Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Cortical thinning and flattening in schizophrenia and their unaffected parents
Authors Yan J , Cui Y, Li Q , Tian L, Liu B, Jiang T , Zhang D, Yan H
Received 19 November 2018
Accepted for publication 1 March 2019
Published 12 April 2019 Volume 2019:15 Pages 935—946
DOI https://doi.org/10.2147/NDT.S195134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Jing Yan,1 Yue Cui,2,3 Qianqian Li,1 Lin Tian,4,5 Bing Liu,2,3 Tianzi Jiang,2,3 Dai Zhang,1,6 Hao Yan1
1Peking University Sixth Hospital/Institute of Mental Health, NHC Key Laboratory of Mental Health (Peking University), National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), Beijing 100191, People’s Republic of China; 2Brainnetome Center/National Laboratory of Pattern Recognition, Institute of Automation, Chinese Academy of Sciences, Beijing 100190, People’s Republic of China; 3University of Chinese Academy of Sciences, Beijing 100049, People’s Republic of China; 4Department of Psychiatry, Wuxi Mental Health Center, Nanjing Medical University, Wuxi 214151, People’s Republic of China; 5Wuxi Mental Health Center, Wuxi Tongren International Rehabilitation Hospital, Nanjing Medical University, Wuxi, 214151, People’s Republic of China; 6Peking-Tsinghua Joint Center for Life Sciences & PKU-IDG/McGovern Institute for Brain Research, Peking University, Beijing 100871, People’s Republic of China
Background: Schizophrenia is a neurodevelopmental disorder with high heritability. Widespread cortical thinning has been identified in schizophrenia, suggesting that it is a result of cortical development deficit. However, the findings of other cortical morphological indexes of patients are inconsistent, and the research on their relationship with genetic risk factors for schizophrenia is rare.
Methods: In order to investigate cortical morphology deficits and their disease-related genetic liability in schizophrenia, we analyzed a sample of 33 patients with schizophrenia, 60 biological parents of the patients, as well as 30 young controls for patients and 28 elderly controls for parents with age, sex and education level being well-matched. We calculated vertex-wise measurements of cortical thickness, surface area, local gyrification index, sulcal depth, and their correlation with the clinical and cognitive characteristics.
Results: Widespread cortical thinning of the fronto-temporo-parietal region, sulcal flattening of the insula and gyrification reduction of the frontal cortex were observed in schizophrenia patients. Conjunction analysis revealed that patients with schizophrenia and their parents shared significant cortical thinning of bilateral prefrontal and insula, left lateral occipital and fusiform regions (Monte Carlo correction, P<0.05), as well as a trend-level sulcal depth reduction mainly in bilateral insula and occipital cortex. We observed comprehensive cognitive deficits in patients and similar impairment in the speed of processing of their unaffected parents. Significant associations between lower processing speed and thinning of the frontal cortex and flattening of the parahippocampal gyrus were found in patients and their parents, respectively. However, no significant correlation between abnormal measurements of cortical morphology and clinical characteristics was found.
Conclusion: The results suggest that cortical morphology may be susceptible to a genetic risk of schizophrenia and could underlie the cognitive dysfunction in patients and their unaffected relatives. The abnormalities shared with unaffected parents allow us to better understand the disease-specific genetic effect on cortical development.
Keywords: schizophrenia, first-degree relatives, cortical thickness, sulcus depth, insula, speed of processing
Introduction
Schizophrenia is a complex neurodevelopmental mental disorder.1 Significant gray matter deficit, particularly the widespread reduction in volume, provides relatively direct evidence of the impaired cortical neurodevelopment in schizophrenia.2,3 Volume is a product of cortical thickness and surface area. Cortical thinning has been previously described in the prefrontal, temporal, parietal and occipital cortices, and limbic areas in both chronic and first-episode schizophrenia patients.4–8 Patterns of cortical thinning may vary across different clinical characteristics of patients with chronic schizophrenia.9 Cortical thinning of the superior temporal gyrus may be associated with positive symptoms10 and prefrontal thinning has been linked to negative symptoms in schizophrenia.11 Surface area reductions, however, have only been sparsely and inconsistently reported in schizophrenia. For example, no significant case-control surface area differences were found between adolescent-onset and first-episode schizophrenia in three vertex-wise studies.12,13 However, another study with a larger sample size found surface area of the frontal, temporal, parietal and occipital regions were reduced in a more circumscribed pattern, compared with that of cortical thinning, suggesting that reduced cortical thickness and surface area makes distinct and complementary contributions to the cortical volume reduction.14
Surface area is related to several measures of gyrification, including sulcal depth and surface features (eg, sulcal and gyral curvature, and gyral complexity).15 Some measures of gyrification abnormalities have also been reported in schizophrenia, mostly using regions of interest (ROI) analysis. Cortical folding was found to have decreased in the left precentral gyrus, right middle temporal gyrus, and right precuneus,16 as well as in most prefrontal regions, except the fronto-marginal region showing the hypergynia,17 while it was found to have increased in the right superior frontal cortex of male patients with schizophrenia.18 The curvature was found to be significantly more flattened in the sulci and indicated a larger peak in the gyri in the childhood- and adolescent-onset patients with schizophrenia.15 Another study on first-episode schizophrenia however reported increased curvature of the right parahippocampal-lingual cortex area.19 Only one study measured the sulcal depth of the bilateral parietal operculum was shallower in schizophrenia patients.20
Gray matter abnormalities are a well-established fact in schizophrenia. Schizophrenia has a high heritability rate21,22 and brain structural abnormalities are observed in both first episode and chronic patients with schizophrenia,5–7 and unaffected siblings as well.23–25 Therefore, it is necessary to determine whether these abnormalities are due to the genetic predisposition and/or disease-related factors. Recently, a large-scale genome-wide association study of schizophrenia and of subcortical brain volumes examined the relationship between the genetic variants and brain imaging phenotypes of patients with schizophrenia and controls.26 However, they found no evidence of genetic overlap between schizophrenia risk, and the volume of seven subcortices (amygdala, caudate nucleus, hippocampus, nucleus accumbens, pallidum, putamen and thalamus) and intracranial volume (ICV). Their findings suggest that it is unlikely that the subcortical volumetric alternation in schizophrenia is due to genetic risk factors.27 However, evidence of the relationship between cortical measurements and schizophrenic genetic risk still needs to be verified. Only a few studies have investigated cortical measurements, mainly thickness, in siblings of patients with schizophrenia. Widespread cortical thickness reductions have been reported in unaffected siblings, especially in the frontal and temporal cortex.23–25 Studies on individuals with high genetic risk factors for schizophrenia, such as unaffected first-degree relatives of the patients, can clarify some causes of brain abnormalities observed in patients and help disentangle disease-related genetic risks shared between probands and relatives from other disease-related factors, including illness state and antipsychotic medication.
Therefore, the current study comprehensively investigated cortical measurements, including cortical thickness, surface area, local gyrification index (LGI) and sulcal depth, in patients with schizophrenia and their unaffected parents, using high-resolution structural MRI data in a vertex-wise whole brain analysis. The associations between the cortical measures and the clinical and cognitive characteristics were preliminarily explored. We hypothesized that unaffected parents of patients with schizophrenia would share similar characteristics in cortical thickness or even more cortical morphological measures because of the disease-related genetic risks buried in their genes that they shared with the patients.
Methods
Subjects
In total, 153 Chinese Han subjects were recruited for the current study, including 33 patients with schizophrenia (SZ), 61 of their unaffected biological parents (PA) (30 fathers and 31 mothers), 30 young healthy controls for the patients (HC1) and 29 old healthy controls for the unaffected parents (HC2).
The SZ and PA groups were recruited from the Peking University Sixth Hospital. The diagnosis of schizophrenia was made according to ICD-10 diagnostic criteria for paranoid schizophrenia by at least two trained and skilled psychiatrists. All patients were under antipsychotic medication when they were enrolled in the study, and the dosages were converted to the equivalent dose of chlorpromazine. More than half of the patients were under combination medication. The Positive and Negative Syndrome Scale (PANSS)28 was used to assess the severity of disease. Patients who had a history of serious medical illness or those who were treated with electroconvulsive therapy during the previous 6 months were excluded from the study. The HC1 and HC2 groups were recruited from the local community. They were well matched with the patients and parents group with regard to age, gender and education level. The exclusion criteria for the healthy controls included having any first- or second-degree relatives with schizophrenia spectrum disorders, intracranial pathology, history of brain injury, neurological disorders and alcohol/substance abuse. All first-degree relatives and normal controls were interviewed by a psychiatrist in order to exclude a previous or current diagnosis of any mental illnesses. The right-handedness of all participants was assessed using the Edinburgh Handedness Inventory.29
The study was approved by the Medical Ethics Committee of Peking University Sixth Hospital. Prior to written consent being obtained, the research objective and procedures were explained in detail to all participants. All available biological parents of the patients were invited to participate in this study, and therefore written consent was given by the patients and their parents, as well as all healthy participants enrolled in this study. This study was conducted in accordance with the Declaration of Helsinki.
Cognitive function assessment
Several commonly impaired cognitive abilities in schizophrenia30,31 were assessed in the current study:1) speed of processing assessed using the Trail Making Test (TMT), Part A and Category Fluency Test (CFT): Animal Naming; 2) working memory; Digital Span (DS) forwards and backwards of the Wechsler Memory Scale-Chinese Revised (WMS-CR); 3) episodic memory: Logical Memory (LM) from the WMS-CR. Other than for the TMT, a higher score indicates better cognitive ability. The detailed cognitive assessments and scoring are given in the supplementary material. All subjects completed the cognitive assessments, with the exception of one patient.
Neuroimaging
MRI data acquisition
Structural magnetic resonance imaging (MRI) scans were conducted at the Department of Radiology, Peking University Third Hospital, using a 3.0-Tesla MAGNETOM Trio MR system (Siemens Medical System, Erlangen, Germany). Head motion was minimized using restraining foam pads. Three dimensional T1-weighted images were acquired at a sagittal orientation, employing a 3D-MPRAGE sequence with the following parameters: time repetition=2350 ms, time echo=3.44 ms, field of view=256×256 mm2, flip angle=7°, 192 sagittal slices, slice thickness=1 mm, matrix size=256×256, total acquisition time=363 seconds. Visual quality control was performed to check movement artifacts, brain lesions or significant changes. After extensive quality control of the brain imaging data, the data of 33 SZ, 60 PA (29 fathers, 31 mothers), 30 HC1 and 28 HC2 subjects, amounting to 151 subjects in total, were included in subsequent analyses.
Data analysis
The publicly available FreeSurfer software package, version 5.3.0 (
Statistical analysis
Differences in basic demographics between groups were examined using two-tailed t-tests and chi-square tests, with the aid of PASW Statistics 18.0 (IBM Corporation, Armonk, NY, USA).
Complete vertex-wise analyses of various morphological measurements were performed for the SZ and PA groups using FreeSurfer 5.3.0. We used a general linear model, controlling for the effect of age and sex, in order to estimate differences in each morphological measure between group SZ and group HC1, as well as group PA and group HC2, at each vertex of the surface. In order to correct for multiple comparisons, a cluster analysis was performed using a Monte Carlo simulation with 10,000 iterations. A vertex-wise and cluster-forming threshold of P<0.05 was used for simulation . These analyses were performed using the “commond-line” group analysis stream of FreeSurfer. The anatomical overlap of brain regions with significant impairments between the SZ and PA groups was determined using conjunction analysis,36 which allows us to summarize two comparisons that show an effect. Specifically, the minimum of the absolute T values across two statistical maps at each vertex is used to create a conjunction map that shows the minimum T values with the true sign of the minimum values. This facilitated the inspection of neuropathological features shared by patients with schizophrenia and their first-degree relatives. The conjunction analyses were performed using FreeSurfer 5.3.0.
The associations between cortical measurements shared by patients and their relatives and clinical characteristics (PANSS scores, course of illness and medication dose), as well as cognitive assessments were investigated using partial correlations controlling for the effect of age and sex, with the aid of MATLAB (R2012a; The MATHWORKS Inc., Natick, MA, USA).
Results
Demographics and cognition
Sociodemographic, psychopathological and cognitive data of the SZ and PA groups are shown in Table 1. No statistically significant differences were noted among the SZ and HC1 groups, or the PA and HC2 groups, in terms of age, sex and years of education.
 | Table 1 Demographic, cognitive and clinical characteristics of the patients, their unaffected parents and healthy controls |
The patients were associated with cognitive deficits in all tested cognitive domains. Additionally, the PA group showed significantly poorer performance than the HC2 group for the TMT and CFT associated with speed of processing.
Group comparisons of morphological measurements of the SZ group
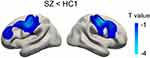
After correction for multiple comparisons at cluster level using Monte Carlo simulation, we observed significant abnormalities in cortical thickness, LGI and sulcal depth in the SZ group, compared with that of the HC1 group. Specifically, widespread cortical thinning was found in SZ patients, involving bilateral frontal, temporal, parietal and occipital regions (P=0.0002 after correction) (Figure 1A). Reduced LGI was found bilaterally in the postcentral/precentral gyrus (P=0.002 after correction), extending to the caudal middle/inferior frontal regions (Figure 2). We also found a decrease in the sulcal depth of the left insula (P=0.020 after correction) (Figure 3A). No increase in cortical thickness, LGI or sulcal depth was observed among the patients. There was no significant difference in surface area between the SZ group and the HC1 group.
Group comparisons of morphological measurements of the PA group
After correction for multiple comparisons, we observed significant abnormalities in cortical thickness and sulcal depth of the PA group, compared with that of the HC2 group. Specifically, cortical thinning was found in bilateral prefrontal and insula, left lateral occipital and fusiform regions of subjects in the PA group (cluster-wise P=0.0002 after correction) (Figure 1B). We also found a decrease in the sulcal depth of the right insula (cluster-wise P=0.023 after correction) (Figure 3B). No increase in cortical thickness and sulcal depth were observed among the parents. There was no significant difference in surface area and LGI between the PA group and the HC2 group.
Conjunction analysis of SZ<HC1 and PA<HC2 for cortical thickness and sulcal depth
Conjunction analysis identified that patients with schizophrenia and their first-degree relatives share regions with significant cortical thinning. These regions include bilateral prefrontal and insula cortices, left lateral occipital and fusiform areas (P<0.001 corrected). (Figure 1C and Table 2).
 | Table 2 Brain regions with significant abnormality shared between patients with schizophrenia and their unaffected parents |
Conjunct regions were not identified for sulcal depth after correction for multiple comparisons. However, we observed a trend towards a shallow sulcus in both patients and their parents, using a more liberal statistical threshold for conjunction analysis of sulcal depth. Group comparisons of sulcal depth in the SZ and PA groups are shown in Additional Figure S1A and B (uncorrected P<0.05). Conjunction analysis revealed that SZ patients and their first-degree relatives share a shallow sulcus in 7 clusters, involving bilateral insula and occipital, left precuneus, parahippocampus and medial orbitofrontal regions (Additional Figure S1C and Table 2).
Correlation between abnormal cortical morphology and clinical and cognitive data
No significant correlation was found in the patients group between any of the abnormal cortical measures and clinical characteristics (PANSS scores, course of illness and medication dose) (P>0.05). However, we did find that a thinner cortex and shallow sulcus in some brain areas are related to the poor cognitive function of patients and that a shallow sulcus in the left parahippocampal gyrus is related to the poor TMT performance of parents (P<0.05, Table 3). However, only the correlation between the TMT and left middle frontal cortex and left calcarine of the SZ and NC1 groups were able to survive FDR correction.
 | Table 3 Partial correlation between abnormal brain regions and cognitive characteristics of the patients, their unaffected parents and healthy controls controlling for age and sex |
Discussion
In the current study, we investigated abnormalities in cortical morphology and cognitive function of patients with schizophrenia and their unaffected parents. The abnormalities shared with their unaffected parents allowed us to better understand the disease-related genetic effect on cortical development and its potential behavioral significance. Our main findings included the finding that patients and their unaffected parents both demonstrate a decrease in cortical thickness and sulcal depth (ie, cortical thinning and flattening), compared with their respective controls, while only patients show decreased LGI. No significant surface area alternation and no increase in cortical morphological measurements were observed in either patients or their unaffected parents. The changes in the cortical morphology of patients and their unaffected relatives are associated with similar deficits in the speed of processing.
Cortical thickness is thought to be a morphological measure of the number of neurons within a cortical layer.37 Some studies on post-mortem materials and animal models have reported that schizophrenia is associated with a reduction in neuron number of the thalamus,38,39 hippocampus40 and visual cortex.41 In the current study, we observed widespread cortical thinning of the bilateral frontal, temporal, parietal and occipital regions in patients with schizophrenia, and in a more circumscribed pattern for their unaffected parents. Patients with schizophrenia and their first-degree relatives share significant cortical thinning of bilateral prefrontal and insula, left lateral occipital and fusiform regions. Previous studies have consistently reported of a widespread decrease in cortical thickness of the fronto-temporo-parietal region of schizophrenic patients, compared with controls, which is most pronounced in the frontal lobe and temporal cortex.4–7,42 For the relatives, though there has been no consensus among researchers as yet, a majority of studies on cortical thickness of relatives report a thinner cortex in the prefrontal cortex, anterior cingulate cortex, temporal and occipital regions.24,25,43 In addition, a study with a large sample size of patients with schizophrenia and their unaffected siblings reported a trend-level reduction in thickness for the unaffected siblings.42 The research findings from the same group suggest that neocortical volume is under genetic control and that the cortical volumetric reductions are related to a familial risk of schizophrenia, but the measurements of subcortical volumes per se did not represent a disease-related intermediate biological phenotype,44 which was verified in a very recent associated study on the genetic risk of schizophrenia and subcortical volume.26 Therefore, cortical morphology is probably more of a genetic risk of schizophrenia than subcortical brain structures. Our findings further support evidence that widespread cortical thinning could be a disease-intermediate phenotype of schizophrenia, which undergoes the volumetric changes that have been repeatedly reported in patients and their first-degree relatives.
Abnormalities in sulcal morphology have been previously reported mostly in the cingulate or paracingulate sulcus of schizophrenia patients.45,46 Only one study measured vertex-wise sulcal depth of the whole brain and found that patients with schizophrenia have a shallow sulcus in bilateral parietal operculum.20 In the current study, significantly shallow sulci were observed in the left insula of patients and right insula of unaffected parents. Moreover, patients and parents shared a trend-level sulcal depth reduction mainly in bilateral insula and occipital cortex. An interesting finding was that sulcal depth reduction of the insula was found in both patients and relatives despite a relative liberal threshold. The insular cortex is bilaterally located deep within the lateral fissure. Insula have been shown to play an important role in cognition and their dysfunction underlies many psychiatric disorders, such as schizophrenia.47 A systematic meta-analysis of insula volume found medium-sized reduction in insula volume in schizophrenia patients, which may suggest its important role in the neuropathology of schizophrenia.48 Recently, two meta-analyses on structural and functional imaging studies across multiple psychiatric diagnoses have identified bilateral insula as a common neurobiological substrate for mental illnesses, which may relate to executive function deficits observed across diagnoses.49,50 It has been suggested that patients with schizophrenia and their biological relatives may have deficits in executive control processing and common activity changes of the insula.51 Sulcal depth has been reported to decline with age,52 while the common shallow sulcus in insula of young patients and their unaffected parents might demonstrate that insula flattening could be a disease-intermediate phenotype. However, studies on sulcal morphology in schizophrenia and their relatives are rare, and the current findings need to be verified in other cohorts using a larger sample size.
In addition, we also found that the local gyrification index (LGI) decreased only in the patients group for bilateral postcentral/precentral gyrus, extending to the caudal middle/inferior frontal regions. LGI is a measure of the brain folding pattern, which depicts one aspect of brain surface complexity. There have been relatively few studies that have investigated gyrification in schizophrenia patients, especially in a voxel-wise way. These studies have yielded mixed results, including higher,18 as well as lower16,17 gyrification of the frontal and temporal lobe, or no gyrification differences between patients and controls.53 Although the findings are inconsistent, the frontal and temporal lobes still seem to be the most susceptible regions in schizophrenia.54 These discrepancies in the findings may point to the heterogeneity of gyrification, or to heterogeneity within populations (medication, demographics) and the methodologies used. A very interesting hypothesis of gyrification is that the tension produced by neuronal connections are involved in the mechanisms of gyrification, which means that changes in structural connectivity between brain regions lead to changes in the cortical folding pattern; while the disconnection hypothesis has been well accepted in the pathogenesis of schizophrenia.54 Recent studies are inclined to confirm that LGI is a neurological marker of schizophrenia.55 Abnormal LGI might represent vulnerability to psychopathology.56 We found that LGI decreased only in the patients group, which indicates that it might be a biomarker of individuals who will actually develop the disorder or be effected by disease progression. Therefore, LGI is a very interesting measurement that needs to be studied further in a prospective manner, with first-episode drug- naïve patients, in order that we can find out its relationship with the neuropathology of disconnection in schizophrenia and other disease-related factors, such as disease-state and medication.
Schizophrenia is associated with cognitive deficits in comprehensive domains.57–59 These deficits have been found not only in patients, but also in their unaffected first-degree relatives.60 In line with previous investigations, we also found significantly poor performance of patients and their unaffected parents in cognitive tests, compared with similar aged controls. These results suggest that these deficits may reflect brain abnormalities that are associated with a genetic risk of schizophrenia.
In the patients group, correlation analysis revealed significant associations between abnormal cortical morphology and cognitive tests, especially in the speed of processing domain, but not with any clinical characteristics, which is partly in line with prior findings.61 These findings further suggest that cognitive dysfunction is an important and stable symptom of schizophrenia patients. Investigation of unaffected parents of patients show the association between reduced speed of processing and a shallower sulcus in the left parahippocampal gyrus, which in a previous study showed less pronounced cortical thinning in unaffected relatives of patients with schizophrenia.62 However, we did not observe similar areas in patients with schizophrenia in the correlation analysis between cortical morphology and cognitive function, which suggests that patients may engage a compensatory network of brain regions other than the normal cortex used for recall. Speed of processing impairment in unaffected relatives of patients may be related to the genetic risk of the disease. However, the neuropathological mechanisms still need to be further investigated.
Several issues need to be considered before understanding our findings. First, unaffected parents of patients, as first-degree relatives of the probands, were mostly living together with the patients and were probably affected by disease-related negative emotions and the stigma attached to the disease. The situation they had been born in might have been worse than that experienced by the siblings of the patients. Adding necessary assessments of emotional status may help exclude confounding factors. Second, some genetic heterogeneity still exists in the parent group, such as that of the presumed obligate carrier who appears to transmit genetic predisposition to their affected children. Third, the small sample size, especially of patients, limits further analysis of this data set, such as that of the gender effect and genetic association analysis of significant measurements. Finally, we used a liberal threshold of the conjunction analysis of sulcal depth, and the validity of our findings remains uncertain without replication in larger samples.
Conclusion
In summary, our study revealed widespread cortical thinning and relatively localized cortical flattening in both patients with schizophrenia and their unaffected parents. The abnormal cortical morphology shared between patients and relatives indicate that specific cortical morphological measurements, such as cortical thickness and sulcal morphology, may be intermediate phenotypes of schizophrenia and may explain the impaired speed of processing in patients and their unaffected relatives.
Ethics approval and consent to participate
The study was approved by the Medical Ethics Committee of Peking University Sixth Hospital. All available biological parents of the patients were invited to participate in this study, and therefore written consent was given by the patients and their parents, as well as all healthy participants enrolled in this study. This study was conducted in accordance with the Declaration of Helsinki.
Abbreviation list
ROI, regions of interest; ICV, intracranial volume; LGI, local gyrification index; SZ, schizophrenia; PA, unaffected biological parents; HC1, young healthy controls for the patients; HC2, old healthy controls for the unaffected parents; PANSS, Positive and Negative Syndrome Scale.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors gratefully acknowledge Qiang Zhao from the Radiology Department of Peking University Third Hospital for his assistance in image acquisition. This study was supported by the National Natural Science Foundation of China grant 81370032 and 81771443 presented to H Yan, grant 91432304 presented to D Zhang, and grant 31771076 presented to Y Cui.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed). 1987;295(6600):681–682.
2. Goldstein JM, Goodman JM, Seidman LJ, et al. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56(6):537–547.
3. Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–52):1–2.
4. Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60(9):878–888. doi:10.1001/archpsyc.60.9.878
5. Narr KL, Bilder RM, Toga AW, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15(6):708–719. doi:10.1093/cercor/bhh172
6. Narr KL, Toga AW, Szeszko P, et al. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005;58(1):32–40. doi:10.1016/j.biopsych.2005.03.043
7. van Haren NE, Schnack HG, Cahn W, et al. Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68(9):871–880. doi:10.1001/archgenpsychiatry.2011.88
8. Sugihara G, Oishi N, Son S, Kubota M, Takahashi H, Murai T. Distinct patterns of cerebral cortical thinning in schizophrenia: a neuroimaging data-driven approach. Schizophr Bull. 2017;43(4):900–906. doi:10.1093/schbul/sbw176
9. Nenadic I, Yotter RA, Sauer H, Gaser C. Patterns of cortical thinning in different subgroups of schizophrenia. Br J Psychiatry. 2015;206(6):479–483. doi:10.1192/bjp.bp.114.148510
10. Walton E, Hibar DP, van Erp TGM, et al. Positive symptoms associate with cortical thinning in the superior temporal gyrus via the ENIGMA Schizophrenia consortium. Acta Psychiatr Scand. 2017;135(5):439–447. doi:10.1111/acps.12718
11. Walton E, Hibar DP, van Erp TGM, et al. Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium. Psychol Med. 2018;48(1):82–94. doi:10.1017/S0033291717001283
12. Voets NL, Hough MG, Douaud G, et al. Evidence for abnormalities of cortical development in adolescent-onset schizophrenia. Neuroimage. 2008;43(4):665–675. doi:10.1016/j.neuroimage.2008.08.013
13. Xiao Y, Lui S, Deng W, et al. Altered cortical thickness related to clinical severity but not the untreated disease duration in schizophrenia. Schizophr Bull. 2015;41(1):201–210. doi:10.1093/schbul/sbt177
14. Rimol LM, Nesvåg R, Hagler DJ, et al. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry. 2012;71(6):552–560. doi:10.1016/j.biopsych.2011.11.026
15. White T, Andreasen NC, Nopoulos P, Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biol Psychiatry. 2003;54(4):418–426.
16. Nesvag R, Schaer M, Haukvik UK, et al. Reduced brain cortical folding in schizophrenia revealed in two independent samples. Schizophr Res. 2014;152(2–3):333–338. doi:10.1016/j.schres.2013.11.032
17. Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Folding of the prefrontal cortex in schizophrenia: regional differences in gyrification. Biol Psychiatry. 2011;69(10):974–979. doi:10.1016/j.biopsych.2010.12.012
18. Narr KL, Bilder RM, Kim S, et al. Abnormal gyral complexity in first-episode schizophrenia. Biol Psychiatry. 2004;55(8):859–867. doi:10.1016/j.biopsych.2003.12.027
19. Schultz CC, Koch K, Wagner G, et al. Increased parahippocampal and lingual gyrification in first-episode schizophrenia. Schizophr Res. 2010;123(2–3):137–144. doi:10.1016/j.schres.2010.08.033
20. Csernansky JG, Gillespie SK, Dierker DL, et al. Symmetric abnormalities in sulcal patterning in schizophrenia. Neuroimage. 2008;43(3):440–446. doi:10.1016/j.neuroimage.2008.07.034
21. Tsuang M. Schizophrenia: genes and environment. Biol Psychiatry. 2000;47(3):210–220. doi:10.1016/S0006-3223(99)00289-9
22. McGuffin P, Owen MJ, Farmer AE. Genetic basis of schizophrenia. Lancet. 1995;346(8976):678–682.
23. Calabrese DR, Wang L, Harms MP, et al. Cingulate gyrus neuroanatomy in schizophrenia subjects and their non-psychotic siblings. Schizophr Res. 2008;104(1–3):61–70. doi:10.1016/j.schres.2008.06.014
24. Goghari VM, Rehm K, Carter CS, Macdonald AW. Sulcal thickness as a vulnerability indicator for schizophrenia. Br J Psychiatry. 2007;191:229–233. doi:10.1192/bjp.bp.106.034595
25. Goghari VM, Rehm K, Carter CS, MacDonald AW. Regionally specific cortical thinning and gray matter abnormalities in the healthy relatives of schizophrenia patients. Cereb Cortex. 2007;17(2):415–424. doi:10.1093/cercor/bhj158
26. Franke B, Stein JL, Ripke S, et al. Genetic influences on schizophrenia and subcortical brain volumes: large-scale proof of concept. Nat Neurosci. 2016;19(3):420–431. doi:10.1038/nn.4228
27. Arguello PA. Schizophrenia and brain volume genetic covariation. Nat Neurosci. 2016;19(3):419. doi:10.1038/nn0316-419
28. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276.
29. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113.
30. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. doi:10.1176/appi.ajp.2007.07010042
31. Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013;150(1):42–50. doi:10.1016/j.schres.2013.07.009
32. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi:10.1006/nimg.1998.0395
33. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97(20):11050–11055. doi:10.1073/pnas.200033797
34. Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran J-P. A surface-based approach to quantify local cortical gyrification. IEEE Transactions on Medical Imaging. 2008;27(2):161–170. doi:10.1109/TMI.2007.903576
35. Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8(4):272–284.
36. Nichols T, Brett M, Andersson J, Wager T, Poline J-B. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25(3):653–660. doi:10.1016/j.neuroimage.2004.12.005
37. Rakic P. Specification of cerebral cortical areas. Science. 1988;241(4862):170–176.
38. Byne W, Fernandes J, Haroutunian V, et al. Reduction of right medial pulvinar volume and neuron number in schizophrenia. Schizophr Res. 2007;90(1–3):71–75. doi:10.1016/j.schres.2006.10.006
39. Young KA, Manaye KF, Liang C, Hicks PB, German DC. Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry. 2000;47(11):944–953.
40. Benes FM, Sorensen I, Bird ED. Reduced neuronal size in posterior hippocampus of schizophrenic patients. Schizophr Bull. 1991;17(4):597–608.
41. Dorph-Petersen K-A, Pierri JN, Wu Q, Sampson AR, Lewis DA. Primary visual cortex volume and total neuron number are reduced in schizophrenia. J Comp Neurol. 2007;501(2):290–301. doi:10.1002/cne.21243
42. Goldman AL, Pezawas L, Mattay VS, et al. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009;66(5):467–477. doi:10.1001/archgenpsychiatry.2009.24
43. Byun MS, Kim JS, Jung WH, et al. Regional cortical thinning in subjects with high genetic loading for schizophrenia. Schizophr Res. 2012;141(2–3):197–203. doi:10.1016/j.schres.2012.08.028
44. Goldman AL, Pezawas L, Mattay VS, et al. Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry. 2008;63(5):475–483. doi:10.1016/j.biopsych.2007.06.006
45. Fornito A, Yücel M, Wood SJ, et al. Surface-based morphometry of the anterior cingulate cortex in first episode schizophrenia. Hum Brain Mapp. 2008;29(4):478–489. doi:10.1002/hbm.20412
46. Fornito A, Wood SJ, Whittle S, et al. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume, and sulcal depth. Hum Brain Mapp. 2008;29(2):222–236. doi:10.1002/hbm.20381
47. Namkung H, Kim S-H, Sawa A. The insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci. 2017;40(4):200–207. doi:10.1016/j.tins.2017.02.002
48. Shepherd AM, Matheson SL, Laurens KR, Carr VJ, Green MJ. Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry. 2012;72(9):775–784. doi:10.1016/j.biopsych.2012.04.020
49. McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. 2017;174(7):676–685. doi:10.1176/appi.ajp.2017.16040400
50. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305–315. doi:10.1001/jamapsychiatry.2014.2206
51. Camchong J, Dyckman KA, Austin BP, Clementz BA, McDowell JE. Common neural circuitry supporting volitional saccades and its disruption in schizophrenia patients and relatives. Biol Psychiatry. 2008;64(12):1042–1050. doi:10.1016/j.biopsych.2008.06.015
52. Shen X, Liu T, Tao D, et al. Variation in longitudinal trajectories of cortical sulci in normal elderly. Neuroimage. 2017;166:1–9. doi:10.1016/j.neuroimage.2017.10.010
53. Highley JR, DeLisi LE, Roberts N, et al. Sex-dependent effects of schizophrenia: an MRI study of gyral folding, and cortical and white matter volume. Psychiatry Res. 2003;124(1):11–23.
54. White T, Hilgetag CC. Gyrification and neural connectivity in schizophrenia. Dev Psychopathol. 2011;23(1):339–352. doi:10.1017/S0954579410000842
55. Hirjak D, Kubera KM, Wolf RC, et al. Local brain gyrification as a marker of neurological soft signs in schizophrenia. Behav Brain Res. 2015;292:19–25. doi:10.1016/j.bbr.2015.05.048
56. Sasabayashi D, Takayanagi Y, Takahashi T, et al. Increased occipital gyrification and development of psychotic disorders in individuals with an at-risk mental state: a multicenter study. Biol Psychiatry. 2017;82(10):737–745. doi:10.1016/j.biopsych.2017.05.018
57. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi:10.1037/a0014708
58. Nuechterlein KH, Subotnik KL, Green MF, et al. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr Bull. 2011;37(Suppl 2):S33–S40. doi:10.1093/schbul/sbr084
59. Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39(6):889–905. doi:10.1017/S0033291708004558
60. Mucci A, Galderisi S, Green MF, et al. Familial aggregation of MATRICS consensus cognitive battery scores in a large sample of outpatients with schizophrenia and their unaffected relatives. Psychol Med. 2018;48(8):1359–1366. doi:10.1017/S0033291717002902
61. Oertel-Knöchel V, Knöchel C, Rotarska-Jagiela A, et al. Association between psychotic symptoms and cortical thickness reduction across the schizophrenia spectrum. Cereb Cortex. 2013;23(1):61–70. doi:10.1093/cercor/bhr380
62. Yang Y, Nuechterlein KH, Phillips O, et al. The contributions of disease and genetic factors towards regional cortical thinning in schizophrenia: the UCLA family study. Schizophr Res. 2010;123(2–3):116–125. doi:10.1016/j.schres.2010.08.005
Supplementary materials
1. Methods for cognitive assessments and scoring
Several commonly impaired cognitive abilities in schizophrenia were assessed in current study:1) speed of processing assessed with Trail Making Test, Part A (TMT) and Category Fluency Test: Animal Naming (CFT); 2) working memory; Digital Span (DS) forward and backward from the Wechsler Memory Scale-Chinese Revised (WMS-CR); 3) episodic memory: Logical Memory (LM) from the WMS-CR. Except for the TMT, higher rating scores indicated better cognitive abilities. The detailed methods of assessments and scoring were described as follows:
TMT:
Participants were presented some consecutive numbers that are arranged in irregular locations and were asked to draw a line connecting a sequence of 25 numbers in order. Participants need to finish it as quickly as possible while still maintaining accuracy and should not lift the pencil from the paper until they finish this task. We record the completion time. The test can provide information about speed of processing.
CFT:
Participants were asked to produce as many animal words as possible in 1 min. A higher number of correct answers reflects a faster processing speed.
DS:
For Digital Span forward, participants were asked to hear a sequence of numerical digits and tasked to recall the sequence correctly, with increasingly longer sequences being tested in each trial. For Digital Span backward, participants were asked to recall the sequence numbers in reverse order. Two trials for each sequence length are administered. Both trials of an item are administered even if the respondent passes the first trial.
LM:
Participants were presented auditorily two story passages and were asked to recall each story immediately after hearing it using as many of the same words of the original passage as they could remember. There are 50 gists (important story ideas units) in the two story passages. The gist recall was evaluated in each participant. The full score was 25 points (0.5 for each gist), then the raw score was converted to scale score according to converting table in the manual of WMS-CR, which was defined on the basis of the common model of Chinese adults.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.