Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Cost-Effectiveness Analysis of a Once-Daily Single-Inhaler Triple Therapy for Patients with Chronic Obstructive Pulmonary Disease (COPD) Using the FULFIL Trial: A Spanish Perspective
Authors Schroeder M , Benjamin N, Atienza L, Biswas C , Martin A , Whalen JD , Izquierdo Alonso JL , Riesco Miranda JA , Soler-Cataluña JJ , Huerta A , Ismaila AS
Received 30 November 2019
Accepted for publication 24 May 2020
Published 10 July 2020 Volume 2020:15 Pages 1621—1632
DOI https://doi.org/10.2147/COPD.S240556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Melanie Schroeder,1 Nicole Benjamin,2 Laura Atienza,3 Chandroday Biswas,4 Alan Martin,5 John D Whalen,6 José Luis Izquierdo Alonso,7 Juan Antonio Riesco Miranda,8 Juan José Soler-Cataluña,9 Alicia Huerta,3 Afisi S Ismaila10,11
1Value Evidence and Outcomes, GlaxoSmithKline plc., Brentford, UK; 2Global Health Economics, ICON plc., Boston, MA, USA; 3Market Access, GlaxoSmithKline SA, Madrid, Spain; 4Global Health Economics, ICON plc., Bengaluru, Karnataka, India; 5Value Evidence and Outcomes, GlaxoSmithKline plc., Uxbridge, UK; 6Global Health Economics, ICON plc., Abingdon, UK; 7Pneumology, Hospital Universitario de Guadalajara, Guadalajara, Spain; 8Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Servicio de Neumología, Hospital San Pedro de Alcántara, Cáceres, Spain; 9Pneumology Department, Hospital Arnau de Vilanova-Lliria (Valencia), Valencia, Spain; 10Value Evidence and Outcomes, GlaxoSmithKline plc., Collegeville, PA, USA; 11Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON, Canada
Correspondence: Afisi S Ismaila
GlaxoSmithKline Plc., 1250 South Collegeville Road, Collegeville, PA 19426-0989, USA
Tel +1 (919) 315 8229
Email [email protected]
Purpose: To evaluate the cost-effectiveness of once-daily fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) vs twice-daily budesonide/formoterol (BUD/FOR) in patients with symptomatic chronic obstructive pulmonary disease (COPD) at risk of exacerbations, from the Spanish National Healthcare System perspective.
Patients and Methods: The validated GALAXY-COPD model was used to simulate disease progression and predict healthcare costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) over a 3-year time horizon for a Spanish population. Patient characteristics from published literature were supplemented by data from FULFIL (NCT02345161), which compared FF/UMEC/VI vs BUD/FOR in patients with symptomatic COPD at risk of exacerbations. Treatment effects, extrapolated to 3 years, were based on Week 24 results in the FULFIL intent-to-treat population, including change in forced expiratory volume in 1 second, St. George’s Respiratory Questionnaire score, and exacerbation rates. Treatment, exacerbations, and COPD management costs (2019€) were informed by Spanish public sources and published literature. A 3% discount rate for costs and benefits was applied. One-way sensitivity and scenario analyses, and probabilistic sensitivity analysis (PSA), were performed.
Results: FF/UMEC/VI treatment led to fewer moderate and severe exacerbations (2.126 and 0.306, respectively) vs BUD/FOR (2.608 and 0.515, respectively), with a mean incremental cost of € 69 and gain of 0.107 QALYs, which resulted in an ICER of € 642 per QALY gained. In sensitivity analyses, the ICER was most sensitive to treatment effect variations in exacerbations and healthcare resource utilization/event costs. Overall, 95% of 1000 PSA simulations resulted in an ICER less than € 11,000 per QALY gained for FF/UMEC/VI vs BUD/FOR, confirming robustness of the results. The probability of FF/UMEC/VI being cost-effective vs BUD/FOR was 100% at a willingness-to-pay threshold of € 30,000 per QALY gained.
Conclusion: At the accepted Spanish ICER threshold of € 30,000, FF/UMEC/VI represents a cost-effective treatment option vs BUD/FOR in patients with symptomatic COPD at risk of exacerbations.
Keywords: cost-utility analysis, health-related quality of life, incremental cost-effectiveness ratio, fluticasone furoate, vilanterol, umeclidinium
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide. Despite the availability of bronchodilator and anti-inflammatory therapies, COPD is expected to become the world’s third leading cause of death by 2020.1 In Spain, the prevalence of COPD was approximately 10% among adults aged 40–80 years in 2009.2
In 2011, the annual direct and indirect costs of COPD in the European Union were estimated to be €23.3 billion and €25.1 billion, respectively,3 and costs worldwide are predicted to increase over time due to continued exposure to risk factors and an aging population.4 In 2018, the average annual direct cost of COPD in Spain was estimated to be €1645 per patient, with an additional €2112 in indirect costs.5
Healthcare costs in COPD vary according to the patient’s level of symptoms,1,6,7 with the frequency and severity of exacerbations having the greatest economic impact.8,9 In 2016, the cost of COPD in Spain was €3200 per patient per year (PPPY) in individuals who experienced exacerbations, and €1403 PPPY in those without exacerbations.10 Exacerbations leading to hospitalization contributed to 41% of total COPD expenditure in Spain in 20047 and, in 2010, totaled €167.9 million.11
In addition to its financial burden, COPD places a strain on patient health and well-being, with many individuals experiencing poor health-related quality of life (HRQoL) and high levels of depression and anxiety due to their condition.12 Exacerbations of COPD are associated with increased symptom burden and poor prognosis. Due to the negative impacts that exacerbations have on both healthcare costs and HRQoL, the prevention of exacerbations is currently one of the main focuses of COPD treatment.1
Three widely used inhaled maintenance treatment options for COPD are long-acting β2-agonists (LABA), long-acting muscarinic antagonists (LAMA), and inhaled corticosteroids (ICS).1 Inhaled triple therapy combining all three of these treatment classes (ICS/LAMA/LABA) can improve lung function, symptoms, and health status, and can reduce exacerbations compared with dual therapy (LAMA/LABA or ICS/LABA) or monotherapy (LAMA).1,13–15 Given the importance of reducing exacerbations in improving treatment for patients with COPD, inhaled triple therapy is recommended by Spanish COPD guidelines for high-risk patients whose exacerbations are not controlled by dual therapy: ie, a combination of two of ICS, LAMA, or LABA.16
The Lung Function and Quality of Life Assessment in Chronic Obstructive Pulmonary Disease with Closed Triple Therapy (FULFIL) trial (NCT02345161) was a randomized, multicenter, Phase III, 24-week, double-blind, parallel-group trial comparing once-daily single-inhaler triple therapy (SITT) with fluticasone furoate/umeclidinium/vilanterol 100/62.5/25 μg (FF/UMEC/VI, via the ELLIPTA inhaler) with twice-daily dual therapy with budesonide/formoterol 400/12 μg (BUD/FOR, via the Turbuhaler inhaler) in patients with symptomatic COPD at risk of exacerbations.13 To minimize the impact of different dosing regimens, patients randomized to the FF/UMEC/VI ELLIPTA study arm also received placebo twice daily, delivered via the Turbuhaler inhaler, while patients randomized to the BUD/FOR Turbuhaler study arm also received placebo once daily, delivered via the ELLIPTA inhaler.13 Patients taking FF/UMEC/VI had significant improvements in lung function and significant reductions in exacerbation risk at 24 and 52 weeks compared with those taking BUD/FOR.13
To help inform decisions on the choice of COPD treatments for patient prescriptions, cost-utility analyses are commonly used.17 Cost-utility analyses help decision makers understand how the clinical benefit observed in randomized trials could improve patient HRQoL, and whether any benefits justify additional costs, by providing clinicians with a more complete analysis of total benefits (eg, changes in HRQoL and health outcomes) rather than focusing on costs alone. The present analysis aimed to evaluate the cost-effectiveness of FF/UMEC/VI vs BUD/FOR from the Spanish National Healthcare System (NHS) perspective, and to investigate the potential economic and health benefits to the Spanish NHS from introducing FF/UMEC/VI SITT to Spanish patients with symptomatic COPD at risk of exacerbations.
Methods
Study Design
A cost-effectiveness analysis of FF/UMEC/VI vs BUD/FOR was performed using the GALAXY-COPD disease model. GALAXY was originally conceived as a generalized disease progression model of COPD,18,19 and consists of a set of linked risk equations developed using data from the large patient cohort of the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study.20 The equations predict status, at each model cycle, for key aspects of COPD: lung function measured by percentage predicted forced expiratory volume in 1 second (FEV1), exacerbation frequency, dyspnea measured as modified Medical Research Council (mMRC) dyspnea score, cough/sputum, and exercise capacity measured by 6-minute walk distance (6MWD), as well as the final outcome of HRQoL measured by the St. George’s Respiratory Questionnaire (SGRQ) score. Covariates for baseline values of various patient characteristics (such as: age, sex, prior exacerbations, fibrinogen concentration, cardiovascular disease comorbidity, “other” comorbidity, 6MWD score, mMRC dyspnea score, and SGRQ total score) are included in the first model cycle.18,19 The equations are then linked by including covariates for values predicted by the other equations from the previous model cycle (moderate exacerbations, severe exacerbations, FEV1, FEV1% predicted, dyspnea, cough and/or sputum, 6MWD, and SGRQ total score).18,19 (Figure 1) Using modifiers that reflect treatment effects (increased lung function, reduction in exacerbation risk, and improved SGRQ score with FF/UMEC/VI vs BUD/FOR), the model predicts disease progression, patient survival, and HRQoL, measured as quality-adjusted life years (QALYs) for each comparator treatment. The GALAXY model has been validated and is a well-established, published tool for the assessment of the cost-effectiveness of COPD treatments.18,19,21–26
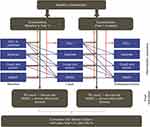 |
Figure 1 Linked-risk equation model. Blue lines indicate the relationship between central attributes in different time periods and orange lines indicate the relationship between intermediate outcomes and exacerbations. Black lines indicate the relationship between the central attributes and the final health outcomes. Notes: *Calculated (in mL) using the risk equation at 1 year and converted to FEV1% predicted based on the cohort profile. Adapted with permission from Briggs AH, Baker T, Risebrough NA, et al, Med Decis Making, 37(4) 469–480. Copyright © 2017, Sage Publishing.19Abbreviations: 6MWD, 6-minute walk distance; FEV1, forced expiratory volume in 1 second; LY, life year; QALY, quality-adjusted life year; RU, resource utilization; SGRQ, St. George’s Respiratory Questionnaire. |
Model Inputs
Spanish Population Characteristics
The baseline “base-case” population characteristics used in the analysis as model input values reflected those from three previous studies of patients with COPD in Spain,27–29 representing a total of 8788 individuals (Table 1). Where a required model parameter was not reported in any of the Spanish studies, values were taken from the FULFIL intent-to-treat (ITT) population (Table 1) or were estimated (mMRC dyspnea score, 6MWD, fibrinogen concentration). Specifically, for mMRC dyspnea score, it was assumed that the proportion of patients with a baseline mMRC dyspnea score ≥2 was equal to the proportion of patients in FULFIL at baseline responding with “2” (breathless during light activity) or “3” (breathless when washing or dressing) to the question, “Describe how breathless you were today” of the EXAcerbations of Chronic pulmonary disease Tool Patient-Reported Outcome (EXACT-PRO) questionnaire.31 Baseline 6MWD scores were estimated using the GALAXY model risk equation, and fibrinogen values were estimated using an additional risk equation previously developed using baseline data from the ECLIPSE study.20 These estimations were validated by a panel of clinical experts.
 |
Table 1 Model Input Parameters, Representing the Spanish Population and FULFIL ITT Population |
Treatment Effects
The model considers treatment effects on FEV1, moderate exacerbations, severe exacerbations, and SGRQ score. Base-case values were sourced from the FULFIL ITT population,13 and are shown in Table 2.
 |
Table 2 Base-Case Treatment Effectsa |
The GALAXY model uses a linked-equation approach, meaning that treatment effects applied to FEV1 will also impact predicted exacerbation rates and, likewise, effects on either exacerbation rates or SGRQ scores will affect predictions for FEV1. To ensure that the model predictions of FEV1, exacerbation rates, and SGRQ aligned with the observed effects in FULFIL, the magnitudes of each treatment effect entered in the model were adjusted until the model-predicted clinical outcomes for the first year matched the observed trial data from the extension population of the FULFIL trial, for whom data were collected for up to 52 weeks.
The model assumed that treatment effects were immediate and thus applied from the initiation of treatment. Since the relative benefits of FF/UMEC/VI over BUD/FOR remained constant between 24 and 52 weeks in FULFIL,13 the base-case analysis assumed treatment effect to remain consistent while patients remained on therapy.
Treatment switching or discontinuation can affect costs and treatment effects, and therefore impact the modeled cost-effectiveness estimates. Rates of treatment discontinuation were assumed to be the same as in the FULFIL ITT population (8% per year with FF/UMEC/VI, 13% per year with BUD/FOR) (FULFIL Clinical Study Report [CSR], GlaxoSmithKline plc. 2017).32 Following discontinuation, all patients were assumed to receive subsequent therapy, with the proportion of patients assigned to each treatment class based on the distribution observed in the EPOCONSUL study28 (Table 3). All assumptions were validated by a panel of clinical experts.
 |
Table 3 Treatment Class Costs for Subsequent Treatment |
Utilities
In the base-case, the model estimated utilities with a risk equation developed using data from an observational study in Spain,26 which factors in the proportion of a patient’s week when they experienced dyspnea symptoms. A calibration factor was applied to ensure that predicted utility at baseline was consistent with previous SGRQ-based algorithms.19
Costs
Drug acquisition costs included on-treatment and post-discontinuation maintenance therapy, as well as rescue medication. Drug costs were obtained from those published by the Spanish Ministry of Health, Consumption and Social Welfare in March 2019 and are expressed as Price to Public plus Value Added Tax (Table 4). The model assumes patients discontinue treatment at a constant rate; therefore, for patients discontinuing treatment, drug costs were calculated as the sum of 6 months each of study treatment and subsequent treatment. Subsequent treatment costs for each class of therapy were calculated as weighted average costs, based on market share data from the IQVIA prescription database33 multiplied by the cost of each treatment class (Table 3). Rescue medication costs were calculated using the cost of salbutamol (100 µg twice daily) and the mean daily number of rescue inhaler uses observed in FULFIL for FF/UMEC/VI and BUD/FOR (1.6 [95% CI 1.6–1.7] vs 1.8 [95% CI 1.8–1.9], respectively) (FULFIL CSR, GlaxoSmithKline plc. 2017).32 No rescue medication costs were applied after discontinuation.
 |
Table 4 Treatment Costs |
To generate healthcare resource utilization (HRU) costs that were not related to everyday pharmacologic treatment, the model utilized a health-state costing approach, with additional costs applied for exacerbation events. All costs were sourced from the literature26 and inflated to 2019€ according to the Instituto Nacional de Estadística General Consumer Price Index.34 Three health states were defined for costing purposes, based on categories of dyspnea symptom frequency. The model calculated the proportion of the population in each health state over time, then applied the appropriate cost to generate annual general disease management costs. Exacerbation events were defined as moderate or severe and were costed individually (Table 5).
 |
Table 5 Costs Applied to Exacerbation Events and Health Status26 |
Model Outputs
The model outputs included the number of moderate and severe exacerbations, life years (LYs), QALYs, and incremental cost-effectiveness ratios (ICERs), presented as incremental cost per QALY gained.
Base-Case, Scenario, and Sensitivity Analyses
The base-case analysis was carried out with a time horizon of three years and a cycle length of one year. An annual discount rate of 3% was applied to costs and benefits, in line with Spanish guidelines.35
Deterministic sensitivity analyses and scenario analyses examined the effects of alternative assumptions, model settings, and parameter values on the base-case results.
A total of nine possible scenarios were considered in these analyses. The first scenario analysis was an estimation of utilities from SGRQ total scores, according to the original algorithm developed by Starkie et al, 2011:36
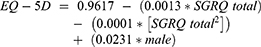
Additional scenario analyses were as follows: both 0% and 5% discount rates for costs and HRQoL (scenarios 2 and 3); 3- and 5-year time horizons with 1- and 3-year durations of treatment effects, respectively (scenarios 4 and 5); lifetime horizon (25 years) and ongoing duration of treatment effects (scenario 6); a variation of clinical parameters in the Spanish target population (scenario 7); distribution of each treatment class after discontinuation observed in FULFIL (scenario 8); and rates of discontinuation observed in the FF/VI arm of the Salford Lung Study in COPD (scenario 9).37
A one-way sensitivity analysis was used to investigate uncertainty around the input parameters. A probabilistic sensitivity analysis (PSA) was conducted to address the uncertainty in the parameters used within the model by assigning distributions to input parameters and risk equation coefficients, and randomly sampling from these distributions over 1000 Monte Carlo simulations. Details of distributions used in PSA simulations are provided in Table 6.
 |
Table 6 Distribution of Input Parameters Used in Probabilistic Sensitivity Analysis |
Results
Base-Case
Over the 3-year time horizon, the predicted cumulative total number of exacerbations per patient was lower with FF/UMEC/VI compared with BUD/FOR (2.126 vs 2.608 moderate exacerbations, and 0.306 vs 0.515 severe exacerbations, respectively; Table 7). Total 3-year costs were almost identical between FF/UMEC/VI (€6660) and BUD/FOR (€6591), with a difference of €69 in favor of BUD/FOR. Drug costs were higher in the FF/UMEC/VI cohort compared with the BUD/FOR cohort (€2820 vs €1763; difference €1057), but non-drug costs were lower (€3840 vs €4828; difference €−988). Overall, treatment with FF/UMEC/VI resulted in an additional 0.017 LYs, and 0.107 QALYs gained at an additional per-patient cost of €69 compared with BUD/FOR, resulting in an ICER of €642 per QALY gained (Table 7).
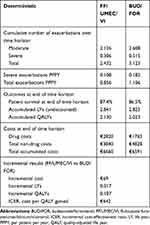 |
Table 7 Base-Case Results (3-Year Time Horizon, Spanish Population) |
Scenario and Sensitivity Analyses
Across the scenario analyses, the ICER per QALY gained ranged from €547 to €17,663 (Table 8). All values remained within the Spanish willingness-to-pay threshold of €30,000 per QALY.38,39
 |
Table 8 Scenario Analyses Results for FF/UMEC/VI Vs BUD/FOR (Spanish Population) |
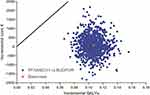
Deterministic sensitivity analyses showed that the results were most susceptible to change by varying the exacerbation treatment effect within the 95% confidence interval (Figure 2), though FF/UMEC/VI remained cost-effective vs BUD/FOR. Overall, 95% of 1000 PSA simulations resulted in an ICER less than €11,000 per QALY gained for FF/UMEC/VI vs BUD/FOR. For all PSA simulations, treatment with FF/UMEC/VI resulted in an increase in QALYs vs BUD/FOR (Figure 3) and was below the cost-effectiveness threshold of €30,000 per additional QALY.
Discussion
This study used the GALAXY-COPD disease progression model to assess the cost-effectiveness, from a Spanish healthcare perspective, of treating Spanish patients with symptomatic COPD at risk of exacerbations with once-daily FF/UMEC/VI vs twice-daily BUD/FOR. In this analysis, the starting FEV1 was 45.3% of predicted normal, indicating COPD severity of Grade 3 according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria.1 Within the refined GOLD ABCD assessment tool,1 the eligibility criteria for the FULFIL trial (a post-salbutamol FEV1/forced vital capacity [FVC] ratio of <0.7 and FEV1 <50% of predicted normal or <80% of predicted normal along with a documented history of ≥1 severe exacerbation [requiring hospitalization] or ≥2 moderate exacerbations [requiring treatment with oral/systemic corticosteroids and/or antibiotics]) in the previous year13 equate to GOLD group C–D symptom severity. Furthermore, 43.0% of patients had the equivalent of a baseline mMRC dyspnea score ≥2, indicating GOLD group D symptom severity. FF/UMEC/VI was found to improve health outcomes and to be cost-effective compared with BUD/FOR. While overall drug costs were higher for patients receiving FF/UMEC/VI compared with BUD/FOR, non-drug costs were reduced, reflecting the lower rates of moderate and severe exacerbations experienced when patients were treated with FF/UMEC/VI. With ICERs for the base-case and all scenario and sensitivity analyses all within the willingness-to-pay threshold of €30,000 per QALY that is generally considered to denote cost-effectiveness in Spain,38–40 our study results suggest the higher acquisition costs of FF/UMEC/VI vs BUD/FOR are justified by the additional health gains with triple therapy in patients with advanced, symptomatic COPD at risk of exacerbations.
A range of different therapies, including ICS, LAMA, and LABA, are available for the treatment of COPD, which can be prescribed alone or in combination depending on individual patient needs.1 Until recently, triple therapy required multiple inhalers (multiple-inhaler triple therapy [MITT]) to be used at different times of the day, but it can now be delivered via a single device (SITT)41,42 which has been shown to be associated with improved treatment adherence.43 Improved adherence does, in turn, have the potential to improve effectiveness, as do other factors of SITTs such as a user-friendly inhaler design.44 FULFIL was a double-blind, double-dummy trial focused on two single-inhaler therapies, therefore it did not address differences in adherence between devices, but the potential impact of differences in adherence, if comparing FF/UMEC/VI SITT to therapy delivered in multiple inhalers, could be considered in future studies evaluating the cost-effectiveness of FF/UMEC/VI in the treatment of COPD.
Consistent with the findings of our study, a similar cost-effectiveness analysis conducted in the United Kingdom (UK), which used the GALAXY model to compare FF/UMEC/VI with BUD/FOR based on FULFIL and using UK unit costs, demonstrated that FF/UMEC/VI SITT was also cost-effective vs dual therapy in the UK.45 Using data from the InforMing the PAthway of COPD Treatment (IMPACT) trial (NCT02164513),14 two further studies have also demonstrated the cost-effectiveness of FF/UMEC/VI vs another ICS/LABA combination (FF/VI)46 and LAMA/LABA dual therapy (UMEC/VI).47 These findings suggest that the data on cost-effectiveness in the UK and Spain may extend to other countries.
One limitation of our analysis is that it relies on the FULFIL trial alone for efficacy vs BUD/FOR and, at the time of the analysis, real-world effectiveness data were not available. It will be important to further explore the cost-effectiveness of FF/UMEC/VI in Spain based on the efficacy observed vs FF/VI and UMEC/VI in the recent IMPACT trial,14 which was conducted in a larger patient population (N=10,355) and over a longer time period (52 weeks) than the entire FULFIL trial. A study by Izquierdo et al48 examining prescribing practices in Spain highlighted the usefulness of understanding of the cost-effectiveness of FF/UMEC/VI vs dual therapies for informing decision-making on COPD treatment in Spain.
The model made a number of assumptions which, although validated by clinical experts, could nevertheless have presented a further study limitation by introducing uncertainty into the findings. Treatment effect and patient discontinuation observed at 24 weeks in FULFIL were assumed to remain consistent over the 3-year time horizon. In the scenario analysis, limiting the treatment effect to 1 year was shown to increase the ICER from €642 to €17,663 per QALY gained. However, it is reasonable to assume that the treatment effect would be maintained for the 3-year period, as findings from the Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) trial showed that patients experienced 4-year sustained efficacy with a LAMA.49 In this study, baseline values for fibrinogen and 6MWD could not be sourced directly from the target population or FULFIL, and thus had to be estimated using risk equations. This could also have been a further limitation in our analysis, although it has been indicated previously that uncertainty in these parameters does not impact upon modeling results.45
These analyses showed that, from the perspective of the Spanish NHS, FF/UMEC/VI is a cost-effective option for the treatment of adult patients with symptomatic COPD who are at risk of exacerbations, when compared to treatment with BUD/FOR. These results may help to inform future decision-making processes in the Spanish NHS.
Data Sharing Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Acknowledgments
Editorial support (in the form of writing assistance, collating author comments, assembling tables/figures, grammatical editing, and referencing) during the development of this manuscript was provided by David Mayes, MChem, and Joanna Wilson, PhD, of Gardiner–Caldwell Communications (Macclesfield, UK), and was funded by GlaxoSmithKline plc.
Author Contributions
All authors contributed to study conception or design, and/or data analysis and interpretation. All authors contributed to drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
MS, LA, AM, and ASI are employed by GlaxoSmithKline plc.; MS, AM, and ASI hold shares in GlaxoSmithKline plc. ASI is also an unpaid part-time professor at McMaster University, Canada. AH was employed by GlaxoSmithKline plc. and held shares in GlaxoSmithKline plc. at the time of the study. CB, NB and JDW were employees of ICON plc. at the time of the study. ICON is a consulting company that received research funds from GlaxoSmithKline plc. to conduct this study, but they were not paid for development of this manuscript. JLIA reports speaker fees, travel grants, and advisory board fees from AstraZeneca, Bayer, Boehringer Ingelheim, Chiesi, GlaxoSmithKline plc., Menarini, Novartis, Pfizer, Sandoz, and Teva. JARM has received speaker fees, travel grants, and consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GlaxoSmithKline plc., Menarini, Novartis, and Pfizer. JJS-C has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GlaxoSmithKline plc., Merck, Menarini, and Novartis, and consulting fees from Boehringer Ingelheim, GlaxoSmithKline plc., AstraZeneca, Ferrer, and Novartis. Trademarks are the property of their respective owners. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020. Available from https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf.
2. Miravitlles M, Soriano JB, Garcia-Rio F, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64(10):863–868. doi:10.1136/thx.2009.115725
3. European Respiratory Society. The economic burden of lung disease. In: European Lung White Book. 2019.
4. Khakban A, Sin DD, FitzGerald JM, et al. The projected epidemic of chronic obstructive pulmonary disease hospitalizations over the next 15 years. a population-based perspective. Am J Respir Crit Care Med. 2017;195(3):287–291. doi:10.1164/rccm.201606-1162PP
5. Merino M, Villoro R, Hidalgo-Vega A, Carmona C. Social economic costs of COPD in Extremadura (Spain): an observational study. Int J Chron Obstruct Pulmon Dis. 2018;13:2501–2514. doi:10.2147/COPD.S167357
6. Rutten-van Mölken M, Lee TA. Economic modeling in chronic obstructive pulmonary disease. Annals ATS. 2006;3(7):630–634.
7. Masa JF, Sobradillo V, Villasante C, et al. Costes de la EPOC en España. Estimación a partir de un estudio epidemiológico poblacional. Arch Bronconeumol. 2004;40(2):72–79. doi:10.1016/S0300-2896(04)75476-9
8. Blasi F, Cesana G, Conti S, et al. The clinical and economic impact of exacerbations of chronic obstructive pulmonary disease: a cohort of hospitalized patients. PLoS One. 2014;9(6):e101228. doi:10.1371/journal.pone.0101228
9. Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7(3):214–228. doi:10.3109/15412555.2010.481697
10. Pérez M, Puig-Peiró R, Aceituno S, Lizán L. Impacto económico de las exacerbaciones agudas en EPOC desde la perspectiva del SNS español. Rev Patol Respir. 2016;19(3):88–95.
11. de Miguel-diez J, Jimenez-Garcia R, Hernandez-Barrera V, et al. Trends in hospital admissions for acute exacerbation of COPD in Spain from 2006 to 2010. Respir Med. 2013;107(5):717–723. doi:10.1016/j.rmed.2013.01.007
12. Soler-Cataluna JJ, Sauleda J, Valdes L, et al. Prevalence and perception of 24-h symptom patterns in patients with stable chronic obstructive pulmonary disease in Spain. Arch Bronconeumol. 2016;52(6):308–315. doi:10.1016/j.arbr.2016.03.019
13. Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446. doi:10.1164/rccm.201703-0449OC
14. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
15. Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, Phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758. doi:10.1016/S2213-2600(18)30327-8
16. Miravitlles M, Soler-Cataluna JJ, Calle M, et al. Guía española de la enfermedad pulmonar obstructiva crónica (GesEPOC) 2017. Tratamiento farmacológico en fase estable. Arch Bronconeumol. 2017;53(6):324–335. doi:10.1016/j.arbres.2017.03.018
17. Philips C, Thompson G What is cost-effectiveness? Hayward Medical Communications. 2009.
18. Exuzides A, Colby C, Briggs AH, et al. Statistical modeling of disease progression for chronic obstructive pulmonary disease using data from the ECLIPSE study. Med Decis Making. 2017;37(4):453–468. doi:10.1177/0272989X15610781
19. Briggs AH, Baker T, Risebrough NA, et al. Development of the galaxy chronic obstructive pulmonary disease (COPD) model using data from ECLIPSE: internal validation of a linked-equations cohort model. Med Decis Making. 2017;37(4):469–480. doi:10.1177/0272989X16653118
20. Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE). Eur Respir J. 2008;31(4):869–873. doi:10.1183/09031936.00111707
21. Tabberer M, Gonzalez-McQuire S, Muellerova H, et al. Development of a conceptual model of disease progression for use in economic modeling of chronic obstructive pulmonary disease. Med Decis Making. 2017;37(4):440–452. doi:10.1177/0272989X16662009
22. Risebrough NA, Briggs A, Baker TM, et al. Validating a model to predict disease progression outcomes in patients with COPD. Value Health. 2014;17(7):A560–561. doi:10.1016/j.jval.2014.08.1852
23. Shah D, Driessen M, Risebrough N, et al. Cost-effectiveness of umeclidinium compared with tiotropium and glycopyrronium as monotherapy for chronic obstructive pulmonary disease: a UK perspective. Cost Eff Resour Alloc. 2018;16(1):17. doi:10.1186/s12962-018-0101-3
24. Hoogendoorn M, Feenstra TL, Asukai Y, et al. External validation of health economic decision models for chronic obstructive pulmonary disease (COPD): report of the third COPD modeling meeting. Value Health. 2017;20(3):397–403. doi:10.1016/j.jval.2016.10.016
25. Driessen MT, Whalen J, Seewoodharry Buguth B, et al. Cost-effectiveness analysis of umeclidinium bromide/vilanterol 62.5/25 mcg versus tiotropium/olodaterol 5/5 mcg in symptomatic patients with chronic obstructive pulmonary disease: a Spanish national healthcare system perspective. Respir Res. 2018;19(1):224. doi:10.1186/s12931-018-0916-7
26. Miravitlles M, Galdiz JB, Huerta A, Villacampa A, Carcedo D, Garcia-Rio F. Cost-effectiveness of combination therapy umeclidinium/vilanterol versus tiotropium in symptomatic COPD Spanish patients. Int J Chron Obstruct Pulmon Dis. 2016;11:123–132. doi:10.2147/COPD.S94006
27. Calle Rubio M, Casamor R, Miravitlles M. Identification and distribution of COPD phenotypes in clinical practice according to Spanish COPD guidelines: the FENEPOC study. Int J Chron Obstruct Pulmon Dis. 2017;12:2373–2383. doi:10.2147/COPD.S137872
28. Calle Rubio M, Alcazar Navarrete B, Soriano JB, et al. Clinical audit of COPD in outpatient respiratory clinics in Spain: the EPOCONSUL study. Int J Chron Obstruct Pulmon Dis. 2017;12:417–426. doi:10.2147/COPD.S124482
29. Almagro P, Martinez-Camblor P, Soriano JB, et al. Finding the best thresholds of FEV1 and dyspnea to predict 5-year survival in COPD patients: the COCOMICS study. PLoS One. 2014;9(2):e89866. doi:10.1371/journal.pone.0089866
30. Encuesta de Salud Nacional Espanola. 2011–2012. Available from https://www.mscbs.gob.es/estadEstudios/estadisticas/encuestaNacional/encuesta2011.htm.
31. Leidy NK, Wilcox TK, Jones PW, et al. Development of the exacerbations of chronic obstructive pulmonary disease tool (EXACT): a patient-reported outcome (PRO) measure. Value Health. 2010;13(8):965–975. doi:10.1111/j.1524-4733.2010.00772.x
32. GlaxoSmithKline plc. FULFIL Clinical Study Report. 2017.
33. IQVIA prescribing data for COPD. 2017. Available from: https://www.iqvia.com/.
34. INEbase INdE. 2019. Available from: https://www.ine.es/dyngs/INEbase/en/categoria.htm?c=Estadistica_P&cid=1254735976607.
35. Lopez-Bastida J, Oliva J, Antonanzas F, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11(5):513–520. doi:10.1007/s10198-010-0244-4
36. Starkie HJ, Briggs AH, Chambers MG, Jones P. Predicting EQ-5D values using the SGRQ. Value Health. 2011;14(2):354–360. doi:10.1016/j.jval.2010.09.011
37. Vestbo J, Leather D, Diar Bakerly N, et al. Effectiveness of fluticasone furoate-vilanterol for COPD in clinical practice. N Engl J Med. 2016;375(13):1253–1260. doi:10.1056/NEJMoa1608033
38. De Cock E, Miravitlles M, González-Juanatey JR, Azanza-Perea JR. Valor umbral del coste por año de vida ganado para recomendar la adopción de tecnologías sanitarias en España: evidencias procedentes de una revisión de la literatura. PharmacoEconomics Spanish Res Art. 2007;4(3):97–107. doi:10.1007/BF03320930
39. Sacristan JA, Oliva J, Del Llano J, Prieto L, Pinto JL. What is an efficient health technology in Spain? Gaceta sanitaria. 2002;16(4):334–343. doi:10.1016/s0213-9111(02)71933-x
40. Atienza L, Benjamin N, Schroeder M, et al. Impact of once-daily single inhaler triple therapy on healthcare resource utilization and associated costs in COPD patients in Spain. Eur Respir J. 2018;52(suppl 62):PA3155.
41. Chiesi Farmaceutici. TRIMBOW Summary of Product Characteristics. 2017.
42. GlaxoSmithKline plc. Trelegy ELLIPTA Summary of Product Characteristics. 2017.
43. Yu AP, Guerin A, Ponce de Leon D, et al. Therapy persistence and adherence in patients with chronic obstructive pulmonary disease: multiple versus single long-acting maintenance inhalers. J Med Econ. 2011;14(4):486–496. doi:10.3111/13696998.2011.594123
44. van der Palen J, Moeskops-van Beurden W, Dawson CM, et al. A randomized, open-label, single-visit, crossover study simulating triple-drug delivery with Ellipta compared with dual inhaler combinations in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2515–2523. doi:10.2147/COPD.S169060
45. Schroeder M, Shah D, Risebrough N, et al. Cost-effectiveness analysis of a single-inhaler triple therapy for patients with advanced chronic obstructive pulmonary disease (COPD) using the FULFIL trial: a UK perspective. Respir Med X. 2019;1(2019):100008.
46. Martin A, Shah D, Schroeder M, et al. P250 Informing the pathway of COPD treatment (the IMPACT study): single inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with COPD – cost-effectiveness in the UK. Thorax. 2018;73(Suppl 4):A237–A237.
47. Anley G, Shah D, Schroeder M, et al. P249 Informing the pathway of COPD treatment (the IMPACT study): single inhaler triple therapy (FF/UMEC/VI) versus dual bronchodilator therapy (UMEC/VI) in patients with COPD – cost-effectiveness in the UK. Thorax. 2018;73(Suppl 4):A236–A237.
48. Izquierdo JL, Miravitlles M, Esquinas C, et al. Characteristics of COPD patients managed in respiratory medicine departments in Spain, according to GOLD groups and GesEPOC clinical phenotypes. Archivos de Bronconeumología. 2018;54(11):559–567. doi:10.1016/j.arbr.2018.09.006
49. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi:10.1056/NEJMoa0805800
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.