Back to Journals » Cancer Management and Research » Volume 11
Cost-Effectiveness Analysis Of Ceritinib And Alectinib Versus Crizotinib In The Treatment Of Anaplastic Lymphoma Kinase-Positive Advanced Non-Small Cell Lung Cancer
Authors Liu M , Zhang L, Huang Q, Li N, Zheng B, Cai H
Received 16 July 2019
Accepted for publication 14 September 2019
Published 25 October 2019 Volume 2019:11 Pages 9195—9202
DOI https://doi.org/10.2147/CMAR.S223441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Maobai Liu,1,* Longfeng Zhang,2,* Qishu Huang,3 Na Li,1 Bin Zheng,1 Hongfu Cai1
1Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, Fujian Province, People’s Republic of China; 2Department of Medical Oncology, Fujian Provincial Cancer Hospital, Fuzhou, Fujian Province, People’s Republic of China; 3College of Pharmacy, Fujian Medical University, Fuzhou, Fujian Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongfu Cai
Department of Pharmacy, Fujian Medical University Union Hospital, No. 29 Xinquan Road, Fuzhou 350001, Fujian Province, People’s Republic of China
Email [email protected]
Background: This study aimed to analyze the cost-effectiveness of crizotinib versus ceritinib or alectinib as first-line-targeted drug therapy for anaplastic lymphoma kinase-positive advanced non-small cell lung cancer in China.
Methods: The Markov model was used to simulate the medical cost and quality-adjusted life years (QALYs) of patients using crizotinib, ceritinib, or alectinib over a 10-year period by establishing three health states: progression-free, post-progression, and death. Randomized controlled clinical data were collected from the open-label, randomized phase 3 trials ALEX and ASCEND-4. Cost and utility values were derived from local charges and literature. Sensitivity analyses included one-way and probabilistic sensitivity analyses.
Results: Compared with patients who used crizotinib as first-line treatment, patients in the ceritinib and alectinib groups yielded an additional 1.32 and 3.30 QALYs with an incremental cost of $84,728.20 and $339,114.36, respectively. Thus, the incremental cost-effectiveness ratio (ICER) was $64,398.83 and $102,675.74 per QALY in the ceritinib and alectinib groups, respectively. Alectinib was estimated to be more effective (4.68 QALY) and more costly ($432,063.06) with an ICER of $128,019.42 per QALY compared with ceritinib (2.69 QALY and $177,676.90). Results were robust to deterministic and probabilistic sensitivity analyses.
Conclusion: As a first-line treatment regimen, ceritinib and alectinib can extend the survival time of patients compared with crizotinib, but the medical cost also increases accordingly. According to the World Health Organization’s three-percent GDP measurement, first-line treatment with Crizotinib is the most cost-effective.
Keywords: crizotinib, ceritinib, alectinib, NSCLC, cost-effectiveness
Introduction
Lung cancer is the most common cancer in China.1 It can be classified into small cell lung cancer and non-small cell lung cancer (NSCLC) based on pathological type; and NSCLC accounts for approximately 80% to 85% of lung cancer.2 Anaplastic lymphoma kinase (ALK) positive is a specific subtype of NSCLC with an incidence of 3–5% in NSCLC.3,4 Thus, the ALK gene can be a key therapeutic target for NSCLC patients. An international multicenter, randomized phase III clinical trial (PROFILE 1014; N = 343)5 has compared the efficacy of the ALK inhibitor of crizotinib and chemotherapy in previously untreated ALK-positive advanced NSCLC. Its findings show that median progression-free survival (mPFS) and overall survival (OS) in patients treated with ALK inhibitors significantly improved compared with those who underwent chemotherapy. 2019 NCCN guidelines6 recommend crizotinib, ceritinib, and alectinib as first-line treatment options for ALK-positive advanced NSCLC. Given the great breakthroughs on research on ALK inhibitors and its better therapeutic effect compared with chemotherapy, the survival time of patients with ALK-positive advanced NSCLC is significantly prolonged, further improving the health of patients.
Crizotinib is the first-stage-targeted inhibitor of ALK-positive NSCLC. Although crizotinib significantly affects the treatment of NSCLC, with its promotion in clinical application, patients develop acquired resistance during the 10–12 months of treatment.7 Alectinib and ceritinib are second-generation-targeted inhibitors of ALK-positive NSCLC. For a previously untreated ALK-positive NSCLC, a head-to-head international, multicenter randomized phase III clinical trial (ALEX; N = 303)8 has shown that alectinib (600 mg, twice daily) was higher than crizotinib (250 mg, twice daily) in safety and efficacy. The results of phase III clinical studies (ASCEND4; N = 376)9 showed that ceritinib (750 mg, once daily) significantly increased mPFS relative to chemotherapy.
A pharmacoeconomic evaluation of the first-line treatment of crizotinib in the Chinese population compared with traditional standard chemotherapy shows that the first-line treatment of crizotinib is more cost-effective.10,11 However, ceritinib and alectinib have better efficacy than crizotinib, and the economic impact and value of these three drugs in the first-line treatment environment in China have not been explored. In the era of rising medical costs, the value of prescription drugs is receiving increasing attention. This study aimed to provide a cost-effective analysis of the first-line treatment of Chinese ALK-positive advanced NSCLC with crizotinib, ceritinib, and alectinib and to provide reference for clinical decision-making and adjustment of the national medical insurance catalogue.
Methods
Model Structure
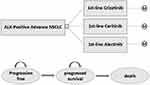
This study established a decision tree and a Markov model, setting three states of progression-free survival (PFS), progressive survival (PS), and death. From the perspective of the Chinese medical system, the model simulated the lifetime medical cost and quality-adjusted life years (QALYs) of patients taking crizotinib, ceritinib, and alectinib and assessed the total cost of treatment and health outcome of patients under each treatment regimen. The patient was diagnosed with untreated ALK-positive NSCLC at the time of enrollment, initially designated to be in the PFS state, and then assigned to PFS or PS on the basis of transition probability. Patients entering the PS state can stay only in this state or develop into death (Figure 1). According to the 2019 version of the NCCN6 and Chinese Society of Clinical Oncology lung cancer guidelines,12 the study made the following three hypotheses: (1) in the first-line treatment group of crizotinib using targeted drug therapy or chemotherapy after disease progression, 50% of patients were assumed to be treated with alectinib and 50% of patients were treated with chemotherapy; (2) in the first-line treatment group of ceritinib, patients were treated with chemotherapy after disease progression; and (3) in the first-line treatment group of alectinib, patients were treated with chemotherapy after disease progression.
The chemotherapy cycle for patients with NSCLC was 21 days. To facilitate parameter calculation, this study set the period of the Markov model to 21 days. According to clinical studies, patients with ALK-positive advanced NSCLC rarely survive more than 10 years after diagnosis, and all patients die after 10 years of simulation in model. Thus, the Markov model cycle was set to 10 years and used a 3% discount rate. All cost units were converted to USD, with an average RMB exchange rate of $1 to 6.6174 Yuan for the full year of 2018.13 This study used the TreeAge 2015 (TreeAge Software, Inc., Williamstown, MA, USA) to build a Markov model and perform basic analysis. Incremental cost-effectiveness ratio (ICER) is a measure of the economics of each option. ICER is the added cost of each additional QALY.
Clinical Data
The probability of PFS and OS in each treatment group was derived from published literature. Clinical data for crizotinib and alectinib were obtained from the ALEX trial,8 and clinical data for ceritinib were acquired from the ASCEND-4 trial.9 The GetData Graph Digitizer (Version 2.26) software was used to extract PFS probabilities and OS probabilities from the PFS and OS curves and to construct individual-level data for the KM curve.14 The KM curve was fitted and extrapolated by Weibull distribution S(t) = exp(-λtγ). The estimated parameters of the model are shown in Table 1. The duration of the PFS and progressed disease phases was calculated using the area under the PFS and OS survival curves. The difference between the PFS and OS estimated by the parametric survival model was used to calculate the probability from PF to death.14,15 The incidence of grade 3–5 adverse effects of crizotinib, ceritinib, and alectinib was 41%, 65%, and 50%, respectively.8,9
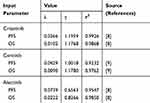 |
Table 1 Weibull Survival Model |
Cost And Utility
The cost of this study only covered direct medical costs, including drug costs, supportive care costs, follow-up costs, chemotherapy costs, treatment costs associated with adverse events, and hospice care costs (Table 2).16,17 The cost of drug came from the national medical insurance negotiation price and local charge, and other costs and utility values were derived from previously published research and related literature. The dosage of the drug taken by the patient every day was based on the drug label and Chinese guide.12 The doses of crizotinib and alectinib were costs twice daily, respectively, whereas the dose of ceritinib was 450 mg once daily. The first-line treatment of the above three drugs was taken until the disease progressed.
 |
Table 2 Base-Case Costs Estimates |
To reduce burden on patients, the China Cancer Foundation has developed the alectinib assistance program for ALK-positive NSCLC patients.18 In the first phase, the patient takes alectinib for five treatment cycles at his own expense (each treatment cycle is 28 days) and can obtain up to 8 treatment cycles. In the second phase, the patient takes alectinib for four treatment cycles at their own expense, and the follow-up drug can be used for up to nine treatment cycles. The patient’s third phase of the assistance program is the same as the second phase, but the assistance project is terminated, and the patients are all treated at their own expense. Utility values are based on preferences and reflect the degree to which people expect a state of health. The general value of death is 0, the value of a fully healthy is 1, and the health of a patient is often between 0 and 1. Utility values are derived from published related literature.19
Basic And Sensitivity Analyses
The Markov model was established to assess QALYs and the long-term cost of treatment in patients with NSCLC who were treated with three ALK inhibitors. The important endpoint of the assessment was ICER. This study compares ICER based on the metrics established by the World Health Organization, which is a threshold of three times the per capita GDP of China in 2018.20 When the ICER was less than the threshold, the increased cost was deemed acceptable; when the ICER was greater than the threshold, the increased cost was not worthwhile. China’s per capita GDP in 2018 was $9678.79,13 so the threshold was $29,306.37.
To assess the impact of model uncertainty on treatment options, this study conducted one-way sensitivity analyses and probabilistic sensitivity analyses (PSA). The range of parameters used in the one-way sensitivity analyses was derived from published literature. When the data range in the literature was unavailable, the baseline value was used to float up and down by 20%. Given the high cost of medicines, the Chinese government works hard to reduce the price of medicines. Thus, the price of medicines was limited only by a downward fluctuation of 50%. The discount rate ranged from 0 to 8%. PSA was further performed through the cost-effectiveness distribution of the quality of life model parameters and cost different distribution by 1000 second-order Monte Carlo simulations. The utility value was set to different distributions in accordance with the ISPOR-SMDM Modeling Good Research Practices Task Force recommendation for probability sensitivity. The final result was presented in a scatter plot and a cost acceptable curve.
Results
Basic Analysis
The results of the basic analysis are shown in Table 3. Compared with first-line crizotinib group, the ceritinib group showed increased QALYs by 1.32, increased cost of $84,728.20, and ICER of $64,398.83/QALY. The alectinib group exhibited increased QALYs by 3.30, increased cost of $339,114.36, and ICER of $102,675.74/QALY. When ICER was compared with a threshold of $29,306.37, the first-line crizotinib group was found to be the most cost-effective. Compared with first-line ceritinib, alectinib had better effect and higher cost, with an ICER value of $128,019.42/QALY, and crizotinib was more cost-effective.
 |
Table 3 Summary Of Cost (US$) And Outcome Results From A Base-Case Analysis |
Sensitivity Analysis
The stability of the results was verified by one-way sensitivity analysis. The tornado diagram is shown in Figure 2. Comparison of the first-line treatment regimen of crizotinib and ceritinib revealed that the cost of second-line chemotherapy was the main factor influencing the model results. Comparison of the first-line treatment regimen of crizotinib and alectinib revealed that the most important factor affecting the results of the model was also the cost of second-line chemotherapy. Among the first-line treatment schemes of ceritinib and alectinib, the cost of alectinib had the greatest influence on the results of the model. Based on the above analysis results, the model results were relatively stable.
The results of probability sensitivity analysis are shown in Figure 3 to reflect the influence of different distribution assumptions on the results. In the first-line treatment of ceritinib or alectinib compared with crizotinib, the 95% confidence interval for the ICER was above the willingness–to-pay threshold of $29,306.37. The cost-effectiveness acceptance curve (Figure 4) showed that when the threshold reaches $25,000, the probability of crizotinib economy was 100%. When the threshold reached $75,000, the probability of crizotinib economy was 21%. Ceritinib rose to 79%. When the threshold reached $150,000, the probability of crizotinib economy fell to 0%, ceritinib was 17%, and alectinib rose to 83%.
Discussion
To the best of our knowledge, this study was the first cost-effectiveness analysis of crizotinib, ceritinib, and alectinib for ALK-positive patients with advanced NSCLC in China. A previously published study compared only crizotinib and alectinib and showed that while alectinib can prolong the average time of mPFS, it was not cost-effective in China.21 This finding was consistent with the results of our study. The clinical data of Guan et al21 for transition probabilities were obtained from the ALEX trial. Guan et al21 hypothesized that patients in the crizotinib arm could receive second-line alectinib (50%) or ceritinib (50%), this hypothesis was inconsistent with the ALEX trial and may result in the deviation of the transition probability. Several economic evaluations have been published in the US.22,23,24 From the perspective of a US payer, alectinib may be more cost-effective than crizotinib.22 From the perspective of a US third-party payer, ceritinib was more cost-effective than crizotinib and chemotherapy.24 Given that our study differed from those conducted in other countries in terms of cost data source and threshold, the results differed. Although the cost of chemotherapy was one of the most sensitive factors, sensitivity analysis showed that our results were stable.
This study suffered from the following limitations. First, the clinical trial data used in the model were not solely from the Chinese population. A randomized controlled phase III clinical ALESIA trial for Asian patients was conducted,25 and the results of this RCT were highly consistent with the Japanese J-ALEX26 and the global ALEX.8 However, the data of OS curve of alectinib have not yet been released. Second, The ALEX8 and ASCEND-49 trials were independent study and had heterogeneity. A head-to-head RCT of ceritinib versus two other drugs has yet to be conducted. The ASCEND-4 trial9 used in the study was a comparison of first-line treatment with ceritinib versus standard chemotherapy, which increased the uncertainty of the results. The inclusion and exclusion criteria in ALEX and ASCEND-4 were consistent. The demographic and clinical characteristics of patients from the two RCTs were also similar. Sensitivity analysis showed that our results and conclusions were robust even though transition probability values varied widely. Third, in the ALEX trial,8 the OS curve for alectinib was immature and incomplete, and the data in the study were extrapolated using software simulations of the currently published OS curves. Therefore, when the ALEX test becomes fully mature, the results of this study must be retrospectively verified. Fourth, this study assumed that the ceritinib dose was 450 mg once a day, which was not consistent with the dose of 750 mg once daily in the ASEND-4 trial.9 The ASCEND-8 study27 explored the dose and mode of administration of ceritinib. When the dose of ceritinib was adjusted to 450 mg once daily, the effect was similar to the dose of ceritinib 750 mg once daily. The adverse reactions were also significantly improved. Therefore, the amount assumed in this study was the recommended dose of 450 mg once daily in the reference drug label. Fifth, according to the national conditions of China, the study assumed that 50% of the patients were treated with alectinib second-line therapy, and the rest were treated with chemotherapy after patients became resistant to crizotinib in the first line. This hypothesis may differ from the actual situation. In the ALEX trial,8 crizotinib was given second-line treatment in accordance with the actual situation in each country. In countries where alectinib has been marketed, alectinib is used for second-line treatment, whereas unlisted countries directly enter second-line chemotherapy. Thus, the actual proportion of second-line treatment after crizotinib resistance was difficult to obtain. The proportion of targeted drug therapy after the progress of crizotinib was between 0% and 100% in sensitivity analysis to reduce uncertainty caused by the hypothesis. The one-way sensitivity analysis showed that the effect of the ratio on the results was small. Moreover, the utility value of NSCLC in the Chinese population has not been explored, and the utility of PFS and PS status was obtained from previous literature. Despite the above-mentioned limitations, the results of sensitivity analysis showed that the proposed model was stable.
Conclusion
From the perspective of Chinese medical care, crizotinib is the most suitable first-line-targeted drug treatment option for patients with ALK-positive advanced NSCLC. For patients who are newly diagnosed with advanced NSCLC and prefer the administration of ceritinib or alectinib, ceritinib is more cost-effective than alectinib.
Acknowledgments
This study was supported by Fujian Provincial Department of Science & Technology (grant no. 2018Y9037 and grant no. 2019R0054) of the People’s Republic of China and National Natural Science Foundation of China (grant no. 71804025). The funders have no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132.
2. Liu X, Cho WC. Precision medicine in immune checkpoint blockade therapy for non-small cell lung cancer. Clin Transl Med. 2017;6(1):7.
3. Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–1203.
4. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566.
5. Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177.
6. National Comprehensive Cancer Network. Non-small cell lung cancer. Version 5. June 7, 2019. NCCN Clinical Practice Guidelines in Oncology: (NCCN Guidelines); 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
7. Zhang Y, Peng F. Research advances of crizotinib in treatment of advanced non-small cell lung cancer. Canc Res Prev Treat. 2017;44(11):774–778.
8. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–838.
9. Soria JC, Tan D, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389(10072):917–929.
10. Lu S, Yu Y, Fu S, Ren H. Cost-effectiveness of ALK testing and first-line crizotinib therapy for non-small-cell lung cancer in China. PLoS One. 2018;13(10):e0205827.
11. Lu S, Zhang J, Ye M, Wang B, Wu B. Economic analysis of ALK testing and crizotinib therapy for advanced non-small-cell lung cancer. Pharmacogenomics. 2016;17(9):985–994.
12. Chen Y, Wu Y, Lu S, et al. Chinese society of clinical oncology. Guidelines of Chinese society of clinical oncology(CSCO) lung cancer. Pmph. 2018;1:15.
13. National Bureau of Statistics. Statistical Bulletin of National Economic and Social Development in 2018. National Bureau of Statistics, February 28, 2019. Available from: http://www.stats.gov.cn/tjsj/zxfb/201902/t20190228_1651265.html.
14. Wu B, Zhang Q, Sun J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J Immunother Cancer. 2018;6(1):124.
15. Wan X, Zhang Y, Tan C, Zeng X, Peng L. First-line nivolumab plus ipilimumab vs sunitinib for metastatic renal cell carcinoma: a cost-effectiveness analysis. JAMA Oncol. 2019;5(4):491–496.
16. Cai H, Zhang L, Li N, et al. Cost-effectiveness of osimertinib as first-line treatment and sequential therapy for EGFR mutation-positive non-small cell lung cancer in China. Clin Ther. 2019;41(2):280–290.
17. Wu B, Gu X, Zhang Q, Xie F. Cost-effectiveness of osimertinib in treating newly diagnosed, advanced EGFR-mutation-positive non-small cell lung cancer. Oncologist. 2019;24(3):349–357.
18. Cancer Foundation of China. Introduction to the alectinib patient assistance project of china cancer foundation. Cancer Foundation of China., 2018. Available from: http://www.cfchina.org.cn/show.php?contentid=1663.
19. Bertranou E, Bodnar C, Dansk V, Greystoke A, Large S, Dyer M. Cost-effectiveness of osimertinib in the UK for advanced EGFR-T790M non-small cell lung cancer. J Med Econ. 2018;21(2):113–121.
20. World Health Organization. Choosing interventions that are cost effective(WHO-CHOICE). Cost Effectiveness Thresholds. Geneva: World Health Organization. 2011. Available from: http://www.who.int/choice/en/
21. Guan H, Sheng Y, Guo W, Han S, Shi L. Cost-effectiveness of alectinib for patients with untreated ALK-positive non-small cell lung cancer in China. Adv Ther. 2019;36(5):1114–1125.
22. Carlson JJ, Suh K, Orfanos P, Wong W. Cost effectiveness of alectinib vs. crizotinib in first-line anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer. Pharmacoeconomics. 2018;36(4):495–504.
23. Claxton L, O’Connor J, Woolacott N, Wright K, Hodgson R. Ceritinib for untreated anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer: an evidence review group evaluation of a NICE single technology appraisal. Pharmacoeconomics. 2019;37(5):645–654.
24. Zhou ZY, Mutebi A, Han S, et al. Cost-effectiveness of ceritinib in previously untreated anaplastic lymphoma kinase-positive metastatic non-small cell lung cancer in the United States. J Med Econ. 2018;21(6):577–586.
25. Zhou C, Kim SW, Reungwetwattana T, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019;7(5):437–446.
26. Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29–39.
27. Cho BC, Kim DW, Bearz A, et al. ASCEND-8: a randomized Phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with Anaplastic Lymphoma Kinase (ALK)-Rearranged Metastatic Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol. 2017;12(9):1357–1367.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.