Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
Cost-effectiveness analysis of baricitinib versus adalimumab for the treatment of moderate-to-severe rheumatoid arthritis in Spain
Authors Schlueter M, Finn E, Díaz S, Dilla T, Inciarte-Mundo J , Fakhouri W
Received 16 January 2019
Accepted for publication 9 April 2019
Published 6 June 2019 Volume 2019:11 Pages 395—403
DOI https://doi.org/10.2147/CEOR.S201621
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Max Schlueter,1 Elaine Finn,1 Silvia Díaz,2 Tatiana Dilla,2 José Inciarte-Mundo,3 Walid Fakhouri4
1IQVIA, Real World Evidence, London N1 9JY, UK; 2Lilly Spain, Health Outcomes & RWE, Alcobendas 28108, Spain; 3Lilly Spain, Medical, Alcobendas 28108, Spain; 4Lilly, Global Patient Reported Outcomes and Real World Evidence (GPORWE) International, Windlesham, Surrey GU20 6PH, UK
Background: Baricitinib is an oral janus kinase inhibitor for the treatment of rheumatoid arthritis (RA) and is approved in Europe for use in adults with moderately-to-severely active RA and an inadequate response or intolerance to conventional synthetic disease-modifying antirheumatic drug (csDMARD) therapy. To date, no economic evaluations have assessed the cost-effectiveness of baricitinib in the Spanish setting.
Objectives: To evaluate the cost-effectiveness of baricitinib versus adalimumab for the treatment of moderately-to-severely active RA in the Spanish setting.
Methods: A discrete event simulation model was developed in Microsoft Excel. Costs and outcomes were estimated over a lifetime horizon using the Spanish national payer perspective. The model compared baricitinib 4 mg once daily in combination with methotrexate with adalimumab 40 mg every other week in combination with methotrexate. Effectiveness and physical function were captured using the American College of Rheumatology criteria and the Health Assessment Questionnaire–Disability Index, input values of which were derived from a phase 3, double-blind, placebo- and active-controlled trial (RA-BEAM; funded by Eli Lilly and Incyte; ClinicalTrials.gov number, NCT01710358). Costs are presented in Euros, 2018 values.
Results: In the base case analysis, baricitinib was associated with a quality-adjusted life year gain of 0.09 years over a lifetime horizon, at an incremental cost of –€558 versus adalimumab. Results of various scenario analyses and probabilistic sensitivity analysis generally were consistent with the base case analysis.
Conclusion: This analysis suggests that baricitinib is a cost-effective treatment option compared to adalimumab for Spanish patients with moderately-to-severely active RA and a previous inadequate response or intolerance to csDMARD therapy.
Keywords: baricitinib, adalimumab, cost-effectiveness, JAK inhibitor, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is one of the most common autoimmune diseases, with a prevalence of 0.5% in Spain,1 which is similar to the worldwide prevalence of 0.5–1.0%.2 This chronic, progressive and disabling systemic autoimmune disease is caused by an interaction of genetic and environmental factors resulting in an increased activity of the pro-inflammatory pathways and auto-antibodies targeting the synovium, cartilage, and bone, leading to joint damage and loss of function. Though RA affects people at all ages, its likelihood of onset increases with age, with the highest onset seen among adults in their sixties.3,4 Substantial comorbidity can be seen outside of the musculoskeletal system, with excess cardiovascular risk, dyslipidemia, and infection.
New therapeutic strategies, including early therapy, treat-to-target approaches, and biological therapies, have led to substantial improvements in the prognosis of RA patients. The current therapeutic target includes remission or, at the very least, low disease activity, with rapid adaptation of treatment if this target is not reached. Treatment recommendations focus on early diagnosis, followed by early initiation of therapy with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and glucocorticoids. If the therapeutic target is not achieved, a biologic DMARD (bDMARD) is typically added to the regimen, most often a tumor necrosis factor inhibitor (TNFi). If this regimen also fails to adequately control disease activity, a switch to another TNFi or to a bDMARD with a different mechanism of action is usually considered.
RA imposes a substantial health care and economic burden in direct and indirect costs. A recent socioeconomic survey undertaken in 10 European countries – including Spain – found the average annual expenditure to be €3,142 with no therapy or non-steroidal anti-inflammatory drugs (NSAIDs), €4,111 with csDMARDs, and €4,842 with csDMARDs and bDMARDs.5 A 2017 literature review on the burden of RA in Spain found that the annual cost per patient varied across different studies (€3,600 to €11,707 in 2002) and that direct costs account for 70–75% of the total annual cost for treatment of RA. The authors also indicated that most studies were carried out several years ago and that further research was warranted to assess the current situation in Spain.1
Since complete or sustained disease remission is unusual, there remains a substantial unmet need for effective and better-tolerated treatments for RA. Recently, baricitinib has been introduced, an orally administered, selective and reversible Janus kinase (JAK) inhibitor6 that belongs to the new drug class of targeted synthetic DMARDs (tsDMARDs). It is rapidly absorbed, has a half-life of 12.5 h and is dosed once daily. Baricitinib can be given as monotherapy or in combination with methotrexate, with a recommended dosing of 4 mg daily.
To date, there is a lack of health economic analyses comparing baricitinib with the current standard of care in patients with RA in Spain. The objective of this cost-effectiveness analysis (CEA) was to assess the health economic value of baricitinib in comparison with adalimumab, one of the most commonly used first-line biologic therapies in Spain to treat RA,7 for the treatment of moderately-to-severely active RA in patients with prior inadequate response to csDMARD therapy.
Methods
Model structure
An economic model was developed in Microsoft Excel with Visual Basic for Applications (VBA) to capture long-term costs and outcomes. Based on a systematic literature review (SLR) of published economic models in RA8 and their critical appraisal, a discrete event simulation (DES) approach was adopted for the model development. The DES approach9 has the benefit of adopting a continuous time approach which allows more realistic modeling of the patient treatment pathway, and better reflecting patient heterogeneity by simulating individual patients rather than patient cohorts. More recently, a number of individual patient simulation models using a DES approach have been used for health economic evaluations in RA.10
Treatment efficacy estimates were defined based on the American College of Rheumatology (ACR) criteria, a validated categorical variable widely used in RA. Based on efficacy data from RA-BEAM, patients in either treatment arm were categorized into the appropriate, mutually exclusive ACR response category (ACR<20/≥20 to <50/≥50 to <70/≥70). If a patient did not achieve at least ACR20 at Week 24, the treatment was terminated in the model, and the patient was assumed to move to palliative care (defined as a mix of leflunomide and cyclosporine) for the remainder of the model time horizon.
Physical function was captured by the Health Assessment Questionnaire–Disability Index (HAQ-DI, from now on referred to as HAQ). A patient’s initial change in HAQ score was calculated for the initial assessment period based on ACR response category. Long-term HAQ change based on the calculated HAQ trajectory was then applied for the period beyond the initial time of the primary endpoint assessment to the point at which either discontinuation of treatment or death occurred. The HAQ trajectory was assumed to be flat for both therapies, but to deteriorate for patients on palliative care.
Long-term treatment discontinuation was modeled with a parametric Weibull model following Kaplan–Meier data of a Spanish RA registry (BIOBADASER) analysis on the continuation of first-line biologic use in RA.11 This was estimated irrespective of ACR response level due to lack of published data; stratification of long-term discontinuation by treatment was deemed inappropriate due to the risk of confounding bias. Treatment discontinuation rates, therefore, do not differ between the treatments compared. A HAQ rebound effect was applied upon treatment discontinuation, assuming that the treatment effect is lost whenever active treatment is terminated. Mortality was estimated by applying hazard ratios (HR) stratified by baseline HAQ score bands to Spanish life tables. It was assumed that start and end effects could be modeled as one-off deductions, proportional to the change in the quality of life score.
Relevant costs and health benefits were calculated based on treatment-related costs and the HAQ score over the modeled time horizon. HAQ was subsequently mapped to generic EQ-5D in order to estimate quality-adjusted life years (QALYs). The model schematic in Figure 1 depicts the flow of each patient through the model simulation.
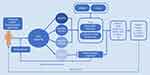 | Figure 1 Schematic representation of the model structure.Abbreviations: ACR, American College of Rheumatology; HAQ, Health Assessment Questionnaire. |
Assessment of uncertainty
Probabilistic sensitivity analysis (PSA) was conducted to test the impact of second-order uncertainty – also referred to as parameter uncertainty – by random, simultaneous variation of the input parameters on the model. The model used predefined ranges around the mean values either based on standard errors sourced from primary sources or by applying a default 10% variation to generate random inputs that follow appropriate sampling distributions. PSA then repeatedly sampled values from these distributions, propagating uncertainty, to estimate the cost–effectiveness ratio. The PSA included 1,000 iterations of 1,000 simulated patients.
In addition, the model explored additional scenario analyses by changing key parameters of the model, including the mapping algorithm used to generate EQ-5D values from HAQ scores, the HAQ trajectory while on treatment, the discount rate for costs and effects, the model time horizon, and the inclusion of serious adverse events (SAE). In addition, a sensitivity analysis was run with hypothetical discount scenarios for baricitinib and adalimumab to account for the fact that list prices in Spain may differ from net prices due to confidential discounts agreed with the pharmaceutical companies.
Model inputs
The model inputs and assumptions selected for the base case were chosen to best represent clinical rationale and best modeling practices. The model was constructed from a Spanish payer perspective with a lifetime horizon and discount rates for costs and benefits set to 3%, according to Spanish guidelines on the economic evaluation of health technologies.12
Patient population
Patient input parameters on age, gender, and baseline HAQ score were taken from the RA-BEAM trial,6 a 52-week, phase 3, double-blind, placebo- and active-controlled trial in RA patients with prior inadequate response to csDMARD therapy, who were randomly assigned to placebo, 4 mg of baricitinib once daily, or 40 mg of adalimumab administered subcutaneously every other week, all with methotrexate background therapy. The exact distribution of the patient-level data from the RA-BEAM trial was used in the model to replicate patient baseline heterogeneity.
Efficacy, safety and mortality
As described above, treatment efficacy estimates were derived from the proportion of patients achieving a mutually exclusive ACR<20/20/50/70 response rate at Week 24 (ie, 6 months) in the RA-BEAM trial;6 see Table 1. In line with previous economic analyses in RA, adverse events were assumed not to be a key driver of the model. This was further validated by clinical specialists and health economists in a consultation workshop conducted prior to model development. To model mortality, hazard ratios reported in a previous RA model10 were applied to the Spanish life tables, sourced from the World Health Organisation.13 Hazard ratios for mortality were assumed to be a function of baseline HAQ only.14
 | Table 1 ACR response rates at week 24 (RA-BEAM, modified intent-to-treat population) |
Quality of life
Health-related quality of life (HRQOL) and specific physical function were captured by HAQ. The HAQ score distribution at baseline was based on the RA-BEAM modified intention-to-treat study population. Initial HAQ change at Week 24 was determined by the ACR response observed at the primary assessment time point. A mean (SE) reduction in HAQ score of 0.80 (0.02) for ACR20 responders, 0.95 (0.02) for ACR50 responders and 1.07 (0.03) for ACR70 responders was applied in the model based on pooled data derived from the baricitinib trials in csDMARD-inadequate responder (csDMARD-IR) populations, RA-BEAM and RA-BUILD.15 A scenario analysis explored the impact of using alternative values from the published literature.16
The long-term HAQ trajectory was assumed to be flat for both active therapies whilst on treatment. This is consistent with previous economic analyses in RA, and supported by data from the RA-BEYOND trial, a long-term extension study.17 In patients who continued treatment with baricitinib 4 mg (N=303) after end of follow-up in the RA-BEAM study, mean (SD) baseline HAQ in RA-BEYOND was 0.73 (0.65), compared to 0.75 (0.68) in patients who were switched from adalimumab 40 mg to baricitinib 4 mg (N=186) at the start of RA-BEYOND. No statistically significant changes in HAQ change from baseline were observed at Week 24 (ie, Week 76 overall) for any of the treatment groups (using mixed models for repeated measures, MMRM).17 HAQ trajectory for patients on palliative care was assumed to deteriorate, replicating the latent class approach derived from a growth mixture model for the base case analysis.18 Scenario analysis explored the impact of an assumption of linear HAQ progression (mean rate of HAQ increase of 0.06/year) as derived from the published literature.19
To generate QALY estimates, HAQ-DI was mapped to EQ-5D using a number of different mapping algorithms. In the base case, an algorithm based on the individual patient data for HAQ-DI and “crosswalked” EQ-5D-5L from the RA-BEAM trial was used, employing a fixed effects regression model. Scenario analyses explored the impact of using other published mapping algorithms frequently used for economic evaluations in RA.19,20
Resource use and unit costs
The model considered direct medical costs relevant to the Spanish health-care payer. Unit costs were taken from national databases and cost sources.21 Drug acquisition costs of baricitinib 4 mg (28-tab pack price: €870.24), and adalimumab 40 mg (2-pen pack price: €951.17) were calculated based on ex-factory prices and a 7.5% discount,22,23 resulting in annual therapy costs of €11,344.20 for baricitinib and €12,365.19 for adalimumab. It was assumed that subcutaneous administration of adalimumab would incur no costs to the payer. Annual costs of palliative care were calculated as €1,791.38. Monitoring resources and frequencies were derived from a Spanish expert panel and assumed to be identical for both adalimumab and baricitinib. Unit costs of monitoring were derived from national sources.21 Hospitalization costs were derived by applying the mean number of hospital days per HAQ band10 to the Spanish inpatient day cost.21 All costs are expressed in 2018 Euros; wherever costs were not available for the most recent year, values were inflated to the most recent year using the inflation rate index provided by the Spanish National Statistics Institute.24 All inputs, sources, and assumptions were validated by an expert panel composed of three Spanish rheumatologists.
Results
Base case analysis
In the base case analysis simulating 50,000 patients, baricitinib was associated with a QALY gain of 0.09 years over a lifetime time horizon, at an incremental cost of –€558 versus adalimumab (Table 2). Baricitinib was therefore considered the cost-effective option, being more effective and less costly than adalimumab (ie, dominant). The breakdown of costs found cost savings with baricitinib to be driven by lower drug acquisition costs and lower hospitalization costs.
 | Table 2 Results of deterministic, probabilistic and sensitivity analyses |
Probabilistic sensitivity analysis
Results of the PSA were consistent with those from the deterministic analysis. The cost–effectiveness scatter plot in Figure 2 illustrates the incremental cost versus the incremental effectiveness for each of the 1,000 simulations. The plot illustrates the uncertainty surrounding the mean estimates of incremental costs and effectiveness, with results showing that the majority of cost–effectiveness pairs were located in the southeastern quadrant, denoting dominance of baricitinib. The cost-effectiveness acceptability curve (CEAC) suggested that baricitinib has a 100% likelihood of being cost-effective compared to adalimumab at all willingness-to-pay (WTP) thresholds greater than €5,000 (Figure 2).
 | Figure 2 Cost–effectiveness plane and cost–effectiveness acceptability curve.Abbreviation: QALY, quality-adjusted life year. |
Scenario analyses
The results from a range of scenario analyses (Table 2) simulating 50,000 patients suggested that the base case is robust to changes in key assumptions and parameters of the model. With the exception of one price discount scenario, all scenario analyses found baricitinib to remain the cost-effective treatment option below a WTP threshold of €30,000/QALY.25
Discussion
To date, no published studies have evaluated the cost-effectiveness of baricitinib for the treatment of moderately-to-severely active RA in patients with prior inadequate response to csDMARDs in Spain. To close this evidence gap, this study developed a cost-effectiveness model considering best modeling practices in RA, adopting a discrete event simulation approach which models individual subjects to account for patient heterogeneity. Efficacy, safety, and quality of life data underpinning the analysis were derived from a Phase 3 randomized controlled trial directly comparing baricitinib and adalimumab, one of the most commonly used first-line bDMARDs in Spain for treatment of moderately-to-severely active RA.
Results of the base case analysis suggest that baricitinib 4 mg is the cost-effective option when compared with adalimumab 40 mg in a csDMARD-IR population. The deterministic analysis found that baricitinib dominated adalimumab and was associated with a modest QALY gain of 0.09 years over a lifetime time horizon, at an incremental cost of –€558 versus adalimumab. Cost savings with baricitinib were largely attributable to lower drug acquisition costs.
Probabilistic analysis confirmed deterministic results while allowing for full, simultaneous parameter variation, providing similar estimates of QALY gains and costs. The CEAC showed baricitinib to be the cost-effective option versus adalimumab at all WTP thresholds greater than €5,000 per QALY gained. In addition, a range of scenario analyses confirmed the base case results, demonstrating that baricitinib is more cost-effective than adalimumab in the csDMARD-IR population in Spain. This included a number of hypothetical discount scenarios to account for potential differences between published list prices and confidential net prices as well as future market entry of biosimilars.25,26
The analysis has a number of limitations. Long-term treatment discontinuation was not stratified by ACR response category due to a lack of published data from the Spanish setting. Following discontinuation of active therapy, patients were assumed to be switched to palliative care for the remainder of the model time horizon. This was deemed justifiable for assessing the cost-effectiveness of baricitinib versus adalimumab. However, in real life, patients are expected to be switched to another TNFi therapy or a bDMARD with a different mechanism of action. In order to calculate generic QALYs, the disease-specific quality of life (QOL) measure, HAQ, was mapped to the EQ-5D measure; a series of mapping algorithms were included in the model in order to test their impact on CEA results. Alternative mapping algorithms were found not to have a notable impact on the incremental cost–effectiveness ratio (ICER). Finally, the generalizability of the RCT population in RA-BEAM to the real world could not be established due to lack of detail regarding patient characteristics in publicly available reporting on the Spanish RA population.11 While patient characteristics in multi-center RCTs may be different from those observed in a local setting in the real world, it is unlikely that use of different patient characteristics in the economic analysis would impact incremental cost-effectiveness results.
In conclusion, results of this economic evaluation suggest that baricitinib, a novel oral JAK1/JAK2 inhibitor, may be a cost-effective treatment option compared to adalimumab 40 mg in patients with moderately-to-severely active RA in Spain who have had prior inadequate response or intolerance to csDMARD therapy. The results from a series of scenario analyses and probabilistic sensitivity analysis suggest that the base case analysis was robust to reasonable parameter variation in the model.
Contributions to scientific literature
This paper represents an original research proposition, being the first publication to synthesize evidence and investigate the use of baricitinib as part of the treatment pathway for moderate-to-severe rheumatoid arthritis patients within Spain.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The project was conducted by IQVIA, and Eli Lilly and Company and funded by Eli Lilly and Company.
Disclosure
Walid Fakhouri, José Inciarte-Mundo, Silvia Diaz and Tatiana Dilla are employees of Eli Lilly and hold Eli Lilly shares. Max Schlueter and Elaine Finn are full-time employees of IQVIA and acted as consultants for this study funded by Eli Lilly and Company. Dr Jose Inciarte reports personal fees from Eli Lilly and Company, during the conduct of the study. The authors report no other conflicts of interset in this work.
References
1. Andrade P, Sacristán JA, Rentero ML, Hammen V, Dilla T. The burden of rheumatoid arthritis in Spain. Health Econ Outcome Res Open Access. 2017;3(126):2. doi:10.4172/2471-268X.1000126
2. Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care. 2012;18(13 Suppl):S295–S302.
3. Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002;4(Suppl 3):S265–S272. doi:10.1186/ar578
4.
5.
6. Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus Placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–662. doi:10.1056/NEJMoa1608345
7. Gomez Reino J, Loza E, Andreu JL, et al. [Consensus statement of the Spanish society of rheumatology on risk management of biologic therapy in rheumatic patients]. Reumatol Clin. 2011;7(5):284–298. doi:10.1016/j.reuma.2011.05.002
8.
9. Karnon J, Stahl J, Brennan A, Caro JJ, Mar J, Moller J. Modeling using discrete event simulation: a report of the ISPOR-SMDM modeling good research practices task force-4. Value Health. 2012;15:821–827. doi:10.1016/j.jval.2012.04.013
10. Stevenson M, Archer R, Tosh J, Simpson E, Everson-Hock E, Stevens J, et al. Adalimumab, etanercept, infliximab,certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess 2016;20(35)
11.
12. López Bastida J, Oliva J, Antoñanzas F, et al. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias. Gac Sanit. 2010;24:154–170. doi:10.1016/j.gaceta.2009.07.011
13.
14. Michaud K, Vera-Llonch M, Oster G. Mortality risk by functional status and health-related quality of life in patients with rheumatoid arthritis. J Rheumatol. 2012;39(1):54–59. doi:10.3899/jrheum.110491
15. Dougados M, van der Heijde D, Chen Y, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis. Epub ahead of print, 2 Nov 2016.
16. Carlson J, Ogale S, Dejonckheere F, Sullivan SD. Economic evaluation of tocilizumab monotherapy compared to adalimumab monotherapy in the treatment of severe active rheumatoid arthritis. Value in Health. 2015;18(2):173–179. doi:10.1016/j.jval.2014.10.013.
17.
18. Norton S, Sacker A, Dixey J, Done J, Williams P, Young A. Trajectories of functional limitation in early rheumatoid arthritis and their association with mortality. Rheumatology (Oxford). 2013;52(11):2016–2024. doi:10.1093/rheumatology/ket253
19. Malottki K, Barton P, Tsourapas A, et al. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: a systematic review and economic evaluation. [Review]. Health Technol Assess. 2011;15(14):1–278. doi:10.3310/hta15140
20. Hernandez AM, Wailoo AJ, Ara R. Tails from the peak district: adjusted limited dependent variable mixture models of EQ-5D questionnaire health state utility values. Value Health. 2012;15(3):550–561. doi:10.1016/j.jval.2011.12.014
21.
22. Base de Datos de medicamentos del Consejo General de Farmacéuticos (Bot PLUS 2.0). Available from:
23. Real Decreto-ley 8/2010, de 20 de Mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público. Boletín Oficial Del Estado.2010;126:45070. Available from:
24.
25. Sacristán JA, Oliva J, Del Llano J, Prieto L, Pinto JL. ¿Qué es una tecnología sanitaria eficiente en España? Gac Sanit. 2002;16:334–343.
26. González A, Ivanova Y, Zozaya N, Jiménez M, Hidalgo Á, eds. La introducción de los biosimilares en España. Estimación del ahorro para el Sistema Nacional de Salud. 1st ed. Madrid: Fundación Weber; 2017.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
