidney, and hematopoietic systems were found among the 2 groups.
Discussion
ST21,22 was a type of administration applied during antibiotic treatment of severe infectious diseases that involves parenteral administration (usually intravenous administration) at the early stage and then, after the patients’ condition was improved (usually 3–5 days after administration), oral antibiotics were used. In general, it was changed among the same medicine with different formulations, from one level of antibacterial drugs to a lower level, or between the same level. The therapeutic and economic value of antibacterial drugs in ST was gaining more attention.
Levofloxacin has been established as one of the leading fluoroquinolone agents during the past 10 years. It was the levo form and active ingredient of ofloxacin. It has the doubled antibacterial activity of ofloxacin, less adverse reaction, and better pharmacokinetic characteristics, gradually replacing ofloxacin. It has shown clinical efficacy in ALRI similar to that of gatifloxacin and is at least as efficacious as the third-generation cephalosporins.23,24 Extensive clinical data have confirmed good tolerability of levofloxacin without the phototoxicity or hepatic and cardiac AEs found with some other newer fluoroquinolone drugs. Therefore, levofloxacin offers a combination of documented efficacy and tolerability and has an established place in the routine treatment of bacterial infections, including ALRI.25,26
Findings of this research showed that either the levofloxacin intravenous infusion group or the levofloxacin “intravenous to oral” group had shown significant difference in clinical efficacy, incidence of adverse reactions, and bacterial clearance rate. However, the pharmacoeconomic cost–benefit analysis showed that the total cost and antimicrobial costs of the ST group were significantly lower than that of the continuous intravenous infusion group. It was also the case with the sensitivity analysis. Patients treated with ST could go home and continue the oral treatment if his/her condition was stable after the change of administration method (oral capsules). This could not only reduce the treatment cost but also lower the probability of complications developed in hospitals. The purpose of ST was to minimize the utilization of resources with the same treatment effect and reduce adverse reactions of the medicine and intravenous medication. Levofloxacin was easy to be absorbed orally with wide distribution in vivo and bioavailability >90%.27,28 Results of this research demonstrated that levofloxacin ST could be applied as an effective method for the treatment of lower respiratory tract infection in the elderly. This treatment method is particularly valuable in countries like China, which has a huge population and insufficient medical resources.
Disclosure
The authors report no conflicts of interest in this work.
References
Mizgerd JP. Lung infection – a public health priority. PLoS Med. 2006;3:e76. | ||
Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. | ||
Blasi F, Cazzola M, Tarsia P, et al. Azithromycin and lower respiratory tract infections. Expert Opin Pharmacother. 2005;6:2335–2351. | ||
Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Int Health. 1998;3:268–280. | ||
Foulongne V, Guyon G, Rodiere M, Segondy M. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J. 2006;25:354–359. | ||
Wolf DG, Greenberg D, Kalkstein D, et al. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr Infect Dis J. 2006;25:320–324. | ||
Lau SK, Woo PC, Yip CC, et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44:2063–2071. | ||
Ball P. The quinolones: history and overview. In: Andriole VT, editor. The Quinolones. 3rd ed. San Diego, CA: Academic Press; 2000:1–31. | ||
Ball P, Fernald A, Tillotson G. Therapeutic advances of new fluoroquinolones. Exp Opin Invest Drugs. 1998;7:761–783. | ||
Ball P. Quinolone-induced QT interval prolongation: a not-so-unexpected class effect. J Antimicrob Chemother. 2000;45:557–559. | ||
Ball P, Mandell L. Treatment of community-acquired respiratory tract infections. In: Hooper DC, Rubinstein E, editors. Quinolone Antimicrobial Agents. 3rd ed. Washington, DC: American Society of Microbiology Press; 2003:227–243. | ||
Abuhammour A, Dajani A, Nounou M, Zakaria M. Standard triple therapy versus sequential therapy for eradication of Helicobacter pylori in treatment naïve and retreat patients. Arab J Gastroenterol. 2016;17:131–136. | ||
Barnes EL, Goldin A, Winter RW, et al. Sequential combination therapy versus monotherapy: a lack of benefit in time to inflammatory bowel disease-related surgery. Dig Dis Sci. 2016;61:3261–3269. | ||
Kuti JL, Le TN, Nightingale CH, Nicolau DP, Quintiliani R. Pharmacoeconomics of a pharmacist-managed program for automatically converting levofloxacin route from i.v. to oral. Am J Health Syst Pharm. 2002;59:2209–2215. | ||
Wasserfallen JB, Erard V, Cometta A, Calandra T, Lamy O. Cost-effectiveness of full-course oral levofloxacin in severe community-acquired pneumonia. Eur Respir J. 2004;24:644–648. | ||
Tachi T, Teramachi H, Asano S, et al. Impact of levofloxacin dose adjustments by dispensing pharmacists on adverse reactions and costs in the treatment of elderly patients. Pharmazie. 2013;68:977–982. | ||
Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Vandervoort MK, Feagan BG. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA. 2000;283:749–755. | ||
Hurst M, Lamb HM, Scott LJ, Figgitt DP. Levofloxacin: an updated review of its use in the treatment of bacterial infections. Drugs. 2002;62:2127–2167. | ||
Vogel F. Intravenous/oral sequential therapy in patients hospitalised with community-acquired pneumonia: which patients, when and what agents? Drugs. 2002;62:309–317. | ||
Liapikou A, Torres A. Pharmacotherapy for lower respiratory tract infections. Expert Opin Pharmacother. 2014;15:2307–2318. | ||
Safavi M, Sabourian R, Foroumadi A. Treatment of Helicobacter pylori infection: current and future insights. World J Clin Cases. 2016;4:5–19. | ||
Xie C, Lu NH. Review: clinical management of Helicobacter pylori infection in China. Helicobacter. 2015;20:1–10. | ||
Huang CH, Lai CC, Chen YH, Hsueh PR. The potential role of nemonoxacin for treatment of common infections. Expert Opin Pharmacother. 2015;16:263–270. | ||
Di Marco F, Braido F, Santus P, Scichilone N, Blasi F. The role of cefditoren in the treatment of lower community-acquired respiratory tract infections (LRTIs): from bacterial eradication to reduced lung inflammation and epithelial damage. Eur Rev Med Pharmacol Sci. 2014;18:321–332. | ||
Grossman RF, Rotschafer JC, Tan JS. Antimicrobial treatment of lower respiratory tract infections in the hospital setting. Am J Med. 2005;118 (Suppl 7A):29S–38S. | ||
File TM Jr, Tillotson GS. Gemifloxacin: a new, potent fluoroquinolone for the therapy of lower respiratory tract infections. Expert Rev Anti Infect Ther. 2004;2:831–843. | ||
Kervezee L, Stevens J, Birkhoff W, et al. Identifying 24 h variation in the pharmacokinetics of levofloxacin: a population pharmacokinetic approach. Br J Clin Pharmacol. 2016;81:256–268. | ||
Zhu L, Zhang Y, Yang J, et al. Prediction of the pharmacokinetics and tissue distribution of levofloxacin in humans based on an extrapolated PBPK model. Eur J Drug Metab Pharmacokinet. 2016;41:395–402. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
