Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 14
Correlations Between TIMD-4 Gene Variants and the Risk and Clinical Features of Rheumatoid Arthritis in a Chinese Population
Authors Yang Z, Liu S, Zhou L, Zhang H , Xu N
Received 23 October 2020
Accepted for publication 11 January 2021
Published 9 February 2021 Volume 2021:14 Pages 221—228
DOI https://doi.org/10.2147/PGPM.S287688
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Zhicheng Yang,1,* Shuaikun Liu,2,* Li Zhou,1 Hui Zhang,1 Nanwei Xu1
1Department of Orthopedics, The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University, Changzhou, People’s Republic of China; 2Graduate School, Dalian Medical University, Dalian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Zhang; Nanwei Xu
The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University, Changzhou, People’s Republic of China
Email [email protected]; [email protected]
Introduction: T-cell immunoglobulin and mucin domain-4 (TIMD-4) are likely to impact autoimmune diseases (e.g., rheumatoid arthritis (RA)). It is hypothesized here that TIMD-4 gene polymorphism is likely to display a correlation with the RA risk.
Methods: For examining the effect exerted by TIMD-4 genetic variant in the RA risk, a case–control study containing 379 RA cases and 432 healthy control groups in Chinese population was performed. This study conducted genotyping with the use of a custom-by-design 48-Plex single nucleotide polymorphism (SNP) Scan™ Kit. Blood serum conditions of TIMD-4 in RA cases and matched control groups were measured by enzyme-connected immunosorbent assay (ELISA).
Results: Our results demonstrated that the TIMD-4 rs7700944 polymorphism could increase the RA risk in Chinese population. According to stratification analyzing processes, the TIMD-4 rs7700944 polymorphism displayed the correlation to the elevated RA risk in the females, smokers and cases aged ≥ 55 years. Cross-over analyses also indicated that the combined effect of smoking or drinking and GA genotype of rs7700944 locus contributed to an elevated risk of RA. In addition, the TIMD-4 rs7700944 polymorphism was also related to RA cases with DAS ≥ 3.20, ESR ≥ 25.00 mm/h and positive anti-ccp. Moreover, compared with the control groups, the average expression level of TIMD-4 in the serum of RA cases was apparently increased.
Conclusion: In conclusion, the TIMD-4 rs7700944 polymorphism may increase the sensitivity to RA in Chinese population.
Keywords: TIMD-4, polymorphism, rheumatoid arthritis, Chinese population
Introduction
Rheumatoid arthritis (RA) refers to one multiple-factorial disease displaying a correlation to synovitis, progressive erosions, and cartilage destruction.1 The globally average prevalence of RA is about 0.5–1.0%, with genetic elements accounting for 60% of RA risk elements.2 Though etiology of RA is poorly understood, there is a consensus that inherited genetic elements may be involved in the development of RA when exposed to environmental triggers.3 Previous study identified that more than 30 genetic areas display a correlation to RA,4 with HLA-DRB1 being the strongest of numerous known genetic risk elements.5 Genome-wide correlation study (GWAS) by Leng et al demonstrated five new sensitivity loci displaying a correlation to RA risk and identified that comprehensive genetic study can provide important information for pathogenesis of RA.6 Gene polymorphism study is likely to clarify RA developing process, such as T-cell immunoglobulin and mucin domain-4 (TIMD-4).
On the whole, B and/or T cell hyperresponsive is likely to facilitate chronic tissue inflammatory process, i.e., a notable characteristic exhibited by autoimmune diseases.7 It is evidenced that TIMD-4 as phosphatidylserine (PS) receptor is capable of maintaining peritoneal macrophages’ homeostatic function8 and regulating adaptive immunity based on the alteration of Ag-particular T cells’ clearance.9 It is noteworthy that TIMD-4’s dysregulating process was identified under several autoimmune states, in particular inside immune cells.7 In addition, TIMD-4 was identified to be over-expressed in Systemic lupus erythematosus (SLE) cases.10 In addition, Abe et al revealed that TIMD-4 has two distinct functions depending on the stage of arthritis and anti-TIMD-4 treatment could inhibit the development of arthritis.11 Based on these observations, we guessed that TIMD-4 may have effects on autoimmune diseases by regulating T cells, e.g., RA and SLE.
Thus far, the TIMD-4 gene polymorphism is proved correlated to numerous diseases, covering childhood atopic dermatitis,12 asthma13,14 as well as RA.15–17 The SNP rs7700944, having the location inside the TIMD-4 gene’s intron region. Introns take up most non-coding DNA inside the genome of humans and have the locations close to coding areas, which may play an important role in genome evolution. They cover function-related vital sequences impacting gene regulating and evolving processes, as well as protein binding motifs regulating particular proteins’ changeable splicing. Polymorphisms in numerous SNPs with the location inside genes’ intron areas display a correlation to sensitivity to RA inside a range of populations.18,19 Previously, Xu et al found that the TIMD-4 rs7700944 gene polymorphism could increase the RA risk in the Ningxia Hui Autonomous Region of China.15 The allele frequency from different areas of China may have a relatively large distinction. In accordance with data from 1000 Genomes database, the frequency of allele A at the TIMD-4 rs7700994 locus was 10.8% and 19.4% in Xishuangbanna Chinese population and Beijing Chinese population, respectively. Therefore, we conducted this hospital-based case–control study involving 379 cases and 432 control groups to examine the correlation between the TIMD-4 rs7700944 polymorphism and RA sensitivity in other Chinese populations.
Cases and Methods
Study Subjects
Totally 379 rheumatoid arthritis (RA) cases were consecutively recruited from the Changzhou Second Hospital – Affiliated Hospital of Nanjing Medical University between September 2015 and October 2019. RA cases were diagnosed in accordance with the criteria for RA set by the American College of Rheumatology (1987).20 Clinical data with potential diagnostic value, e.g., age, sex, age at onset, disease duration, treatment duration, physicians presented function-related class, anti-cyclic peptide containing citrulline (anti-CCP), RA disease Activity Score (DAS28), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and rheumatoid factor (RF) were provided. The respective RA case received the interviewing based on trained personnel through a standardized questionnaire for determining the risk elements. The control groups were free of RA and recruited by the identical institutions in the identical period. The subjects who had other autoimmunological diseases, infectious diseases, a current or previous history of malignancy or a family history of autoimmune diseases were excluded. In brief, the controls were healthy subjects. The control groups were matched with patients by age (± 5 years) and sex. Smoker received the classification to be smoking at least one cigarette per day for at least 1 year. Alcohol consumer had the definition of drinking alcoholic beverages at least once a week for over 1 year. Informed consent was obtained from all cases and control groups prior to their participation. The protocol here gained the approval from the Ethics Committee by the Changzhou Second Hospital – Affiliated Hospital of Nanjing Medical University. This study was conducted in accordance with the Declaration of Helsinki.
DNA Extraction
For the exploration of TIMD-4 polymorphism, all participants of this study presented 2 mL of peripheral blood inside ethylenediaminetetraacetic acid (EDTA) tubes and then conducted the storing process at −80 °C for further application. DNA received the extraction with the use of the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany).
SNP Genotyping
The present study conducted SNP genotyping with the use of a custom-by-design 48-Plex SNP scanTM Kit (Genesky Biotechnologies Inc., Shanghai, China). The primers of rs7700994 polymorphism employed to achieve polymerase chain reacting process (PCR) referred to 5ʹ-CTTAGCTGGCTTAGGAGTAGC-3ʹ (forward); 5ʹ-GGTCCACGATAAAGGAGAAA-3ʹ (reverse). About 10% of the samples received the re-genotyping process for replicating existing outcomes, the 100% uniformity was obtained. The present study employed enzyme-linked immunosorbent assay (ELISA) Kit (Boster, Wuhan, China) for detecting the serum level of TIMD-4 in RA cases and healthy control groups.
Statistical Analyses
The demographic and clinically related features exhibited by participants of this study received the evaluation with the use of the Chi-square (χ2) test and Student’s t-test. The present study employed 95% confidence intervals (CIs) and odds ratios (ORs) for estimating the correlation of TIMD-4 rs7700944 polymorphism and RA risk by logistic regression analyses. This study carried out the stratification analyzing processes in accordance with age, sex, smoking and alcohol. Hardy–Weinberg equilibrium (HWE) for TIMD-4 genotype distributing results in control groups received the testing process with the use of the Chi-square (χ2) test. A cross-over analysis was used to assess the effects of the interactions between environmental factors, such as smoking and/or drinking, and genetic factors on the RA risk. The present conducted all statistics-related investigations with the use of the SAS software package (var. 9.1.3; SAS Institute, Cary, NC, USA). P < 0.05 exhibited statistically related significance.
Results
Clinical Parameters of the Study Population
The clinically related parameters regarding individuals for the case–control analysis are presented in Table 1. The subjects had the full matching in terms of sex and age (p =0.482 and 0.930, respectively). In addition, several clinically related parameters, covering function-related class, anti-CCP antibody, DAS28, RF, CRP, ESR, treatment duration, disease duration and onset age were also listed in the column of cases. HWE analysis revealed no distinction in the control group (P = 0.932). As revealed from the power analyses, the sample size of this study was enough to indicate the association between TIMD-4 gene polymorphism and RA risk.
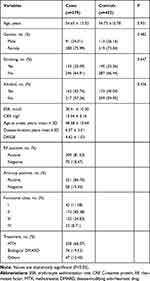 |
Table 1 Patient Demographics and Risk Factors in Rheumatoid Arthritis |
Correlation Between TIMD-4 Gene Polymorphism and RA Risk
Table 2 presents the TIMD-4 rs7700944 polymorphism’s genotyping frequencies in cases and control groups. The minor allele frequency of rs7700944 in the case group reached over that in the control. Taking the GG genotype as a reference group, GA genotype displayed a correlation to the elevated RA risk. (GA vs GG: OR, 1.37, 95% CI, 1.02–1.86, P = 0.039). A significant correlation was found in the dominant model (P = 0.017), not in the recessive model (P = 0.142). Lastly, according to the allelic model, the information suggested that A allele improved the sensitivity of RA as well (A vs G: OR, 1.38; 95% CI, 1.08–1.77; P = 0.011).
 |
Table 2 Logistic Regression Analysis of Associations Between TIMD-4 rs7700944 Polymorphism and Risk of Rheumatoid Arthritis |
Stratification analyzing processes were conducted in accordance with sex, age, smoking and alcohol (Table 3). Our results revealed that the TIMD-4 rs7700944 polymorphism displayed the correlation to the increased RA risk among the females, smokers and cases aged ≥ 55 years. In addition, the TIMD-4 rs7700944 polymorphism was also related to RA cases with DAS ≥ 3.20, ESR ≥ 25.00 mm/h and positive anti-ccp (Table 4). However, no significant relationship was found in the analyses of CRP, RF status and function-related class.
 |
Table 3 Stratified Analyses Between the TIMD-4 rs7700944 Polymorphism and the Risk of Rheumatoid Arthritis |
 |
Table 4 The Associations Between the TIMD-4 rs7700944 Polymorphism and Clinical Characteristics of Rheumatoid Arthritis |
Correlation of the TIMD-4 rs7700944 Polymorphism with the Serum TIMD-4 Levels
Data indicated that the average serum levels of TIMD-4 were significantly higher in RA cases compared with control groups (P < 0.01, Figure 1). Based on the TIMD-4 rs7700944 genotypes, we compared serum TIMD-4 level in RA cases and control groups, and found that there was no significant variation among different genotypes (Figure 1).
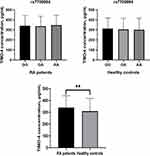 |
Figure 1 Associations among the serum level of TIMD-4 and genotype frequency in RA patients and healthy controls (**P < 0.01). |
Cross-over Analyses
We next analyzed the joint effects of the TIMD-4 rs7700944 polymorphism and either smoking or alcohol consumption on RA risk. Crossover analyses indicated that combined effect of smoking or drinking and GA genotype of rs7700944 locus contributed to an elevated risk of RA (Supplemental Table 1).
TIMD-4 Potential Gene–Gene Interactions
Several genes including BAI1. MFGE8, STAB2, GAS6, JMJD6, CD300LB, PPP1R3B, MERTK, PPP1R8, CMYA5 were involved in the interaction of TIMD-4 (Supplemental Figure 1), which was discovered by the String online tool ((http://string-db.org/).
Discussion
In the correlation analysis between the TIMD-4 rs7700944 polymorphism and RA, we found that the TIMD-4rs7700944 polymorphism displayed a correlation to the elevated RA risk in Chinese population. Furthermore, our results also revealed that the TIMD-4 rs7700944 polymorphism displayed the correlation to the increased RA risk among the females, smokers and cases aged ≥55 years. Crossover analyses also identified that combined effect of smoking or drinking and GA genotype of rs7700944 locus contributed to an elevated risk of RA. In addition, the TIMD-4 rs7700944 polymorphism was related to RA cases with DAS ≥ 3.20, ESR ≥ 25.00 mm/h and positive anti-ccp. Data indicated that the average serum levels of TIMD-4 were significantly higher in RA cases compared with control groups.
TIMD-4, with the major expression inside antigen-presenting cells, regulates T-cell activation and tolerance, in part by mediating the uptake and engulfment of apoptotic cells.21 Yeung et al found that TIMD-4 interaction with its putative ligand promotes Th2 responses.22 In addition, according to Albacker et al, TIMD-4-expressing cells regulate adaptive immunity by mediating the removal of PS-expressing apoptotic, Ag-particular T cells, which regulated the amount exhibited by Ag-particular T cells that remain after the clearance of Ag or infection.9 The blockade of PS receptor function contributed to lymphocyte activation and signs of systemic autoimmunity.21 On the whole, the immune systems related to the autoimmune disease course cover two aspects: in which the pathologically related procedure receives the driving from T cells; in which the humoral B response conducts the disorder mediation through the production of autoantibodies capable of forming immune complexes or to binding tissue self-antigens.23 For this reason, the correlation between the TIMD-4 gene polymorphism and autoimmune disease turns out to be the stress of public attention.
Three existing studies explored the correlation between the TIMD-4 gene polymorphism and RA, but with contradictory findings. In 2012, Xu et al identified the noticeable correlation of the TIMD-4 rs7700944 polymorphism with RA in Chinese population (214 cases and 211 control groups).15 The positive correlation was confirmed in the subsequent study among Egyptian population (128 cases and 125 control groups).17 This study also suggested that the TIMD-4 minor allele A could be viewed as an increased risk factor of RA, with an OR of 3.10 (240 cases and 240 control groups). However, no significant correlation was found in the Iranian population (120 cases and 120 control groups).16 The distinction can be explained in the following aspects. For one thing, the study of Mosaad et al achieved the minimum sample size.21 Small sample size exhibits insufficient statistics-related power, causing false-positive or false-negative results. For another, rs7700944 may have an ethnicity-particular effect. In accordance with information of 1000 Genomes database, allele A’s frequency at the TIMD-4 rs7700994 locus was 19.4% in Beijing Chinese population. Specific to the Chinese Han population, the risk allele A frequency was 16.8% (this study), which was close to the results in the database, indicating that the selection of samples in this study was representative. Given the mentioned, racial groups and sample size critically impact the detection of novel genetic correlation.
Though positive findings were observed, some limitations should be addressed. First, this study refers to one case–control study based on hospitals, and selection bias is inevitable. Second, gene–gene interacting processes are required for in-depth studies. Third, the potential systems of the TIMD-4 rs7700944 polymorphism impacting RA pathology require investigations.
To sum up, an SNP rs7700944 was reported here in the TIMD-4 gene to be a sensitivity locus for RA. Subsequent analyses using greater sample sizes and various racial groups are required for confirming the mentioned findings.
Data Sharing Statement
The data of this study are available from the corresponding authors Hui zhang and Nanwei Xu upon reasonable request.
Acknowledgment
Thanks to Yizhen Liu for his contribution to the article.
Funding
This study was supported in part by Science and Technology Development Fund of Nanjing Medical University (NMUB2019316), and the Changzhou Key Subject Fund (XK201603).
Disclosure
All authors report no conflicts of interest in this work.
References
1. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. doi:10.1056/NEJMra1004965
2. Sparks JA. Rheumatoid arthritis. Ann Intern Med. 2019;170(1):ITC1–ITC16. doi:10.7326/AITC201901010
3. Karlson EW, Deane K. Environmental and gene-environment interactions and risk of rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(2):405–426. doi:10.1016/j.rdc.2012.04.002
4. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi:10.1016/S0140-6736(10)60826-4
5. Raychaudhuri S, Sandor C, Stahl EA, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet. 2012;44(3):291–296. doi:10.1038/ng.1076
6. Leng RX, Di DS, Ni J, et al. Identification of new susceptibility loci associated with rheumatoid arthritis. Ann Rheum Dis. 2020;79(12):1565–1571. doi:10.1136/annrheumdis-2020-217351
7. Fang XY, Xu WD, Pan HF, Leng RX, Ye DQ. Novel insights into tim-4 function in autoimmune diseases. Autoimmunity. 2015;48(4):189–195. doi:10.3109/08916934.2014.983266
8. Carland F, Fujioka S, Nelson T. The sterol methyltransferases SMT1, SMT2, and SMT3 influence arabidopsis development through nonbrassinosteroid products. Plant Physiol. 2010;153(2):741–756. doi:10.1104/pp.109.152587
9. Albacker LA, Karisola P, Chang YJ, et al. TIM-4, a receptor for phosphatidylserine, controls adaptive immunity by regulating the removal of antigen-specific T cells. J Immunol. 2010;185(11):6839–6849. doi:10.4049/jimmunol.1001360
10. Zhao P, Xu L, Wang P, et al. Increased expression of human T-cell immunoglobulin- and mucin-domain-containing molecule-4 in peripheral blood mononuclear cells from patients with system lupus erythematosus. Cell Mol Immunol. 2010;7(2):152–156. doi:10.1038/cmi.2009.118
11. Abe Y, Kamachi F, Kawamoto T, et al. TIM-4 has dual function in the induction and effector phases of murine arthritis. J Immunol. 2013;191(9):4562–4572. doi:10.4049/jimmunol.1203035
12. Page NS, Jones G, Stewart GJ. Genetic association studies between the T cell immunoglobulin mucin (TIM) gene locus and childhood atopic dermatitis. Int Arch Allergy Immunol. 2006;141(4):331–336. doi:10.1159/000095459
13. Zhao B, Abdelmoudjib G, Li J, et al. Two polymorphisms in the TIM-4 gene are associated with asthma in a Chinese Han population. Int J Immunogenet. 2011;38(1):31–35. doi:10.1111/j.1744-313X.2010.00965.x
14. Chen JP, Zhao WY, He NH, He SX, Wang G, Wang WJ. Relationship between TIM-4 polymorphism and childhood asthma. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14(2):120–123.
15. Xu J, Yang Y, Liu X, Wang Y. Genetic variation and significant association of polymorphism rs7700944 G>A of TIM-4 gene with rheumatoid arthritis susceptibility in Chinese Han and Hui populations. Int J Immunogenet. 2012;39(5):409–413. doi:10.1111/j.1744-313X.2012.01103.x
16. Zakeri Z, Hashemi M, Pourhosseini SM, Eskandari-Nasab E, Bahari G, Taheri M. Association between the rs7700944 polymorphism in the TIM-4 gene and rheumatoid arthritis in Zahedan, southeast Iran. Rev Bras Reumatol. 2013;53(4):341–345. doi:10.1590/S0482-50042013000400005
17. Mosaad YM, El-Bassiony SR, El-Ghaweet AE, Elhindawy MM, El-Deek BS, Sultan WA. TIM-1 rs41297579 G>A (−1454) and TIM-4 rs7700944 gene polymorphisms as possible risk factor for rheumatoid arthritis: relation to activity and severity. Int J Immunogenet. 2015;42(4):254–264. doi:10.1111/iji.12201
18. You CG, Li JF, Xie XD, Zhu Y, Li PQ, Chen YR. Association of interleukin-1 genetic polymorphisms with the risk of rheumatoid arthritis in Chinese population. Clin Chem Lab Med. 2007;45(8):968–971. doi:10.1515/CCLM.2007.156
19. Jiang L, Yin J, Ye L, et al. Novel risk loci for rheumatoid arthritis in Han Chinese and congruence with risk variants in Europeans. Arthritis Rheumatol. 2014;66(5):1121–1132. doi:10.1002/art.38353
20. Silman AJ. The 1987 revised American Rheumatism Association criteria for rheumatoid arthritis. Br J Rheumatol. 1988;27(5):341–343. doi:10.1093/rheumatology/27.5.341
21. Rodriguez-Manzanet R, DeKruyff R, Kuchroo VK, Umetsu DT. The costimulatory role of TIM molecules. Immunol Rev. 2009;229(1):259–270. doi:10.1111/j.1600-065X.2009.00772.x
22. Yeung MY, McGrath M, Najafian N. The emerging role of the TIM molecules in transplantation. Am J Transplant. 2011;11(10):2012–2019. doi:10.1111/j.1600-6143.2011.03727.x
23. Heininger K, Stoll G, Linington C, Toyka KV, Wekerle H. Conduction failure and nerve conduction slowing in experimental allergic neuritis induced by P2-specific T-cell lines. Ann Neurol. 1986;19(1):44–49. doi:10.1002/ana.410190109
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
