Back to Journals » Journal of Pain Research » Volume 13
Cortical Thickness Mediates the Association Between Self-Reported Pain and Sleep Quality in Community-Dwelling Older Adults
Authors Montesino-Goicolea S, Valdes-Hernandez PA , Hoyos L , Woods AJ, Cohen R , Huo Z , Riley JL III , Porges EC , Fillingim RB, Cruz-Almeida Y
Received 30 April 2020
Accepted for publication 20 August 2020
Published 24 September 2020 Volume 2020:13 Pages 2389—2400
DOI https://doi.org/10.2147/JPR.S260611
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Soamy Montesino-Goicolea,1,2 Pedro A Valdes-Hernandez,1,2 Lorraine Hoyos,3 Adam J Woods,2,4,5 Ronald Cohen,2,5 Zhiguang Huo,6 Joseph L Riley III,1,7 Eric C Porges,2,5 Roger B Fillingim,1,7 Yenisel Cruz-Almeida1,2,4,7
1Department of Community Dentistry & Behavioral Sciences, University of Florida, Gainesville, FL, USA; 2Center for Cognitive Aging and Memory, McKnight Brain Foundation, University of Florida, Gainesville, FL, USA; 3University of Central, Florida College of Medicine, Orlando, FL, USA; 4Department of Neuroscience, College of Medicine, University of Florida, Gainesville, FL, USA; 5Department of Clinical and Health Psychology, College of Health Professions, University of Florida, Gainesville, FL, USA; 6Department of Biostatistics, College of Public Health and Health Professions College of Medicine, University of Florida, Gainesville, FL, USA; 7Institute on Aging, University of Florida, Gainesville, FL, USA
Correspondence: Yenisel Cruz-Almeida
Pain Research and Intervention Center of Excellence, University of Florida, PO BOX 112610, Gainesville, FL 326010, USA
Tel +1 352-294-5845
Fax +1 352-273-5920
Email [email protected]
Introduction: Musculoskeletal pain is prevalent in older adults representing the leading cause of disability in this population. Similarly, nearly half of older adults complain of difficulty sleeping. We aimed to explore the relationship between sleep quality with self-reported musculoskeletal pain, somatosensory and pain thresholds in community-dwelling older adults and further explore brain regions that may contribute to this association.
Methods: Older adults (> 60 years old, n=69) from the NEPAL study completed demographic, pain and sleep assessments followed by a quantitative sensory testing battery. A subset (n=49) also underwent a 3T high-resolution, T1-weighted anatomical scan.
Results: Poorer sleep quality using the Pittsburgh Sleep Quality Index was positively associated with self-reported pain measures (all p’s > 0.05), but not somatosensory and pain thresholds (all p’s > 0.05). Using a non-parametric threshold-free cluster enhancement (TFCE) approach, worse sleep quality was significantly associated with lower cortical thickness in the precentral, postcentral, precuneus, superior parietal, and lateral occipital regions (TFCE-FWE-corrected at p < 0.05). Further, only postcentral cortical thickness significantly mediated the association between sleep quality and self-reported pain intensity using bootstrapped mediation methods.
Conclusion: Our findings in older adults are similar to previous studies in younger individuals where sleep is significantly associated with self-reported pain. Specifically, our study implicates brain structure as a significant mediator of this association in aging. Future larger studies are needed to replicate our findings and to further understand if the brain can be a therapeutic target for both improved sleep and pain relief in older individuals.
Keywords: chronic pain, sleep quality, sleep, brain, aging
Introduction
Chronic pain is highly prevalent in older adults and represents the leading cause of disability in this cohort. Similarly, nearly half of older adults complain of difficulty sleeping.1 Studies have confirmed that several psychological and physical changes occur with normal aging. While older adults experience changes in sleep architecture, age itself does not result in disturbed sleep.2 However, the ability to get needed sleep does decrease with age.1 Age-related risk factors for poor sleep include psychiatric illnesses, life changes (eg, retirement, bereavement, decreased social interactions), environmental changes (eg, placement in a nursing home) and polypharmacy.1 In turn, poor sleep quality is considered a risk factor for age-related morbidity (eg, cardiovascular disease, diabetes, dementia, depression, pain) and mortality.3–5
Sleep disorders and chronic pain are both tied to significant reductions in quality of life in older adults. A recent meta-analysis estimated the pooled prevalence of sleep disorders to be 44% among adults with chronic pain.6 Although the link between sleep and pain has been widely established,7,8 the mechanisms underlying this relationship have yet to be fully elucidated. Different reports have pointed toward the potential role of endogenous pain modulation, inflammation, affect, mood and other states such as emotional distress or catastrophizing as possible mediators.9 A growing literature has identified brain morphometric changes in chronic pain10–17 Among these, Alshuft and colleagues assessed cortical thickness in persons with chronic knee osteoarthritis and found a negative association between pain duration and cortical thickness mainly in fronto-temporal areas.11 Further, studies reporting brain changes in people with sleep disorders18–25 are increasing. For example, in a longitudinal analysis in cognitively normal older adults,26 those participants who reported average sleep duration <7 h exhibited higher rates of subsequent thinning in the superior temporal sulcus and gyrus, inferior and middle frontal gyrus, and superior frontal sulcus of the left hemisphere, and in the superior frontal gyrus of the right hemisphere. Similar results were reported by Suh and colleagues27 where compared to good sleepers, cortical thinning was found in persistent insomnia symptoms in the anterior cingulate cortex, precentral cortex, and lateral prefrontal cortex. Given the evidence of the independent impact of both conditions on cortical thickness, we propose the brain is an important mediator of the sleep-pain relationship (see Figure 1). To our knowledge, only one study to date has reported how acute sleep deprivation negatively impacts pain sensitivity specifically via its impact on brain function in younger individuals.28 However, similar relationships have not been examined in older adults.
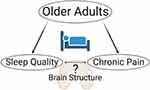 |
Figure 1 Mechanistic working model. |
Therefore, the aim of the present study was to explore the relationship between sleep quality and clinical pain, as well as somatosensory and pain threshold measures in community-dwelling older adults. Further, we explored brain cortical structures that may contribute to this association. We employed quantitative sensory testing (QST) to complement the subjective self-reported pain experience and to noninvasively assess sensory and pain perception pathway function that may help us understand potential pathophysiological mechanisms. The primary hypothesis of the present study was that sleep quality, as measured with the Pittsburgh Sleep Quality Index (PSQI),29 would be significantly associated with measures of self-reported pain as well as experimental somatosensory and pain threshold measures using quantitative sensory testing. Based on previous studies, we also hypothesized that cortical thickness would be negatively correlated with pain and PSQI, specifically in the fronto-temporal and somatosensory regions.
Materials and Methods
Participants
Older adults (age >60 years) who were native English speakers were recruited as part of an ongoing study at the University of Florida (NEPAL: Neuromodulatory Examination of Pain and Mobility Across the Lifespan). Recruitment of participants was accomplished using newspaper and flyers at UF and around the Gainesville community soliciting older participants to study how the brain changes with age. Interested individuals were screened over the phone and again in person to establish preliminary eligibility. Participants were excluded for any of the following conditions: 1) serious psychiatric conditions (eg, schizophrenia, major depression, bipolar disorder); 2) self-reported history of alcohol or drug abuse in the past; 3) Alzheimer’s, Parkinson’s, Epilepsy and other known intra-cerebral pathology; 4) significant cognitive impairment as evidenced by the Modified Mini-Mental State Examination [3 MS] score ≤77; 5) hospitalizations for mental health reasons in the past year; 6) chronic or current use of narcotic medications; 7) serious systemic (uncontrolled diabetes self-reported HbA1C >7), neurological, or cardiovascular disease (uncontrolled hypertension >155/90 mm Hg); 8) systemic rheumatic disorders (ie, rheumatoid arthritis, systemic lupus erythematosus, fibromyalgia); 9) self-reported HIV or AIDS; 10) MRI contraindications (eg, aneurysm clip, cardiac pacemaker, implanted cardioverter defibrillator (ICD), electronic implant or device, magnetically activated implant or device; artificial or prosthetic limb, metallic stent, filter, or coil, any metallic fragment or foreign body, wire mesh implant, surgical staples, clips, or metallic sutures, joint replacement (hip, knee, etc.); 11) excessive anxiety regarding protocol procedures; and 12) inability to consent for study participation. All procedures were conducted in accordance with the declaration of Helsinki and reviewed and approved by the University of Florida’s Institutional Review Board; in addition, all participants provided informed consent prior to undergoing further screening and any experimental procedures. Participants attended separate experimental sessions (health assessment session (HAS), quantitative sensory testing (QST) and neuroimaging). Only measures relevant to the study hypotheses are included and presented below. This is a secondary data analysis as we have previously reported detailed information from our Nepal participants.30,31
Health Assessment Session
A clinical research coordinator obtained written informed consent followed by demographic and general health and pain history information containing non-pathological personal history (ie, diet, exercise and toxic habits like smoking, alcohol, and caffeine use) and personal pathological history (ie, existing systemic diseases, existing sleep disorders like obstructive sleep apnea, restless legs syndrome, narcolepsy and insomnia; previous surgeries or hospitalizations and all medications taken). For the present study, participants that reported any medical condition in the health history questionnaire related to any sleep disorder were excluded. During this session, we also assessed the following constructs of interest: self-reported pain, global cognition, depression and sleep quality.
Participants were categorized as having chronic pain if they reported pain on most days during the preceding 3 months. When pain was reported during the clinical pain history interview, participants drew in a validated body manikin32 the anatomical pain regions where they had experienced pain (head/face, neck, shoulders, arms, hands, chest, stomach, upper and lower back, legs, knees, and feet) including ratings of their worst pain intensity on average using a numerical rating scale (NRS) and their pain duration. We also assessed pain interference during walking, using stairs, in bed, sitting or lying, and standing.33
Global Cognition
The Montreal Cognitive Assessment (MoCA)34 was administered to assess global cognitive abilities including short-term memory, orientation, executive function, language abilities, animal naming, abstraction, attention, clock-drawing test. Scores on the MoCA range from zero to 30, with a score of 26 and higher generally considered normal global cognition.
Depressive Symptoms
The Center for Epidemiologic Studies Depression Scale (CES-D)35 includes twenty items comprising six scales reflecting major facets of depression: depressed mood, feelings of guilt and worthlessness, feelings of helplessness and hopelessness, psychomotor retardation, loss of appetite, and sleep disturbance.
Sleep Quality
At the end of the session, subjects were provided with a copy of the PSQI questionnaire, for them to complete at their convenience and return at their next visit. The instrument is used to measure the quality and patterns of sleep in seven domains: C1: subjective sleep quality, C2: sleep latency (ie, the time it takes to fall asleep), C3: sleep duration, C4: habitual sleep efficiency (the ratio of total sleep time to time in bed), C5: sleep disturbances, C6: the use of sleep-promoting medication (ie, prescribed or over-the-counter), and C7: daytime dysfunction over the last month. Each of the seven domains is a 0 to 3 scale. The sum of the components produces a global score ranging from 0 to 21, where a higher score indicates worse sleep quality. For the present study, those participants who checked question 5j of the PSQI questionnaire because they reported trouble sleeping due to sleep apnea or the use of machine for apnea were excluded (n=1).
Quantitative Sensory Testing (QST) Session
As previously reported,30,31 all QST procedures were performed in a quiet room (temperature 21°C-23°C) with subjects seated in a comfortable chair with armrests and a semi-reclining back. An overview of the testing procedures was explained to the subject and for each different modality, specific instructions were delivered immediately before beginning the test. Measurement of each threshold type was first demonstrated, and at least one practice trial was conducted to ensure that subjects understood the testing procedures. Vibratory and thermal detection and pain threshold measurements were obtained with the TSA-II Neurosensory Analyzer and accompanying software (Medoc Ltd., Ramat Yishai, Israel) tested at the thenar eminence of the hand and at the first metatarsal head of the foot. The method of limits was used to obtain all detection thresholds.
Vibration Detection Thresholds
The handheld VSA-3000 circular probe (contact tip = 1.22 cm2) of the Medoc system was used to measure vibratory thresholds for a 100 Hz stimulus frequency. Subjects were asked to indicate as soon as they felt the vibratory sensation. Three trials, separated by ~10 sec each, began at 0 μm at a rate of 0.5 μm/sec and increased until the subject indicated that the stimulus was felt or until the maximum amplitude of 130 μm was reached. The mean value across the three trials was calculated as the vibratory detection threshold for each site.
Thermal Detection Thresholds
A 30 mm × 30 mm thermode connected to the TSA-II Neurosensory Analyzer was used to deliver thermal stimuli. Each trial began at 32°C, and the temperature decreased (for cool) or increased (for warm) at a rate of 1°C/second until the subject perceived the stimulus or until the stimulus reached the cutoff value (0°C for cool and 50°C for warm). Each trial was separated by approximately 10 seconds. The average of threshold temperatures across 4 trials was calculated as the thermal detection threshold for each modality and test site.
Thermal Pain Thresholds
Subjects were instructed to indicate as soon as the sensation changed from “just being cold to being painfully cold” or from “just being hot to being painfully hot.” Each trial began at 32°C and was either decreased (for cold pain) or increased (for heat pain) at a rate of 1°C/sec until pain threshold was reached or the cutoff value was reached (0°C for cold pain and 50°C for heat pain). Each trial was separated by at least 20 sec. The mean across three trials at each test site was calculated for both the heat and cold pain detection threshold.
Pressure Pain Thresholds
An AlgoMed computerized pressure algometer (Medoc Ltd., RamatYishai, Israel) was used to deliver calibrated pressure through a 10mm rubber tip. Testing was done on the right trapezius and right quadriceps muscles with the order of testing sites randomized and counter-balanced. Pressure applied to each site was increased at a constant rate of 1kg/s until participants clicked a button to indicate when the stimulus “first became painful” and the threshold was recorded. This procedure was repeated 3 times to obtain an average pressure pain threshold for each test site.
Neuroimaging Session
Neuroimaging data were collected at the University of Florida’s McKnight Brain Institute on the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility’s Philips (Best, the Netherlands) 3-Tesla scanner using a 32-channel radiofrequency coil. A high-resolution, T1-weighted, turbo field echo, anatomical scan was collected using the following parameters: repetition time = 7.1 ms, echo time = 3.2 ms, 170 slices acquired in a sagittal orientation, flip angle = 8 deg., resolution = 1 mm isotropic. Head movement was minimized via cushions positioned inside the head coil and instructions to participants.
MRI Data Preprocessing
The structural MRI data preprocessing was performed using the CAT12 Toolbox (http://dbm.neuro.uni-jena.de/cat/) in SPM12 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/), and was implemented on MATLAB software (MathWorks, Natick, Massachusetts, USA). Preprocessing was performed by the CAT 12 Toolbox under the default settings. Firstly, all the T1-weighted anatomical images were manually reoriented in order to place the anterior commissure at the origin of the 3D Montreal Neurological Institute (MNI) space. The images were then segmented into gray matter, white matter, and cerebrospinal fluid.36 Next, they were normalized to the MNI space by using a diffeomorphic non-linear registration algorithm (diffeomorphic anatomical registration through exponentiated lie algebra toolbox-DARTEL).37 The final resulting voxel size was 1.5 mm × 1.5 mm × 1.5 mm. For quality assurance, the resulting images were checked for homogeneity. Given the high correlation values (>0.85) of every image, none were discarded.
Data and Statistical Analysis
Data were entered by one experimenter and checked for accuracy by a blinded experimenter. Quantitative sensory testing data were z-transformed for each modality at each test site and then combined for analysis because of the multicollinearity within mechanical, thermal and pain modalities. Thus, 4 standardized Z-scores were created for vibratory detection, thermal detection, thermal pain, and pressure pain thresholds and used for further statistical analysis. The combination of these modalities is appropriate based on the physiological properties of sensory channels. To compare the clinical and demographic characteristics between subjects with chronic pain and without chronic pain, we used Student’s t-tests for continuous variables, and Chi-square test for categorical variables. Spearman rank correlations were used to examine pair-wise associations between PSQI variables given the ordinal nature of the PSQI total and individual component scores. We explored pair-wise associations between the individual PSQI subscales because of the limited existing literature using the PSQI in relatively healthy community-dwelling older samples like participants in the present study. Since global cognition (ie, MoCA scores) were significantly different between the groups, it was included as a covariate in all subsequent analyses. We performed a logistic regression using maximum likelihood estimates to determine which PSQI components were significantly predictive of self-reported pain (dichotomous dependent variable) adjusted for global cognition (ie, MoCA). To facilitate variable selection, we employed the automatic forward stepwise procedure, which starts with no variables in the model and each step, the most significant variable is entered. At each step the procedure examines the variables included for entry and removal until all variables in the model fulfill the criteria for retention (alpha level = 0.10). The regression coefficient is represented as log odds ratio per unit change of a particular variable, where an odds ratio (OR) indicates the probability of being in the chronic pain group versus being in the non-pain group. Finally, partial correlations adjusting for global cognition (ie, since MoCA scores were significantly different between the groups) were used to examine the associations between total PSQI and individual component scores with clinical pain, somatosensory and pain threshold variables. All tests were two-tailed and we report both uncorrected (i.e, p = 0.05) as well as corrected probability values (ie, corrected p = 0.05) accounting for multiple comparisons applying the Holm-Bonferroni method38 using the calculator by Gaetano.39
Cortical Thickness Analysis
Surface-based analysis was performed using the CAT12 Toolbox using a fully automated method that allows for the measurement of cortical thickness.40 To repair the topological defects, a spherical harmonic method41 was used to reparametrize the cortical surface mesh on the basis of an algorithm that reduces area distortions.42 Prior to the statistical analyses, the individual cortical thickness maps were smoothed by using a Gaussian filter with full-with a half-maximum of 15 mm. In addition, vertex-wise general linear models were fitted to the individual maps, and a multiple regression analysis was performed on the individual cortical thickness maps. The PSQI total score was then included in the design matrix. For the regression analyses, a non-parametric permutation test with 10,000 random permutations was performed. Threshold-Free Cluster Enhancement (TFCE)43 was used to identify the brain regions significantly correlated with PSQI total score. The statistical significance threshold was set at p < 0.05, FWE-corrected, which is a conservative procedure that reduces false positives, allowing for correction for multiple comparisons across space using permutation testing. The anatomical locations of the significant clusters were determined with reference to the Desikan-Killiany atlas.44
Mediation Analysis
A mediation analysis was conducted to test the total indirect effect of sleep quality on pain through cortical thickness measures. To control Type I error only the cortical thickness regions that were statistically significant in the TFCE analyses were included in the mediation. Given that global cognition (ie, MoCA) was significantly different between the pain groups, the mediation analysis also controlled for MoCA scores. We used bootstrapping procedures (n=5000) to obtain estimates and confidence intervals around the indirect effects to overcome potential problems caused by unmet assumptions in mediation analysis.45 We used the Hayes PROCESS macro model445 that provides modern methods for inference about indirect effects including bootstrapped confidence intervals. Given our small sample size for estimating classical mediation effects, we used the percentile bootstrapped confidence intervals for inference about the indirect effects, as its performance is relatively invulnerable to small sample sizes and potential outliers.
Results
Clinical and Demographic Characteristics
A total of 186 individuals were screened over the telephone, and ultimately 85 individuals underwent structural neuroimaging (T1). Of those participants, a subset (n=69) completed the PSQI questionnaire and comprise the present study sample. As shown in Table 1, 60.86% of participants were female and 66.6% reported chronic musculoskeletal pain (ie, pain on most days during the past 3 months). Our participants reported worst pain locations most likely of musculoskeletal origin in the upper and lower back (39%), in the legs, knees, and feet (26%), in the arms and hands (17%), in the neck and shoulders (13%). Two participants reported pain in the head and chest/frontal region of the body (5%). Most pain participants (41%) reported 3 or more pain problems, while 30% and 28% reported two and one pain problems, respectively. There were no significant differences regarding demographic characteristics between participants with and without chronic musculoskeletal pain. MoCA scores were significantly lower among individuals reporting chronic pain (M = 25.93, SD = 3.06) compared to those not reporting chronic pain (M = 27.39, SD = 1.72; t (67) = 2.12, p = 0.038 two-tailed).
 |
Table 1 Demographic and Clinical Characteristics of the Sample (n=69) |
PSQI Characteristics
Table 2 shows the descriptive PSQI information for our sample. Table 3 summarizes associations between individual PSQI component scores and their associations with the total PSQI score. As expected, total PSQI score was significantly associated with each of the component scores, albeit weakly with component C5, and there were less consistent associations between the individual component scores.
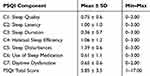 |
Table 2 Descriptive Statistics of the PSQI and Its Component Scores in Our Study Sample |
 |
Table 3 Intercorrelation Matrix Among the PSQI Components (n=69) |
PSQI Components and Presence of Pain
We performed a stepwise logistic regression to determine which of the seven PSQI components (ie, C1 to C7) were significantly predictive of self-reported pain during the past 3 months (ie, dichotomous dependent variable) while controlling for MoCA score given the significant group differences in MoCA reported above. The final model was statistically significant, χ2 (8df, n = 69) = 21.4, p = 0.006. The model as a whole explained between 26.8% (Cox and Snell R2) and 37.2% (Nagelkerke R2) of the variance in self-reported pain status, and correctly classified 78.3% of cases. For this model, C5 (ie, Sleep disturbance) was the only independent variable statistically significant (exp(β) = 5.3, p=0.027) suggesting participants with sleep disturbances had 5 times higher odds to report pain during the past 3 months compared to those who did not report sleep disturbance (ie, per unit increase in C5).
PSQI Components and Clinical Pain Severity
Partial correlations adjusted for MoCA were performed to examine the association between sleep quality with clinical pain measures. Total PSQI and the PSQI component scores (ie, C2, C3, C5, C7) were significantly correlated with self-reported pain measures (see Table 4). These results did not change significantly after correcting for multiple comparisons.
 |
Table 4 Associations Between Total PSQI Score and the PSQI Components with Self-Reported Pain and Somatosensory and Pain Threshold Measures (n=69) |
PSQI Components and Somatosensory and Pain Thresholds
Partial correlations adjusted for MoCA were performed to examine the association between sleep quality with somatosensory and pain thresholds. Total PSQI and the PSQI component scores (ie, C2, C3, C5, C7) were not significantly correlated with these experimental measures (see Table 4).
Total PSQI and Brain Cortical Thickness
A subset of our participants who returned the PSQI questionnaire also completed a structural MRI (n=49). Using a non-parametric threshold-free cluster enhancement (TFCE) approach, higher PSQI total scores reflective of worse sleep quality were significantly associated with lower cortical thickness in the precentral, postcentral, precuneus, superior parietal, and lateral occipital regions (TFCE FWE-corrected at p < 0.05, Figure 2). Further, the indirect effect of PSQI and self-reported pain through cortical thickness was tested using a percentile bootstrap estimation approach with 5000 samples (Shrout & Bolger, 2002), while controlling for MoCA (see Figure 3). These results indicated that only the indirect coefficient (a×b) for the postcentral cortical thickness was significant, B = −0.089, SE = 0.05, CI = −0.170, −0.004; with total effect c (B = 8.45, SE = 4.6, CI = 0.757, 16.14) and direct effect c’ (B = 0.275, SE = 0.12, CI = 0.071, 0.478) also being significant, although the latter total and direct effects were not of interest in the present study.
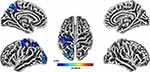 |
Figure 2 Cortical thickness was negatively associated with total PSQI scores (FWE-corrected values after applying threshold-free cluster enhancement (TFCE, p < 0.05)). |
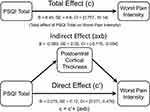 |
Figure 3 Postcentral cortical thickness mediated the association between PSQI total and worst pain intensity. |
Discussion
The present study sought to elucidate the association of sleep quality with self-reported pain and somatosensory and pain thresholds in older adults and further explore the potential mediating role of the brain in this association. As hypothesized, overall sleep quality was significantly associated with self-reported pain intensity, but not laboratory measures. In addition, reduced cortical thickness in fronto-temporal brain regions was negatively associated with overall sleep quality. Further, reduced cortical thickness in the primary somatosensory cortex (ie, postcentral gyrus) mediated the association between sleep quality and pain.
In our sample of community-dwelling older adults, we found that sleep quality was associated with self-reported pain measures. Specifically, our results revealed that those participants reporting trouble getting to sleep, sleeping less than usual, reporting restless sleep and a greater daytime dysfunction also reported greater pain severity, a greater number of painful sites (ie, multisite pain) and more pain while performing daily activities (ie, walking, using stairs, while in bed, sitting or lying, and standing on a daily basis). This is consistent with previous studies in the older population,46 including a recent systematic review where worse sleep was associated with an increased risk of developing a pain condition and worse physical functioning.47 One of the major insights from a recent longitudinal study was that sleep deficiency predicted any pain, multiple pain locations, and pain-related disability, independent of known risk factors for pain.48 Contradictory to studies in younger samples,7,8 we did not find any significant associations between sleep and somatosensory and pain threshold measures. Besides age, it is possible that the low levels of pain and sleep dysfunction in our sample may explain our findings. For example, QST has been examined in individuals with diagnosed insomnia and/or reporting severe chronic pain, while our sample is from the community mostly experiencing mild-to-moderate pain severity and without a diagnosed sleep condition. Future work is needed to examine laboratory somatosensory and pain measures and sleep in various samples to replicate these findings.
Our study also aimed to explore potential associations between cortical thickness and sleep quality. We found that worse sleep quality was associated with lower cortical thickness in the precentral, postcentral, precuneus, superior parietal, and lateral occipital brain regions. These findings are consistent with the emerging literature where evidence of changes in the brain’s gray matter were related to the presence of sleep disorders.21–23,27,49 Still, we cannot directly compare results from previous studies including samples with diagnosed sleep disorders to our generally healthy individuals without any sleep disorder diagnoses. In general, there is a paucity of studies in the literature showing an association between sleep quality and cortical thickness in healthy older individuals. However, our aim was to examine cortical thickness as a potential mechanism linking self-reported pain and sleep quality. Only primary somatosensory cortical thickness significantly mediated the association between sleep quality and self-reported pain. Our findings are consistent with results from the only study to date that has examined the underlying brain and behavioral mechanisms explaining the sleep-pain association.28 Although using fMRI during pain stimulation, Krause and colleagues found that acute sleep deprivation significantly increased pain-evoked activation within the primary somatosensory cortex in healthy younger individuals without chronic pain. Further, both results are consistent with the idea that brain areas involved in somatosensation and pain processing are more tightly linked with sleep than other areas like those involved in movement or reasoning. This makes behavioral sense given our need to wake up to a sensory stimulus (eg, an alarm clock or pain that is potentially dangerous). Indeed, there is mechanistic evidence both in animals50 and humans51 linking somatosensory cortex function with sleep regulation and deprivation. Thus, it is plausible that nervous system changes along the nociceptive pathway including the somatosensory cortex51 and sensory thalamus,50 underlie the bidirectional interaction between chronic pain and sleep dysfunction. Future mechanistic investigations are needed both in animals and humans to replicate our findings and systematically evaluate these associations.
The present findings should be interpreted in light of some study limitations. First, our observational, cross-sectional study design does not support conclusions regarding temporal directionality or causality; thus, future intervention studies are needed. Second, evaluation of sleep in this study was based on self-report, and this may differ from findings using objective sleep measures, such as polysomnography or actigraphy, which would provide a more sensitive exploration of the reciprocal relationship between pain and sleep. Third, our sample consisted of healthy older adults reporting very good sleep quality, on average 5 out 17 on PSQI, where scores below 5 are considered good sleep quality. Thus, future work should include older individuals with worse sleep quality. Fourth, our sample size in those with chronic pain is nearly double that without chronic pain and this could represent a potential selection bias. To decrease this bias in our study, most of our study advertisements were specifically geared towards the study of brain aging processes over time in younger and older adults. Indeed, pain categorization occurred in a post-hoc fashion and participants were informed that the Nepal study was aimed to examine the brain, thinking and memory function, pain sensitivity and walking ability, without giving a special emphasis to pain. Despite these limitations, our study provides novel evidence about the inherent role of the brain in the interaction between sleep and chronic pain in older adults.
Conclusion
To our knowledge, this is the first investigation implicating cortical thickness as a mediator of the association between sleep quality and chronic pain in older individuals. Further mechanistic understanding of the complex, bidirectional relationship between sleep and pain may provide alternative therapeutic targets for treating sleep dysfunction and/or chronic pain conditions, which are both highly prevalent in our aging population. Given that a recent study found sleep improvements after a cognitive behavioral therapy targeted to insomnia in patients with fibromyalgia was associated with increased cortical thickness in several frontal brain regions,52 our findings may have potential clinical implications that are worth exploring in older samples.
Acknowledgments
This work was supported by the NIH (NIA grants K01AG048259, R01AG059809, R01AG067757 to YCA, K01AG050707 to AJW; NIAAA grant K01AA025306 to ECP), the University of Florida Claude D. Pepper Center (P30AG028740), and the University of Florida McKnight Brain Research Foundation and Center for Cognitive Aging and Memory. The authors are also grateful to our older community volunteers for their participation in the NEPAL study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult – a mini-review. Gerontology. 2010;56(2):181–189. doi:10.1159/000236900
2. Roepke SK, Ancoli-Israel S. Sleep disorders in the elderly. Indian J Med Res. 2010;131:302.
3. Gangwisch JE, Heymsfield SB, Boden-albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large US sample. Sleep. 2007;30:1667. doi:10.1093/sleep/30.12.1667
4. Shi L, Chen SJ, Ma MY, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. doi:10.1016/j.smrv.2017.06.010
5. Yin J, Jin X, Shan Z, et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: a systematic review and dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc. 2017;6(9). doi:10.1161/JAHA.117.005947
6. Mathias JL, Cant ML, Burke ALJ. Sleep disturbances and sleep disorders in adults living with chronic pain: a meta-analysis. Sleep Med. 2018;52:198–210. doi:10.1016/j.sleep.2018.05.023
7. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi:10.1016/J.JPAIN.2013.08.007
8. Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–132. doi:10.1016/S1087-0792(03)00044-3
9. Herrero Babiloni A, De Koninck BP, Beetz G, De Beaumont L, Martel MO, Lavigne GJ. Sleep and pain: recent insights, mechanisms, and future directions in the investigation of this relationship. J Neural Transm. 2019. doi:10.1007/s00702-019-02067-z
10. Frøkjær JB, Bouwense SAW, Olesen SS, et al. Reduced cortical thickness of brain areas involved in pain processing in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2012;10(4):434–438.e1. doi:10.1016/j.cgh.2011.11.024
11. Alshuft HM, Condon LA, Dineen RA, Auer DP, Mouraux A. Cerebral cortical thickness in chronic pain due to knee osteoarthritis: the effect of pain duration and pain sensitization. PLoS One. 2016;11(9):1–16. doi:10.1371/journal.pone.0161687
12. Erpelding N, Moayedi M, Davis KD. Cortical thickness correlates of pain and temperature sensitivity. Pain. 2012;153(8):1602–1609. doi:10.1016/j.pain.2012.03.012
13. Jiang Z, Dinov ID, Labus J, et al. Sex-related differences of cortical thickness in patients with chronic abdominal pain. PLoS One. 2013;8(9). doi:10.1371/journal.pone.0073932
14. Borsook D, Upadhyay J, Chudler EH, Becerra L. A key role of the basal ganglia in pain and analgesia – insights gained through human functional imaging. Mol Pain. 2010;6:27. doi:10.1186/1744-8069-6-27
15. Ruscheweyh R, Deppe M, Lohmann H, et al. Pain is associated with regional grey matter reduction in the general population. Pain. 2011;152(4):904–911. doi:10.1016/j.pain.2011.01.013
16. Schwedt TJ, Chong CD, Antal A. Correlations between brain cortical thickness and cutaneous pain thresholds are atypical in adults with migraine. PLoS One. 2014;9(6):e99791. doi:10.1371/journal.pone.0099791
17. Vachon-Presseau E, Tétreault P, Petre B, et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 2016;139(7):1958–1970. doi:10.1093/brain/aww100
18. Amorim L, Magalhães R, Coelho A, et al. Poor sleep quality associates with decreased functional and structural brain connectivity in normative aging: a MRI multimodal approach. Front Aging Neurosci. 2018;10. doi:10.3389/fnagi.2018.00375
19. Kang JM, Joo SW, Son YD, et al. Low white-matter integrity between the left thalamus and inferior frontal gyrus in patients with insomnia disorder. J Psychiatry Neurosci. 2018;43(6):366–374. doi:10.1503/jpn.170195
20. Lee YJG, Kim S, Kim N, et al. Changes in subcortical resting-state functional connectivity in patients with psychophysiological insomnia after cognitive–behavioral therapy: changes in resting-state FC after CBT for insomnia patients. Neuro Image Clin. 2018;17(July2017):115–123. doi:10.1016/j.nicl.2017.10.013
21. Cross NE, Memarian N, Duffy SL, et al. Structural brain correlates of obstructive sleep apnoea in older adults at risk for dementia. Eur Respir J. 2018;52(1):1800740. doi:10.1183/13993003.00740-2018
22. Yeung AWK. Morphometric and functional connectivity changes in the brain of patients with obstructive sleep apnea: a meta-analysis. J Sleep Res. 2019;(January):1–10. doi:10.1111/jsr.12857
23. Joo EY, Noh HJ, Kim J-S, et al. Brain gray matter deficits in patients with chronic primary insomnia. Sleep. 2013;36(7):999–1007. doi:10.5665/sleep.2796
24. Scullin MK. Do older adults need sleep? A review of neuroimaging, sleep, and aging studies. Curr Sleep Med Rep. 2017;3(3):204–214. doi:10.1007/s40675-017-0086-z
25. Sexton CE, Storsve AB, Walhovd KB, Johansen-Berg H, Fjell AM. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology. 2014;83(11):967–973. doi:10.1212/WNL.0000000000000774
26. Spira AP, Gonzalez CE, Venkatraman VK, et al. Sleep duration and subsequent cortical thinning in cognitively normal older adults. Sleep. 2016;39(5):1121–1128. doi:10.5665/sleep.5768
27. Suh S, Kim H, Dang-Vu TT, Joo E, Shin C. Cortical thinning and altered cortico-cortical structural covariance of the default mode network in patients with persistent insomnia symptoms. Sleep. 2016;39(1):161–171. doi:10.5665/sleep.5340
28. Krause AJ, Prather AA, Wager TD, Lindquist MA, Walker MP. The pain of sleep loss: a brain characterization in humans. J Neurosci. 2019;39(12):2291–2300. doi:10.1523/JNEUROSCI.2408-18.2018
29. Buysse Charles F, Reynolds Ill DJ, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
30. Lysne P, Cohen R, Hoyos L, Fillingim RB, Riley JL, Cruz-Almeida Y. Age and pain differences in non-verbal fluency performance: associations with cortical thickness and subcortical volumes. Exp Gerontol. 2019;126(November2018):110708. doi:10.1016/j.exger.2019.110708
31. Cruz-Almeida Y, Fillingim RB, Riley JL, et al. Chronic pain is associated with a brain aging biomarker in community-dwelling older adults. Pain. 2019;160(5):1119–1130. doi:10.1097/j.pain.0000000000001491
32. Cruz-Almeida Y, Martinez-Arizala A, Widerström-Noga EG. Chronicity of pain associated with spinal cord injury: a longitudinal analysis. J Rehabil Res Dev. 2005;42(5):585–594. doi:10.1682/JRRD.2005.02.0045
33. Bilbao A, Quintana JM, Escobar A, Las Hayas C, Orive M. Validation of a proposed WOMAC short form for patients with hip osteoarthritis. Health Qual Life Outcomes. 2011;9:7–10. doi:10.1186/1477-7525-9-75
34. Koski L. Validity and applications of the montreal cognitive assessment for the assessment of vascular cognitive impairment. Cerebrovasc Dis. 2013;36(1):6–18. doi:10.1159/000352051
35. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi:10.1177/014662167700100306
36. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi:10.1016/j.neuroimage.2005.02.018
37. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi:10.1016/j.neuroimage.2007.07.007
38. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70.
39. Gaetano J. Holm-Bonferroni sequential correction: an EXCEL calculator. 2013. doi:10.13140/RG.2.1.4466.9927
40. Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. Neuroimage. 2013;65:336–348. doi:10.1016/j.neuroimage.2012.09.050
41. Yotter RA, Nenadic I, Ziegler G, Thompson PM, Gaser C. Local cortical surface complexity maps from spherical harmonic reconstructions. Neuroimage. 2011;56(3):961–973. doi:10.1016/j.neuroimage.2011.02.007
42. Yotter RA, Thompson PM, Gaser C. Algorithms to improve the reparameterization of spherical mappings of brain surface meshes. J Neuroimaging. 2011;21(2):134–147. doi:10.1111/j.1552-6569.2010.00484.x
43. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi:10.1016/j.neuroimage.2008.03.061
44. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi:10.1016/j.neuroimage.2006.01.021
45. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach.
46. Chen Q, Hayman LL, Shmerling RH, Bean JF, Leveille SG. Characteristics of chronic pain associated with sleep difficulty in older adults: the maintenance of balance, independent living, intellect, and zest in the elderly (MOBILIZE) boston study. J Am Geriatr Soc. 2011;59(8):1385–1392. doi:10.1111/j.1532-5415.2011.03544.x
47. Afolalu EF, Ramlee F, Tang NKY. Effects of sleep changes on pain-related health outcomes in the general population: a systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med Rev. 2018;39:82–97. doi:10.1016/j.smrv.2017.08.001
48. Chen TY, Lee S, Schade MM, Saito Y, Chan A, Buxton OM. Longitudinal relationship between sleep deficiency and pain symptoms among community-dwelling older adults in Japan and Singapore. Sleep. 2019;42(2):1–11. doi:10.1093/sleep/zsy219
49. Macey PM, Haris N, Kumar R, Thomas MA, Woo MA, Harper RM. Obstructive sleep apnea and cortical thickness in females and males. PLoS One. 2018;13(3):e0193854. doi:10.1371/journal.pone.0193854
50. Fernandez LMJ, Vantomme G, Osorio-Forero A, Cardis R, Béard E, Lüthi A. Thalamic reticular control of local sleep in mouse sensory cortex. Elife. 2018;7:1–25. doi:10.7554/eLife.39111
51. Gorgoni M, Ferlazzo F, Moroni F, et al. Sleep deprivation affects somatosensory cortex excitability as tested through median nerve stimulation. Brain Stimul. 2014;7(5):732–739. doi:10.1016/j.brs.2014.04.006
52. McCrae CS, Mundt JM, Curtis AF, et al. Gray matter changes following cognitive behavioral therapy for patients with comorbid fibromyalgia and insomnia: a pilot study. J Clin Sleep Med. 2018;14(9):1595–1603. doi:10.5664/jcsm.7344
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
