Back to Journals » Therapeutics and Clinical Risk Management » Volume 13
Cost-effectiveness analysis of dolutegravir plus backbone compared with raltegravir plus backbone, darunavir+ritonavir plus backbone and efavirenz/tenofovir/emtricitabine in treatment naïve and experienced HIV-positive patients
Authors Restelli U , Rizzardini G, Antinori A, Lazzarin A, Bonfanti M, Bonfanti P, Croce D
Received 2 March 2017
Accepted for publication 5 May 2017
Published 29 June 2017 Volume 2017:13 Pages 787—797
DOI https://doi.org/10.2147/TCRM.S135972
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Umberto Restelli,1,2 Giuliano Rizzardini,3,4 Andrea Antinori,5 Adriano Lazzarin,6 Marzia Bonfanti,1 Paolo Bonfanti,7 Davide Croce1,2
1Centre for Research on Health Economics, Social and Health Care Management, LIUC – Università Cattaneo, Castellanza, Varese, Italy; 2School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; 3First and Second Divisions of Infectious Diseases, “Luigi Sacco” Hospital, Milan, Italy; 4School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; 5National Institute for Infectious Diseases “L Spallanzani”, Rome, 6Department of Infectious Diseases, San Raffaele Scientific Institute, 7Department of Infectious and Tropical Diseases, A Manzoni Hospital, Lecco, Italy
Background: In January 2014, the European Medicines Agency issued a marketing authorization for dolutegravir (DTG), a second-generation integrase strand transfer inhibitor for HIV treatment. The study aimed at determining the incremental cost-effectiveness ratio (ICER) of the use of DTG+backbone compared with raltegravir (RAL)+backbone, darunavir (DRV)+ritonavir(r)+backbone and efavirenz/tenofovir/emtricitabine (EFV/TDF/FTC) in HIV-positive treatment-naïve patients and compared with RAL+backbone in treatment-experienced patients, from the Italian National Health Service’s point of view.
Materials and methods: A published Monte Carlo Individual Simulation Model (ARAMIS-DTG model) was used to perform the analysis. Patients pass through mutually exclusive health states (defined in terms of diagnosis of HIV with or without opportunistic infections [OIs] and cardiovascular disease [CVD]) and successive lines of therapy. The model considers costs (2014) and quality of life per monthly cycle in a lifetime horizon. Costs and quality-adjusted life years (QALYs) are dependent on OI, CVD, AIDS events, adverse events and antiretroviral therapies.
Results: In treatment-naïve patients, DTG dominates RAL; compared with DRV/r, the ICER obtained is of 38,586 €/QALY (6,170 €/QALY in patients with high viral load) and over EFV/TDF/FTC, DTG generates an ICER of 33,664 €/QALY. In treatment-experienced patients, DTG compared to RAL leads to an ICER of 12,074 €/QALY.
Conclusion: The use of DTG+backbone may be cost effective in treatment-naïve and treatment-experienced patients compared with RAL+backbone and in treatment-naïve patients compared with DRV/r+backbone and EFV/TDF/FTC considering a threshold of 40,000 €/QALY.
Keywords: antiretroviral therapy, costs, economic evaluation, cost-utility analysis
Background
In the last decade, several new antiretroviral drugs for the treatment of HIV have been approved by drug regulatory agencies in Europe, US, Canada and Australia (ie, darunavir [DRV], raltegravir [RAL], elvitegravir, rilpivirine, maraviroc). Most of these marketing authorizations are based on non-inferiority Phase III studies that demonstrated the new drug to be non-inferior compared with the comparators taken into consideration.
Antiretroviral therapy (ART) usually consists of a backbone composed of two nucleoside reverse transcriptase inhibitors (NRTIs) and of a third agent, either a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI), a CCR5 receptor antagonist or an integrase strand transfer inhibitor (INSTI). The first-line treatment recommended by the Italian Guidelines On The Use Of Antiretroviral Drugs is a backbone plus an INSTI or a backbone plus an NNRTI or, in particular conditions, a backbone plus a PI.1
The most recent data available on the Italian National Health Service (NHS) expenditure for ARTs show a total of 672.7 million €, of which 336.9 million € for drug combinations, 161.8 million € for PIs (alone or in one pill combination), 52.4 million € for NRTIs, 33.3 million € for NNRTIs and 88.3 million € for other antiretroviral drugs.2
In January 2014, the European Medicines Agency issued a marketing authorization for dolutegravir (DTG), a second-generation INSTI for the treatment of HIV on the European market.3
DTG is the only antiretroviral drug recommended with both abacavir/lamivudine (ABC/3TC) and tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) backbones by the Department of Health and Human Services’ Panel Guidelines.4 It proved to be superior for viral suppression in treatment-naïve patients to DRV plus ritonavir (DRV+r) at 48 and 96 weeks5,6 and to efavirenz/TDF/FTC (EFV/TDF/FTC).7 Compared to RAL, it proved to be non-inferior in treatment-naïve patients at 48 and 96 weeks8,9 and to be superior in treatment-experienced patients at 48 weeks.10
Within the aforementioned studies, the analyses conducted considering as target population a high viral load patients subgroup (HIV RNA >100,000 copies/mL), provided only assuming DRV+r and RAL+backbone as comparators, showed an increased difference in terms of effectiveness due to the use of DTG+backbone vs the comparator compared with the total population.5,6,8,9
In Italy, HIV clinical pathways at a regional level introduced the concept of cost-effectiveness as a parameter to be taken into consideration when selecting ART to be administered.11–13 Therefore, the investigation of the value for money of ARTs is increasingly needed within the Italian context.
Starting from the efficacy results presented earlier, the study aimed at determining the incremental cost-effectiveness ratio (ICER) of the use of DTG+backbone compared with RAL+backbone, DRV+r+backbone and EFV/TDF/FTC in HIV-positive treatment-naïve patients and compared with RAL+backbone in treatment-experienced patients, adopting the point of view of the Italian National Health Service.
Further analyses were conducted considering a subgroup of patients with high viral load (HIV RNA >100,000 copies/mL) at baseline, where such subgroup was considered in clinical trials (vs DRV+r and RAL in treatment-naïve patients).
Materials and methods
Model structure
The analysis was performed using a Monte Carlo Individual Simulation Model, the ARAMIS-DTG model (an updated version of the ARAMIS model used to evaluate the cost-effectiveness of maraviroc+optimized background therapy [OBT] vs OBT).14
This microsimulation model allows patients to pass through successive lines of therapy considering costs and quality of life per monthly cycle in a lifetime horizon. Patients pass through mutually exclusive health states, defined in terms of diagnosis of HIV with or without opportunistic infections (OI) and cardiovascular disease (CVD).
Each patient within the model is assigned to one of 12 mutually exclusive health states: without OIs or with one of five possible OIs combined with the possibility of being affected by CVD. Patients can transition between any of the HIV health states into the CVD health state (if so, they remain within this state until death) and can experience acute OIs. Patients remain in a given health state for at least one cycle (1 month) and are at risk of changes in CD4+ cell count, consequences of the disease (OIs, AIDS-related morbidity), CVD, adverse events (AEs) and treatment failure due to viral rebound.
Figure 1 shows the model structure in terms of transitions among health states.
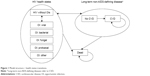  | Figure 1 Model structure – health states transitions. |
For the first 48 weeks in which a patient is assigned to a therapy, the model considers the 48 weeks probability to remain virologically controlled (as reported in Table 1). After this period, the probability of a viral rebound becomes monthly, being estimated by dividing the difference in viral load suppression at 48 and 96 weeks5–10,15 by the viral load suppression at 48 weeks. After 96 weeks, the monthly probability of a viral rebound is derived from the study by Rockstroh et al.16
Patients enter the model with a CD4+ level as observed in the cohort considered for each ART and experience a cell recovery based on the mean CD4+ cell count increase (with a cap of 1,500 cells/μL) of the treatment in use for 24 months, after which the pace of CD4+ cells’ recovery becomes non-treatment-related. Patients failing ART maintain the CD4+ level, since they are switched to the subsequent ART regimen defined within the algorithm implemented. Twelve months after failing the last ART, a decline of CD4+ cell count is observed, as explained later (“Clinical parameters” section).
CD4+ cell count, along with the time since ART started, affects the monthly probability to experience OIs (viral, bacterial, protozoal, fungal, and others), and they were calculated from the study by D’Arminio et al.17 Monthly CVD risk is estimated considering the coronary heart disease’s Framingham score plus equation for stroke. The parameters tracked in the model and used to estimate the score are the total cholesterol, the high-density lipoprotein cholesterol and age of patients. All other parameters derived from clinical trials remain constant. AIDS diagnosis is defined by CD4+ cell count equal to or lower than 200 cells/μL.
AEs’ monthly probability rate is influenced by the ART in use derived from the incidence of AEs emerged from the clinical trials considered.5,7,8,10 The costs and quality-adjusted life years (QALYs) are dependent on OI, CVD, AIDS, AEs and ART (the latter influencing only costs), as explained in the respective paragraphs. The occurrence of specific events is recorded for each patient from entry into the model until death, using tracker variables.
Both outcomes (QALYs) and costs were discounted at an annual rate of 3%.18
Figure 2 illustrates the inter-relationship between the different components of the model.
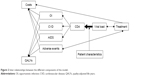  | Figure 2 Inter-relationships between the different components of the model. |
Death may occur due to acute OI, HIV-related disease (including OI), CVD and background mortality (Italian-specific life tables).
Each cost utility analysis has been conducted with a lifetime horizon.
Intervention and comparator
The comparators considered in the analysis are RAL+backbone, the main competitor within the same drug class as DTG (INSTI); DRV+r+backbone, which, along with atazanavir+r, is the PI recommended by international HIV guidelines in first-line therapies;1,4 and EFV/TDF/FTC, which represented the gold standard therapy for years, before the development of protease inhibitors, and is among the cheapest ART options on the market.
Population
Each cost utility analysis was conducted considering a theoretical cohort of 1 million patients.
The cohort characteristics in terms of age, gender, CD4+ cell count, viral load at baseline, hepatitis B and C infection and Framingham score are based on the clinical trials mentioned earlier,5–10 as well as the effectiveness values of the therapies considered.
For naïve patients, the aforementioned studies considered a population with median age between 34 and 37 years, of which males were between 83% and 86%. In Italy, newly diagnosed patients, as reported in the most recent publication by the Italian National Health Institute,19 were 77.4% males with a median age of 39 years for males and 36 years for females. Caucasians represent between 68% and 86% of the cohorts of the studies considered. In Italy, the proportion of Caucasians is estimated to be >80% based on published data19 and experts’ opinion. Median baseline HIV-1 RNA copies/mL and median CD4 cells/μL were between 4.48 log10 and 4.68 log10 and between 338 and 400 cells/μL, respectively, in clinical trials. Unpublished data of the Italian Cohort of Antiretroviral Naïve patients show higher HIV-1 RNA, while CD4 cells copies’ levels are in line with the aforementioned data. This seems coherent considering that clinical trials’ cohorts are selected, presenting lower viral load compared to the general HIV-positive population. Finally, a higher hepatitis B virus (HBV) and hepatitis C virus (HCV) proportion of co-infected patients are observed in Italy, which is due to the higher prevalence of hepatitis virus in Italy, compared with central and north European countries and with United States and Canada.
Model input
Treatment algorithm
Following an algorithm defined by opinion leaders for each arm of the model, based on Italian HIV/AIDS guidelines,20 patients are assigned to a first-line antiretroviral therapy (ART), DTG+backbone or one of the comparators considered, and to subsequent ARTs (the treatment algorithm is reported as Supplementary materials). The treatment algorithm was defined by four directors of hospital Infectious Diseases departments, among which the Medical Director of the Italian National Institute for Infectious Diseases; the Director of Infectious Diseases Division, the Scientific Director for Clinical Research of a clinical research university hospital and the Director of Infectious Diseases Department of a clinical research university hospital. Consensus was reached through discussions within advisory boards. The choice of subsequent lines of treatment depends on the reason of the switch: tolerability failure with no resistance, virologic failure with no resistance or virologic failure with resistance.
Clinical parameters
The therapy in use influences the viral load of each patient, as observed in the clinical trials reported earlier,5–10 affecting the decline of CD4+ cell count21 (also influenced by the patient’s characteristics). The effectiveness value of each therapy (percentage of patients with HIV RNA of <50 copies/mL at week 48) is reported in Table 1.
Mortality ratios for CVD, HIV and background mortality are taken from the Italian Statistics National Institute database,22 and a standardized mortality ratio is considered based on CD4+ cell count, as in the study by Lewden et al.23
Utility
The utility values related to CD4+ cell count, CVD, acute and post-acute OIs and the AEs considered within the model are presented in Table 2. Changes in the quality of life are associated with the following parameters: six CD4+ cell count categories (0–50, 50–100, 100–200, 200–350, 350–500 and >500 cells/μL), acute OIs according to five categories (bacterial, protozoal, fungal, viral, other), long-term non-AIDS-defining diseases and acute AEs leading to ART discontinuation.
Once an acute OI is experienced, the related utility is applied and after 3 months post-OI utilities are applied for the remainder of time that the individual is alive. Within the model, the lowest utility is applied for health states considering CD4+ cell count, OIs and CVD, while utilities decrement are subtracted from the base utility when patients experience one of the AEs considered.
Costs
The costs considered within the model are direct health costs related to ART, OI prophylaxis, treatment of OIs, routine care (hospitalization, outpatient activity, stratified by CD4+ cell count), costs of therapy switch, costs due to acute AEs and cost of death.
The cost of OIs, OIs’ prophylaxis, CVD and AEs were calculated on the basis of interviews submitted to directors of Infectious Diseases departments of Italian hospitals, to assess the resources consumption in real clinical practice for the diagnosis and care of the aforementioned events. These costs were approximated considering the reimbursement tariffs used within the Italian NHS for DRG and outpatient activities.
The costs, referred to year 2014, are reported in Table 3.
ART costs were considered based on Lombardy Region diagnostic clinical pathway.11 INSTI- and PI-based first-line therapies’ cost (INSTI+backbone and PI+backbone) were considered to be the sum of third drug’s cost (INSTI or PI) and of a weighted mean of the cost of the two most relevant backbones for Italian clinical practice on the basis of experts’ opinion (40% cost of ABC/3TC and 60% of emtricitabine/tenofovir).
The price of drugs in Italy is negotiated at a national level by the Italian Medicines Agency (AIFA). The ex-factory price is published in the Official Gazette of the Italian Republic and is then reduced by applying mandatory discounts defined at a national level. Regional prices are then subject to regional tenders; however, limited differences are observed for antiretroviral drugs. The prices used in the analysis, published in the Lombardy Region diagnostic clinical pathway,11 are comparable (with limited differences of <1 €) with those published within the most recent Italian HIV National Guidelines,1 excluding the drugs whose price was changed after 2014. The different prices (related to only three drugs) are, however, at the same level of clinical pathways and publications concerning other regions, as the clinical pathway of Veneto24,25 and that of Calabria,26 with differences of <0.5 €. Therefore, the prices used in the analysis are considered by the authors as representative at a national level.
Sensitivity analysis
Univariate sensitivity analyses were performed to address the uncertainty of the main parameters of the model. The range of each parameter considered is presented in Table 4. A probabilistic sensitivity analysis was not performed due to its estimated running time being, as reported by Pialoux et al,27 ~417 days per each comparator.
  | Table 4 Ranges and values of the parameters modified within the sensitivity analysis |
Based on experts’ opinion, we tested the effects of a decrease of 10% of DTG cost (an increase of the drug cost was considered highly unlikely), of a change of ±10% of the cost of subsequent therapies and salvage therapies (due to the possible decrease of therapies’ costs and increase due to new available drugs), of not considering any discount rate both for costs and QALYs, of a decrease of 2.5% of DTG effectiveness and of the use of alternative utility values associated with each CD4+ cell count level.
Results
Base case results
The results of the analyses conducted are reported in Table 5.
DTG dominates RAL in treatment-naïve patients, leading to lower costs for the Italian National Health Service and being more effective, resulting in a higher number of QALYs per patient. The same result is observed in treatment-naïve patients with high viral load.
Compared with DRV+r, DTG leads to an increase of costs and an increase of QALYs, with an ICER of 38,586 €/QALY in treatment-naïve patients and of 6,170 €/QALY in treatment-naïve patients with high viral load. The same scenario is observed in the comparison of DTG+backbone vs EFV/TDF/FTC, with an ICER of 33,664 €/QALY.
Considering treatment-experienced patients, the use of DTG compared with RAL leads to an increase of costs and to an increase of QALYs with an ICER of 12,074 €/QALY.
Sensitivity analysis results
The results of sensitivity analysis are presented in Table 6.
  | Table 6 Results of the sensitivity analysis |
In treatment-naïve patients, the sensitivity analysis results confirmed the dominance of DTG-based therapies compared with RAL-based therapies; confirmed DTG-based therapies to be cost effective compared with DRV+r-based therapies and with EFV/TDF/FTC considering a 40,000 €/QALY threshold, excluding the analyses in which the effectiveness of DTG is lowered by 2.5%, the cost of subsequent therapies is lowered by 10% and the cost of salvage therapies is raised by 10%; confirmed the dominance of DTG-based therapies compared with RAL-based therapies in high viral load patients and showed ICERs <12,500 €/QALY for DTG-based therapies vs DRV-based therapies in patients with high viral load.
In treatment-experienced patients, the sensitivity analysis confirmed DTG-based therapies to be cost-effective compared with RAL-based therapies considering a threshold of 40,000 €/QALY in all scenarios.
Discussion
The results of the analysis performed show how the use of DTG+backbone may be cost effective in treatment-naïve patients compared with RAL+backbone (being dominant, leading to lower costs and to a higher number of QALYs), DRV/r+backbone and EFV/TDF/FTC, considering the threshold of 40,000 €/QALY identified by the Italian Health Economics Association.18
DTG-based therapies showed a better cost-effectiveness profile compared with RAL+backbone and DRV/r+backbone in treatment-naïve patients subgroup with high viral load.
In 2015, Despiégel et al14 published an article presenting the results of a cost-effectiveness analysis in which, using the ARAMIS-DTG model, they compared the use of DTG in treatment-naïve patients with EFV, RAL, DRV+r, rilpivirine, elvitegravir/cobicistat, atazanavir+r and lopinavir/r and in treatment-experienced patients with RAL. DTG resulted to be dominant (leading to a decrease of costs for the Canadian NHS and to an increase of QALYs) in all the comparisons. These results are consistent with our analysis considering RAL as a comparator in treatment-naïve patients. When DRV+r and EFV/TDF/FTC are assumed as comparators in treatment-naïve patients and RAL is assumed as comparator in treatment-experienced patients, the Italian results showed increased QALYs and costs due to the use of DTG, while in the analysis performed in Canada, DTG led to higher QALYs and lower costs.
In Europe, a similar analysis was conducted in France by Pialoux et al.27 The authors performed a cost-effectiveness analysis, through the use of the ARAMIS-DTG model, comparing the use of DTG vs RAL in treatment-experienced and INSTI-naïve adults with at least two-class resistance. DTG led to an increase of QALYs (+0.35) and of costs (+7,266 €), with a cost per QALY of 21,048 €, leading the authors to consider DTG cost effective compared to RAL in the target population. The results obtained by Pialoux et al are consistent with those obtained in the analysis presented.
A further study concerning the health economics evaluation of DTG is that of Peng et al,28 in which DTG+ABC/3TC was compared with EFV/TDF/FTC as first-line treatment of HIV-1 patients in the United States. The study shows a lifetime better result for DTG in terms of QALY gained (+0.12 per person) and higher costs (+58,188 US$ per person), with an ICER of 482,717 US$/QALY. Even if the analysis shows a higher effectiveness and higher costs for DTG+backbone compared with EFV/TDF/FTC as in the study performed in Italy, it is difficult to compare the results of analyses performed in two health systems with different characteristics as those of Italy (universalistic public system) and of the United States (market-based health insurance system).
The analysis performed is the first to assess the cost-effectiveness of DTG vs its main comparators within the Italian context. The main limitation of our analysis is related to the generalizability of the results, being based on Italian costs and on Italian HIV/AIDS guidelines. The prices of HIV drugs are subject to continuous modifications due to pharmaceutical companies’ market strategies; therefore, the analysis should be updated whenever changes in drugs prices are observed. To estimate the cost of OIs’ management, OIs’ prophylaxis, CVD, AEs and laboratory tests, we considered the reimbursement tariffs of the Italian NHS. This is consistent with the point of view assumed but might underestimate the costs for the Italian NHS. In fact, if the cost to perform a health care service for a public provider is higher than the tariff with which it is reimbursed by the NHS, at the end of the fiscal year, the NHS might have to provide further economic resources to the aforementioned provider. Moreover, concerning the clinical effectiveness of the therapies considered, the analysis presented is based on efficacy data coming from published trials, while future analyses should focus on effectiveness data collected within the Italian health service (not available at present) to ensure the reliability of the results. Furthermore, specific studies on the cost of AEs and complications in Italy in HIV-positive patients’ cohorts should be performed to provide reference data for future analyses. Limitations related to the model used for the analysis are related to the absence of a probabilistic sensitivity analysis and to a possible underestimation of CVD incidence, due to the fact that the model estimates this risk through Framingham equation, not considering the increased risk associated with HIV infection.
Conclusion
The use of DTG+backbone may be cost effective in treatment-naïve and treatment-experienced patients compared with RAL+backbone and in treatment-naïve patients compared with DRV/r+backbone and EFV/TDF/FTC considering the threshold of 40,000 €/QALY identified by AIES.
Acknowledgment
The study has been sponsored by ViiV Healthcare Srl.
Disclosure
UR declares honoraria for conference talks by Janssen-Cilag. GR declares honoraria for conference talks and advisory board participations (Abbvie, BMS, MSD, Gilead, ViiV, Janssen-Cilag). AA declares grants, personal fees and non-financial support from Gilead Sciences, personal fees from Merck, grants and personal fees from Bristol Myers Squibb, personal fees and non-financial support from Abbvie, grants, personal fees and non-financial support from ViiV Healthcare and grants and personal fees from Janssen-Cilag, all outside the submitted work. AL declares honoraria for conference talks and advisory board participations: Abbvie, BMS, MSD, Gilead, ViiV and Jannsen-Cilag. MB declares no conflicts of interest. PB declares honoraria for conference talks and advisory board participations: Abbvie, BMS, MSD, Gilead, ViiV and Jannsen-Cilag. DC declares honoraria for advisory board participations by MSD, Abbvie and Baxter. The authors report no other conflicts of interest in this work.
References
HIV/AIDS Italian Expert Panel. Linee Guida Italiane sull’utilizzo dei farmaci antiretrovirali e sulla gestione diagnostico-clinica delle persone con infezione da HIV-1. (2016). Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2545_allegato.pdf. Accessed November 22, 2016. | ||
The Medicines Utilisation Monitoring Centre. National Report on Medicines Use in Italy. Rome: Italian Medicines Agency; 2015. Available from: http://www.agenziafarmaco.gov.it/sites/default/files/Rapporto_OsMed_2015__AIFA.pdf. Accessed May 19, 2017. | ||
European Medicines Agency – EPAR Summary for the Public – Tivicay. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002753/WC500160681.pdf. Accessed May 19, 2017. | ||
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services. Available from: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed May 19, 2017. | ||
Clotet B, Feinberg J, van Lunzen J, et al; ING114915 Study Team. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–2231. | ||
Molina JM, Clotet B, van Lunzen J, et al. Once-daily dolutegravir is superior to once-daily darunavir/ritonavir in treatment-naïve HIV-1-positive individuals: 96 week results from FLAMINGO. J Int AIDS Soc. 2014;17(4 suppl 3):19490. | ||
Walmsley SL, Antela A, Clumeck N, et al; SINGLE Investigators. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–1818. | ||
Raffi F, Rachlis A, Stellbrink HJ, et al; SPRING-2 Study Group. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–743. | ||
Raffi F, Jaeger H, Quiros-Roldan E, et al; Extended SPRING-2 Study Group. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13(11):927–935. | ||
Cahn P, Pozniak AL, Mingrone H, et al; Extended SAILING Study Team. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700–708. | ||
Directorate General for Health of Lombardy Region D.d.g. 22 December 2014 – n. 12515 – Approvazione del documento avente ad oggetto “Percorso diagnostico terapeutico (PDT) del paziente affetto da malattia HIV/AIDS – anno 2105”. Available from: http://omceomi.it/docs/default-source/leggi-e-norme/percorso-diagnostico-terapeutico-paziente-affetto-da-malattia-hiv-aids-anno-2015-burl-30-12-2014.pdf?sfvrsn=0. Accessed May 15, 2015. | ||
Regione Lazio, Decreto del Commissario ad acta n. U00002 del 13 Gennaio 2014 – Allegato A “Percorso Diagnostico Terapeutico (POT) Regionale sulla Terapia Antiretrovirale”. Available from: http://www.regione.lazio.it/binary/rl_sanita/tbl_normativa/Decr_U00002_13_1_13_aggiornamento_terapia_HIV.pdf. Accessed November 24, 2015. | ||
Decreto del Segretario della Segreteria Regionale per la Sanità n. 148 del 02 Dicembre 2013 – Allegato A “Percorso Diagnostico Terapeutico Assistenziale (PDTA) del paziente adulto affetto da infezione da HIV/AIDS nella Regione Veneto”. Available from: http://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDecreto.aspx?id=263808. Accessed November 24, 2015. | ||
Despiégel N, Anger D, Martin M, et al. Cost-effectiveness of dolutegravir in HIV-1 treatment-naive and treatment-experienced patients in Canada. Infect Dis Ther. 2015;4(3):337–353. | ||
Stellbrink HJ, Reynes J, Lazzarin A, et al. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS. 2013;27(11):1771–1778. | ||
Rockstroh JK, DeJesus E, Saag M, et al. Long-term safety and efficacy of raltegravir (RAL)-based versus efavirenz (EFV)-based combination therapy in treatment-naïve HIV-1 infected patients: final 5-year double-blind results from STARTMRK. Presented at: XIX International AIDS Conference; July 22–27; 2012; Washington, DC. | ||
D’Arminio MA, Sabin CA, Phillips A, et al. The changing incidence of AIDS events in patients receiving highly active antiretroviral therapy. Arch Intern Med. 2005;165:416–423. | ||
Italian Health Economics Association (AIES). Proposta di Linee-Guida per la valutazione economica degli interventi sanitari. Politiche Sanitarie. 2009;10(2):91–99. | ||
Italian National Institute of Health, AIDS Operative Centre. Notiziario dell’Istituto Superiore di Sanità; 2016;29(9 Suppl 1). Available from: http://www.iss.it/binary/ccoa/cont/dic_2015.pdf. Accessed May 19, 2017. | ||
HIV/AIDS Italian expert panel. Linee Guida Italiane sull’utilizzo dei farmaci antiretrovirali e sulla gestione diagnostico-clinica delle persone con infezione da HIV-1. (2014). Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2261_allegato.pdf. Accessed December 18, 2014. | ||
Mauskopf J, Brogan AJ, Talbird SE, Martin S. Cost-effectiveness of combination therapy with etravirine in treatment-experienced adults with HIV-1 infection. AIDS. 2012;26(3):355–364. | ||
Italian Statistics National Institute [homepage on the Internet]. I.Stat Database. Available from: http://dati.istat.it/Index.aspx. Accessed May 19, 2017. | ||
Lewden C, Chene G, Morlat P, et al; Agence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) CO8 APROCO-COPILOTE Study Group; Agence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) CO3 AQUITAINE Study Group. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr. 2007;46(1):72–77. | ||
Regione del Veneto [webpage on the Internet]. Percorso Diagnostico Terapeutico Assistenziale (PDTA) del paziente adulto affetto da infezione da HIV/AIDS nella Regione Veneto. Allegato A al Decreto n. 148 del 2 Dicembre 2013. Available from: http://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDecreto.aspx?id=263808. Accessed May 19, 2017. | ||
Regione del Veneto. Percorso Diagnostico Terapeutico Assistenziale (PDTA) del paziente adulto affetto da infezione da HIV/AIDS nella Regione Veneto – aggiornamento a febbraio 2016. Allegato A al Decreto n. 55 del 8 Giugno 2016. Available from: https://www.regione.veneto.it/c/document_library/get_file?uuid=3beb4023-6e19-4d71-a73e-48a4e6161d3d&groupId=10793. Accessed May 19, 2017. | ||
Regione Calabria. Percorso Diagnostico Terapeutico Assistenziale (PDTA) regionale del Paziente affetto da malattia da HIV/AIDS. Available from: http://www.regione.calabria.it/sanita/allegati/dpgr_2012/allegato_1_dpgr_198_del_20_dicembre_2012.pdf. Accessed May 19, 2017. | ||
Pialoux G, Marcelin AG, Despiégel N, et al. Cost-effectiveness of dolutegravir in HIV-1 treatment-experienced (TE) patients in France. PLoS One. 2015;10(12):e0145885. | ||
Peng S, Tafazzoli A, Dorman E, Rosenblatt L, Villasis-Keever A, Sorensen S. Cost-effectiveness of DTG+ABC/3TC versus EFV/TDF/FTC for first-line treatment of HIV-1 in the United States. J Int AIDS Soc. 2014;17(4 suppl 3):19605. | ||
Kauf TL, Roskell N, Shearer A, et al. A predictive model of health state utilities for HIV patients in the modern era of highly active antiretroviral therapy. Value Health. 2008;11(7):1144–1153. | ||
Simpson KN, Luo MP, Chumney EC, King MS, Brun S. Cost effectiveness of lopinavir/ritonavir compared with atazanavir in antiretroviral-naive patients: modelling the combined effects of HIV and heart disease. Clin Drug Investig. 2007;27(1):67–74. | ||
Paltiel AD, Scharfstein JA, Seage GR 3rd, et al. A Monte Carlo simulation of advanced HIV disease: application to prevention of CMV infection. Med Decis Making. 1998;18(2 suppl):S93–S105. | ||
Schackman BR, Goldie SJ, Freedberg KA, Losina E, Brazier J, Weinstein MC. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making. 2002;22(1):27–38. | ||
Rizzardini G, Restelli U, Bonfanti P, et al. Cost of human immunodeficiency virus infection in Italy, 2007–2009: effective and expensive, are the new drugs worthwhile? Clinicoecon Outcomes Res. 2012;4:245–252. | ||
Raitano M. “The Impact of Death-Related Costs on Health-Care Expenditure: A Survey”, ENEPRI Research Report No. 17/February 2006. | ||
Simpson KN, Luo MP, Chumney E, Sun E, Brun S, Ashraf T. Cost-effectiveness of lopinavir/ritonavir versus nelfinavir as the first-line highly active antiretroviral therapy regimen for HIV infection. HIV Clin Trials. 2004;5(5):294–304. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




