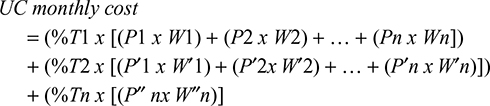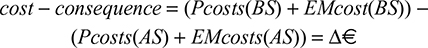Back to Journals » ClinicoEconomics and Outcomes Research » Volume 10
Cost–consequence analysis of fluticasone furoate/vilanterol 92/22 mcg for the management of COPD in the Spanish NHS
Authors Vallejo-Aparicio LA, Peces-Barba G , Gil A, Huerta Hernandez A
Received 24 March 2018
Accepted for publication 22 June 2018
Published 5 September 2018 Volume 2018:10 Pages 501—510
DOI https://doi.org/10.2147/CEOR.S169154
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Xing Lin Feng
Laura Amanda Vallejo-Aparicio,1 Germán Peces-Barba,2 Alicia Gil,3 Alicia Huerta Hernandez1
1Market Access, GlaxoSmithKline, Tres Cantos, Madrid, Spain; 2IIS-Fundación Jiménez Díaz. CIBERES, Madrid, Spain; 3Omakase Consulting, Barcelona, Spain
Objectives: The Salford Lung Study in Chronic Obstructive Pulmonary Disease (SLS COPD) is a 12-month, open-label randomized clinical trial comparing clinical effectiveness and safety of initiating once-daily fluticasone furoate/vilanterol (FF/VI) 92/22 mcg with continuing usual care (UC) in patients with COPD followed in primary care in the UK. The objective of this analysis is to estimate the economic impact of these results when applied to Spain.
Materials and methods: An Excel-based cost–consequence model with a one-year time horizon was populated with SLS COPD results, adopting the Spanish National Health System (NHS) perspective. Patients analyzed were diagnosed COPD patients ≥40 years old, currently managed with maintenance treatment and with a history of exacerbations (total number estimated from Spanish data). Mean least squares annual rates of moderate/severe exacerbations after 1 year for the intention-to-treat population from SLS COPD were included in the model (1.50 [FF/VI] and 1.64 [UC]); serious adverse events were excluded from the analysis as no differences between treatment arms were found. Medication and exacerbation management costs in euros were estimated from Spanish public sources for 2016. Model base-case analysis assumed an increased usage of FF/VI from 4% to 10% within 1 year, and a 100% proportion of days covered with study medications. Deterministic sensitivity analyses were performed for mitigating uncertainty.
Results: At base case, within 50,522 COPD patients analyzed, substitution of UC with FF/VI 92/22 mcg was associated with reduced medication and exacerbation management costs, leading to potential total annual savings of €353,623. Deterministic sensitivity results ranged from €218,333 up to €1,532,366 potential cost savings associated with FF/VI, showing the robustness of base-case results.
Conclusion: The decreased rate of exacerbations with FF/VI 92/22 mcg compared with UC observed in SLS COPD could be translated into potential health care savings for the Spanish NHS. These results may be useful to inform decision-making processes.
Keywords: cost–consequence analysis, COPD, fluticasone furoate/vilanterol, COPD Salford Lung Study
Introduction
COPD is a highly prevalent, chronic and progressive respiratory disease, associated with high health care resource consumption and costs.1 One of the main components of total disease management costs is pharmacologic treatment, representing ~40% of total costs in Spain.1,2
Nowadays, both international and national guidelines for COPD management3,4 are based on evidence that usually comes from efficacy randomized controlled clinical trials. In these studies patients included are selected based on strict inclusion/exclusion criteria, which make it difficult to extrapolate the results to patients seen in everyday clinical practice. Therefore, studies conducted in routine clinical practice are important to guide clinicians and health care providers in their COPD-related decision-making processes.
The Salford Lung Study in COPD (SLS COPD), a 12-month open-label randomized clinical trial, compared the effectiveness and safety of initiating once-daily fluticasone furoate/vilanterol 92/22 mcg (FF/VI, GlaxoSmithKline, Brentford, London, UK) with continuing usual care (UC) in patients diagnosed with COPD followed in primary care in Salford and South Manchester (UK), taking regular maintenance therapy and with one or more (≥1) exacerbations in the last 3 years.5
Results from this study5 showed that, compared to patients continuing on UC, initiating FF/VI statistically significantly reduced the annual rate of moderate/severe exacerbations by 8.4% (95% CI, 1.1–15.2; P=0.02) in the primary effectiveness analysis population (intent-to-treat [ITT] subset of patients having ≥1 moderate/severe exacerbation in the previous year; n=2,269). Results were also consistent across the ITT population. No statistically significant differences were found on the incidence of serious adverse events (SAEs) between treatment groups (29% FF/VI; 27% UC).
Decision-making processes also require studies addressing the economic consequences of COPD maintenance treatments. Therefore, the objective of the present analysis was to estimate the economic impact of substitution of UC by FF/VI on the Spanish health care budget, when applying the SLS COPD results to the Spanish population.
Materials and methods
A cost–consequence economic model was developed in Excel and populated with the SLS COPD results and Spanish data. A one-year time horizon was selected (according to SLS COPD maximum duration) and the perspective chosen was the Spanish National Healthcare System (NHS). The analysis was performed following recommendations of International and Spanish guidelines for economic evaluation.6–8
All assumptions made for the analysis were validated with a panel of three clinical and pharmacoeconomic experts, authors of this manuscript.
Study comparators
Treatment comparators for the economic analysis were those included in the SLS COPD:
- FF/VI 92/22 mcg arm: the addition of a long-acting muscarinic antagonist (LAMA) treatment was allowed if the patient had been previously treated with two long-acting bronchodilators (both LAMA and long-acting beta-agonists [LABA]) and an inhaled glucocorticoids (ICS) prior to randomization.
- UC arm: included LABA and LAMA bronchodilators alone or in combination, ICS alone or in combination with a single long-acting bronchodilator, and ICS in combination with LAMA and LABA.
Results for the ITT population from the SLS COPD were used in the economic analysis and are detailed in the next sections.
Model structure
The model estimates the monetary consequences of substitution of UC by FF/VI on the Spanish health care budget when applying the SLS COPD results to the Spanish population. Results are obtained in terms of pharmacologic treatment costs and exacerbation management costs considering two alternative scenarios:
- Base scenario: represents the “current” usage of FF/VI 92/22 mcg and UC in Spain.
- Alternative scenario: represents a hypothetical situation assuming an increased usage of FF/VI 92/22 mcg with respect to the current scenario.
- Finally, the model estimates differences between both scenarios.
Model structure is shown in Figure 1.
  | Figure 1 Economic model structure. Abbreviation: FF/VI, fluticasone furoate/vilanterol. |
The reference year chosen for the analysis was 2016. For that year, the “current” usage of FF/VI 92/22 mcg in Spain was estimated to be 4% with respect to the rest of COPD maintenance treatments available in the market (UC =96%).9 For the base-case analysis, the model assumes a usage of FF/VI 92/22 mcg of 10% (UC =90%) in the alternative scenario, to study the economic consequences of this increased usage. However, these values were modified in the deterministic sensitivity analyses specified in the next sections to assess potential impact on study results.
Model inputs
Population
The Spanish population was selected based on the inclusion criteria from the SLS COPD for the ITT population: diagnosed COPD patients ≥40 years old, treated with a maintenance treatment and with a history of exacerbations (≥1 moderate/severe exacerbation in the previous 3 years).
Considering these criteria, the number of Spanish patients to be included in the economic model was calculated based on published local epidemiologic data and from international studies whenever local data were not available. Population estimation and references used are detailed in Figure 2.10–13 No epidemiologic data were found for estimating patients having a history of ≥1 exacerbations in the previous 3 years. Therefore, the approach considered was estimating patients having a history of ≥1 exacerbations in the previous year.11,13
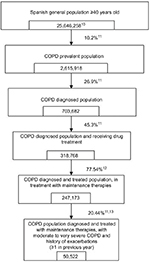  | Figure 2 Spanish COPD target population estimation. |
Finally, some deterministic sensitivity analyses were carried out on the data used for the Spanish population calculations and are detailed in the corresponding section.
Clinical inputs
Results from SLS COPD for the ITT population (n=2799) were 8.4% reduction in exacerbations with FF/VI 92/22 mcg vs UC (95% CI, 1.4–14.9; P=0.02). Annual number of moderate/severe exacerbation events per patient were considered in the analysis. For the ITT population, mean least squares annual rates of moderate/severe exacerbations after 1 year were included in the model: 1.50 (for FF/VI 92/22 mcg) and 1.64 (for UC).
The incidence of SAEs was excluded from the analysis, as results from SLS COPD showed that its frequency was similar between treatment groups (29% FF/VI 92/22 mcg; 27% UC).
Cost inputs
Exacerbation management costs
Total cost per moderate/severe exacerbation event was estimated taking into account management costs per type of exacerbation and the distribution of each type of exacerbation per comparator arm, based on the SLS COPD results.5 Costs per type of exacerbation (moderate or severe) were obtained from literature14 and adjusted with inflation up to 2016 (in euros, €),15 and are shown in Table 1.
  | Table 1 Model inputs for base-case analysis Notes: aBased on ITT population data; bUC cost calculated as an average drug cost at Price to Public plus Value Added Tax; cPrice to Public plus Value Added Tax of FF/VI 92/22 mcg; dSerious adverse events of pneumonia were excluded from the analysis as its incidence was similar between treatment groups in the Salford Lung Study in COPD.5 Abbreviations: UC, usual care; FF/VI, fluticasone furoate/vilanterol; ITT, intent to treat. |
Based on annual rates of moderate/severe exacerbations after 1 year, the model calculates annual total costs for moderate/severe exacerbations in both scenarios.
Pharmacologic costs
The model obtains annual pharmacologic treatment costs for each scenario. This is based on monthly treatment costs, calculated as acquisition cost per medication packs delivered at the retail pharmacies and expressed as Price to Public plus Value Added Tax (PTP + VAT). All prices per pack were obtained from the Spanish Ministry of Health, Equality and Social Policy published in December 2016.16
Triple therapy group (ICS in combination with two long-acting bronchodilators) was excluded from monthly cost calculations for both UC and FF/VI arms, as the proportion of patients using triple therapy in the SLS COPD was similar in both arms.5
This means that monthly cost for the FF/VI arm was established as price per pack from published PTP + VAT (see Table 1).
For the UC arm, monthly cost was established in three steps:
- Determination of therapeutic groups and relative market shares within UC in Spain: therapeutic groups were classified into two types, long-acting bronchodilator treatments (LAMA or LABA alone or in combination) and ICS containing treatments (ICS alone or in combination with a long-acting bronchodilator). Their relative market shares within UC were obtained from a Spanish market research study performed for the year 2016 with values of 61% and 39%, respectively.13
- Monthly cost estimation: all active ingredients, brands, and presentations available in the Spanish market for each therapeutic class were considered. Cost estimation was based on the following:
- Overall UC monthly cost estimation: considering the calculations from steps 1 and 2, a weighted overall UC monthly cost was obtained using the following formula:
|
where
%T1 = relative weight of therapeutic class 1 within UC;
P1 = price of presentation 1 from therapeutic class 1;
W1 = relative weight of presentation 1 within therapeutic class 1;
%T2 = relative weight of therapeutic class 2 within UC;
P′1 = price of presentation 1 from therapeutic class 2;
W′1 = relative weight of presentation 1 within therapeutic class 2.
In the base-case analysis, complete patient compliance with both treatment arms was assumed for pharmacologic cost calculations, meaning a proportion of days covered (PDC) with study medications of 100%.
Main assumptions, variables and inputs used for the base-case analysis in the economic model are summarized in Table 1.
Results reporting
Results were expressed as cost differences of both scenarios (in euros [€] for 2016), obtained through the following formula:
|
where
PCosts = pharmacologic costs;
EMcosts = exacerbation management costs;
BS = base scenario;
AS = alternative scenario.
Sensitivity analyses
To minimize the impact of uncertainty, and to determine the robustness of the results obtained for the base-case analysis, some deterministic sensitivity analyses were performed through individually modifying several parameters of the economic model.
Parameters selected for these analyses were:
- Patient compliance to drug treatments: in an everyday clinical practice COPD patients are not thought to be completely compliant with their medication, moreover in the case of elderly polymedicated patients. As a consequence, PDC was modified to ascertain the impact of different treatment compliance rates (such as 80% and 60%) on the base-case analysis results.
- Percentage of moderate-to-very-severe COPD patients with a history of exacerbations: in the base-case analysis, target COPD population is obtained taking into account a history of ≥1 exacerbations in the previous year,11,13 as no epidemiologic data were found allowing estimation of patients having a history of ≥1 exacerbations in the previous 3 years as per the inclusion criteria in the SLS COPD. Results from the SLS COPD showed that the ITT population had a mean of 2.01 exacerbations in the year prior to the study, so the impact of considering a history of ≥2 exacerbations11,13 was analyzed.
- Percentage of COPD-diagnosed patients under pharmacologic treatment: this value was taken from the EPISCAN study,11 an epidemiologic study performed in Spain in 2007. More recent epidemiologic studies in COPD in Spain are not available, although it is recognized that COPD management has evolved during the last 10 years. To address this issue, the percentage value from EPISCAN was modified with data from a more recent publication performed in newly COPD diagnosed patients,12 in which the percentage of diagnosed COPD patients undergoing drug treatment is higher than the selected value for the base-case analysis (45.3% [base case] vs 78.8% [alternative value]).
- Usage of FF/VI 92/22 mcg in the alternative scenario: for the base-case analysis it was assumed that usage of FF/VI 92/22 mcg would increase from 4% to up to 10%. This 10% base value was increased to alternative values, such as 20% and 30%, to assess the impact of this parameter on base-case analysis results.
All deterministic sensitivity analyses performed and values modifications undertaken are detailed in Table 3.
  | Table 3 Deterministic sensitivity analyses performed Abbreviations: PDC, proportion of days covered; FF/VI, fluticasone furoate/vilanterol; UC, usual care. |
Results
Base-case analysis results
A total of 50,522 COPD Spanish patients were estimated and included in the model according to the specifications detailed in the Methods section (Figure 1).
For the base-case analysis, substituting UC with FF/VI 92/22 mcg was associated with:
- Decreased drug treatment costs: accounting for €212,804.12 cost savings.
- Decreased exacerbation management costs: accounting for €140,818.86 cost savings.
As a result, substitution of UC with FF/VI 92/22 mcg could lead to potential total annual cost savings of €353,622.98 for the Spanish NHS.
Base-case results and all deterministic analyses performed are presented in Table 4.
  | Table 4 Economic model results Abbreviations: PDC, proportion of days covered; FF/VI, fluticasone furoate/vilanterol 92/22 mcg; NA, not applicable. |
Deterministic sensitivity analyses results
Results ranged from €218,332.78 up to €1,532,366.23, being “usage of FF/VI 92/22 mcg according to alternative-scenario” and “percentage of COPD diagnosed patients under treatment” the study parameters with the highest impact on results. The impact of each parameter modification with respect to the base-case analysis results is shown in Table 4.
Results from these analyses contributed to mitigate uncertainty around model parameters and assumptions, also demonstrating the robustness of the base-case analysis results.
Discussion
In Spain, COPD is a highly prevalent disease11 being the fifth cause of death among men and the seventh among women.18 It also generates high annual costs to the NHS, representing an important public health care problem and, consequently, being one of the NHS priorities.19
It is estimated that the total management costs of COPD patients in Spain represent almost 0.2% of gross domestic product,19 so the availability and use of health care interventions that may contribute to reduce not only the clinical but also the economic burden of illness seems crucial.
The aim of the present analysis was to show that the health benefit in terms of exacerbation rate reduction with FF/VI compared to UC seen in the SLS COPD could be translated into economic benefits for the Spanish NHS. It has been shown that substituting the use of UC by FF/VI 92/22 mcg in Spanish COPD patients could lead to potential total annual savings of €353,623 to the NHS, in terms of reduced drug treatment costs and reduced exacerbation management costs. In this sense, an increase in FF/VI 92/22 mcg usage among COPD Spanish patients could contribute to reduce the total economic burden associated with the management of COPD in Spain.
One limitation of this analysis could be related to the extrapolation of SLS COPD results to the Spanish setting, mainly due to potential differences in baseline patient characteristics. However, when comparing results from the SLS COPD with two studies performed in Spain,12,20 baseline patient’s characteristics could be considered similar and applicable, in terms of age, gender, body mass index, and COPD severity measured following GOLD classification.3 These results indicate plausible and relevant similarities between the population included in the SLS COPD and the overall, Spanish COPD population.
Another limitation could be related to pharmacologic cost calculations for the UC group. Relative weights between therapeutic classes assumed for Spain could present some differences from those observed in the SLS COPD.5 These differences could be related to when the studies were performed, as the SLS COPD was conducted between 2012 and 2015,5 and treatments usage trends could have varied between that time period and 2016, the reference year for the present analysis.
The structure of the present economic model was designed to easily translate the health benefit in terms of exacerbation rate reduction showed by FF/VI treatment in the SLS COPD into economic benefits for the Spanish NHS. This model represents a static view of the disease in terms of costs and consequences, which is the main difference compared to other models based on disease progression,21 which offers a dynamic perspective. Economic models are developed depending on the objective of the analysis that should be addressed; therefore, the current model structure chosen for the present analysis is considered the best approach as the intention was not to represent the natural course of the disease.
Finally, another limitation that should be mentioned could be related to the general uncertainty around the estimation of the total target Spanish patient population and the general assumptions made for model calculations, which is a common concern in economic evaluations because of the general lack of publicly available epidemiologic and cost data. The approach to mitigate as much as possible this uncertainty has been twofold: through performing various deterministic sensitivity analyses by individually modifying selected parameters and through the involvement of clinical and pharmacoeconomic experts for validation of all analysis assumptions.
Results from the SLS COPD5 could have significant relevance for both clinicians and health care providers, as this represents the largest to date effectiveness study conducted in routine clinical practice, which may help to support their everyday decision-making processes. Additionally, the aim of the economic evaluation is to provide tools supporting informed health care decisions regarding medicines recommendations and health care resources distribution, being particularly relevant in the current economic context where resources are scarce.
At present, this is the first analysis in Spain estimating the economic impact of an increased usage of FF/VI 92/22 mcg, based on the results of an effectiveness study (SLS COPD).5 A previous study performed in Spain assessed the economic consequences of exacerbation reduction in COPD, showing relevant avoided costs to the NHS with FF/VI 92/22 mcg compared with VI alone in 1 year (€1,869,430 [€37,669]).22 Despite the intrinsic differences between both studies, it could be considered that results from the present study are in line with this previous research, showing the economic benefits that the use of FF/VI 92/22 mcg for COPD management could offer to the Spanish NHS. Additionally, the present cost–consequence analysis has also been performed in the UK, showing the economic benefits of FF/VI 92/22 mcg compared with UC for a hypothetical population of 1,000 COPD patients,23 showing the consistency with the results obtained in the present analysis.
Despite the above-mentioned limitations, and in addition to demonstrating clinical effectiveness, studies addressing the economic consequences of COPD treatments are needed to support informed health care decision-making processes. In this sense, results from this study may be useful for evaluators and decision-makers when considering selection of COPD maintenance treatments in Spain.
Conclusion
Results from the present study showed that an increased usage of FF/VI 92/22 mcg treatment in COPD patients in an everyday clinical setting could result in potential cost savings to the Spanish NHS, reducing the associated burden of the disease. In this sense, these results could be considered useful to inform decision-making processes about COPD maintenance treatments selection in Spain.
Acknowledgments
Special thanks to Carl Llor from Via Roma Health Centre, Barcelona (Spain), for his contributions to the present analysis and to this manuscript. Additionally, special thanks to Isabel Pérez-Escolano from Departamento de Evaluación de Medicamentos in GlaxoSmithKline Spain, for her contributions to this manuscript. This analysis has been performed by GlaxoSmithKline (code HO-16–17343).
Author contributions
All authors made substantial contributions to this study:
Laura Amanda Vallejo-Aparicio: conception and design, data acquisition, analysis, and interpretation. Germán Peces-Barba: conception and design, and data analysis and interpretation. Alicia Gil: conception and design, and data analysis and interpretation. Alicia Huerta Hernandez: conception and design, and data acquisition, analysis, and interpretation. All authors have critically revised this manuscript for important intellectual content. All authors have approved the final version of the manuscript to be published. All authors agree to being accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Disclosure
Laura Amanda Vallejo-Aparicio is an employee of GlaxoSmithKline. Germán Peces-Barba received fees for his involvement on the present study, not receiving payment for writing the manuscript. He additionally declares not having other conflicts of interest. Alicia Gil received fees for her involvement on the present study, not receiving payment for writing the manuscript. Alicia Huerta Hernandez is an employee of and holds stocks in GlaxoSmithKline. The authors report no other conflicts of interest in this work.
References
Masa JF, Sobradillo V, Villasante C, et al. Costs of chronic obstructive pulmonary disease in Spain. Estimation from a population-based study. Arch Bronconeumol. 2004;40(2):72–79. | ||
Miravitlles M, Murio C, Guerrero T, Gisbert R. Costs of chronic bronchitis and COPD: a 1-year follow-up study. Chest. 2003;123(3):784–791. | ||
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2017). Available from: http://golcopd.org. Accessed May, 2017. | ||
Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Guía española de la enfermedad pulmonar obstructiva crónica (GesEPOC) 2017. Tratamiento farmacológico en fase estable [Spanish COPD Guidelines(GesEPOC) 2017. Pharmacological treatment of stable COPD]. Arch Bronconeumol. 2017;53(6):324–335. Spanish. | ||
Vestbo J, Leather D, Diar Bakerly N, et al. Effectiveness of fluticasone furoate-vilanterol for COPD in clinical practice. N Engl J Med. 2016;375(13):1253–1260. | ||
López Bastida J, Oliva J, Antoñanzas F, et al. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias [A guideline proposal for economic evaluation applied to health technologies]. Gac Sanit. 2010;24(2):154–170. Spanish. | ||
Weinstein MC, O’Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices--modeling studies. Value Health. 2003;6(1):9–17. | ||
Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc. 2013;11(1):6. | ||
Asthma Monitor study. COPD results. 2016. Kantar Health, S.A., Spain. Available from: www.kantarhealth.com | ||
National Institute of Statistics (INE). Resident population by date, sex and age at July 2016. National results. Detailed series since 2002. Available from: http://www.ine.es/inebaseDYN/cp30321/cp_inicio.htm. Accessed February, 2017. | ||
Soriano JB, Miravitlles M, Borderías L, et al. Diferencias geográficas en la prevalencia de EPOC en España: relación con hábito tabáquico, tasas de mortalidad y otros determinantes [Geographic differences in COPD prevalence in Spain: relationship with smoking habit,mortality rates and other determinants]. Arch Bronconeumol. 2010;46(10):522–530. Spanish. | ||
Barrecheguren M, Monteagudo M, Ferrer J, et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir Med. 2016;111:47–53. | ||
Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. | ||
Sicras A, Huerta A, Navarro R, Ibañez J. Uso de recursos y costes asociados a las exacerbaciones de enfermedad pulmonar obstructiva crónica: estudio retrospectivo de base poblacional [Resource use and associated costs of chronic obstructive pulmonary disease exacerbations:a retrospective population-based study]. SEMERGEN - Medicina de Familia. 2014;40(4):189–197. Spanish. | ||
National Institute of Statistics (INE).General Consumer Price Index (IPC). National results. Available at. http://www.ine.es/dynt3/inebase/es/index.htm?padre=3470&capsel=3466. | ||
Ministry of Health, Equality and Social Policy [Catalogue of sanitarian products included in the Spanish National Health System pharmaceutical provision]. Available from: http://www.msssi.gob.es/profesionales/nomenclator.do. Accessed December 1, 2016. | ||
Quintiles IMS Health databases [Medical dispensing database]. Total annual moving (MAT) December 2015–2016. Available from: www.iqvia.com. Accessed February, 2017. | ||
Soriano JB, Miravitlles M. Datos epidemiológicos de EPOC en España [COPD epidemiological data in Spain]. Arch Bronconeumol. 2007;43:2–9. Spanish. | ||
COPD strategy for the National Health System Documents. Available from: http://www.msps.es/organizacion/sns/planCalidadSNS/EPOC.htm. Accessed May, 2017. | ||
Izquierdo JL, Barcina C, Jiménez J, Muñoz M, Leal M. Study of the burden on patients with chronic obstructive pulmonary disease. Int J Clin Pract. 2009;63(1):87–97. | ||
Tabberer M, Gonzalez-Mcquire S, Muellerova H, et al. Development of a conceptual model of disease progression for use in economic modeling of chronic obstructive pulmonary disease. Med Decis Making. 2017;37(4):440–452. | ||
Mayoralas Alises S, Huerta A, Parrondo J. Costes sanitarios evitados con furoato de fluticasona/vilanterol debido a la reducción de la tasa de exacerbaciones en pacientes con enfermedad pulmonar obstructiva crónica [Healthcare costs avoided with fluticasone furoate/vilanterol due to exacerbation rate reduction in patients with COPD]. Pharmacoecon Span Res Artic. 2016;13(3):97–104. Spanish. | ||
Driessen MT, Barnfather S, Mulley PJ, Boucot II, Ignacio T, van de Wetering G. P57 The cost-consequence of fluticasone furoate/vilanterol 100/25 MCG in the UK using the results from the COPD Salford lung study. Thorax. 2016;71(Suppl 3):A114–A115. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.