Back to Journals » International Journal of General Medicine » Volume 15
Correlation of SIDT1 with Poor Prognosis and Immune Infiltration in Patients with Non-Small Cell Lung Cancer
Authors Tian Y , Zhou Y , Liu J, Yi L, Gao Z, Yuan K , Tong J
Received 12 November 2021
Accepted for publication 5 January 2022
Published 22 January 2022 Volume 2022:15 Pages 803—816
DOI https://doi.org/10.2147/IJGM.S347171
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yubin Tian,1,2,* Yong Zhou,2,* Junhui Liu,1,2 Lei Yi,2 Zhaojia Gao,2,3 Kai Yuan,1,3 Jichun Tong2,3
1School of Medical, Dalian Medical University, Dalian, People’s Republic of China; 2The Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University, Changzhou, People’s Republic of China; 3Heart and Lung Disease Laboratory, The Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University, Changzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kai Yuan; Jichun Tong, Email [email protected]; [email protected]
Purpose: To investigate the expression profile of systemic RNA interference deficient-1 transmembrane family member 1 (SIDT1) in patients with non-small cell lung cancer (NSCLC) and assess its prognostic value and immune infiltration.
Materials and Methods: A tissue microarray (TMA) was purchased containing 60 paired NSCLC tissues and matched para-tumor tissues, and another TMA was constructed comprising 140 NSCLC tissues. Then, immunohistochemical staining and scoring were performed. Finally, we evaluated the expression profiles and prognostic values of SIDT1 in NSCLC. Correlation between SIDT1 gene expression and the immune microenvironment of NSCLC was investigated using the ESTIMATE and CIBERSORT algorithms.
Results: SIDT1 was mainly detected in the cytoplasm and the cell membrane. SIDT1 expression was significantly higher in tumor tissues than in para-tumor tissues. High expression of SIDT1 was correlated with the NSCLC stage. High SIDT1 expression signals worsening overall survival (OS) in NSCLC patients, especially in stage I. In LUSC, high SIDT1 expression is an independent prognostic factor for worsening OS.
Conclusion: High SIDT1 expression predicted a low survival rate in LUSC patients, suggesting it may be a potential target for prognostic assessment of NSCLC patients.
Keywords: NSCLC, prognosis, TMA, immune infiltration, SIDT1
Introduction
Lung cancer is one of the most common malignancies worldwide and has been on the rise globally in the last decade, and is the leading cause of cancer-related deaths. In addition, the incidence and mortality rates of lung cancer are increasing year by year in China.1 Histologically, non-small cell lung cancer (NSCLC) is classified into several subtypes, including lung squamous cell carcinoma (LUSC), lung adenocarcinoma (LUAD), large cell lung cancer, and other rare types. Despite some progress in early diagnosis and systemic therapy, the 5-years survival rate of NSCLC patients remains relatively low.2 Therefore, it is necessary to find more sensitive and specific biomarkers and therapeutic targets for NSCLC patients by studying the pathogenesis of NSCLC.
The SID transmembrane family member 1 (SID-1), a member of the systemic RNA interference-deficient (SID) channel family, is encoded by the systemic RNA interference defective-1 (sid-1) gene.3 It is a double-stranded RNA (dsRNA) specific gated channel protein, identified initially after screening for mutants in C. elegans. The Systemic RNA interference-defective-1 transmembrane family member 1 (SIDT1), the mammalian homolog of the SID-1 gene, encodes an integral membrane protein.4 SIDT1 acts as a transmembrane channel for small interfering RNA (siRNA) that localize to the plasma membrane on the plasma membrane promotes the uptake of extracellular siRNA by human cells and may lead to highly specific post-transcriptional gene silencing.5 SIDT1 is essential for normal tissue organization, but it is also involved in tumour progression and chemoresistance. In addition, SIDT1 can act as a mediator of intercellular communication. Indeed, there are few studies on the role of SIDT1 in tumors, and its role in breast and pancreatic cancer has been reported,4 but according to our literature search, the role of SIDT1 in lung cancer has not been reported. This study aimed to elaborate on the expression level and prognostic value of SIDT1 in NSCLC.
The prognosis of NSCLC is closely related to the altered immune microenvironment. We assessed the level of immune cell infiltration and explored the correlation between SIDT1 expression and immune infiltration in NSCLC in an in-depth study, and the relationship between SIDT1 and immune infiltration may help improve the prognosis of NSCLC.
Materials and Methods
Ethics Approval
This study was reviewed and approved by the Research Ethics Committee of The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University. Written informed consent was provided by all patients or their families. All specimens were processed and anonymized according to ethical and legal standards.
Patients and Construction of TMA
A tissue microarray (TMA) (ZL-HLugC120PT01) including 60 paired NSCLC tumor tissues and para-tumor tissues were purchased from Superbiotek (Shanghai, China). It was used to estimate the difference of SIDT1 expression between the tumor and para-tumor group. From January 2005 to December 2005, 140 NSCLC tissues and 10 normal adjacent tissue samples were collected from patients with lung cancer who underwent surgery at the Department of Thoracic Surgery of Zhongshan Hospital, Fudan University Hospital. Complete clinical data were obtained, and clinical follow-up continued until July 2013, and patients were classified according to TNM classification, a system established jointly by the American Joint Committee on Cancer and the Union for International Cancer Control. Patients included in this study did not receive chemotherapy, radiotherapy, or biologic therapy before surgery. The tissue samples from the 140 primary NSCLC cases were arranged in rows and columns to construct a TMA, as previously described by Gao et al.6
Immunohistochemical Staining
Immunohistochemistry was performed using standard indirect immunoperoxidase procedure (Envision Plus; Dako; Agilent Technologies, Inc.) to detect the expression of SIDT1 in NSCLC. Paraffin specimens were cut into slices (4-µm thick), mounted on slides, baked, dewaxed, and hydrated according to conventional methods.7 Furthermore, the sections were incubated with 3% H2O2 for 20 min at room temperature to quench the endogenous peroxidase activity.8 Then, these sections were placed in a 10mM citrate buffer (pH 6.0) for 10 minutes at 100°C for heat-induced antigen retrieval. Next, slides were placed in 10 mM TBS containing 4% normal goat serum (Abcam) and incubated for 1 hour at room temperature, followed by the addition of primary antibody to SIDT1 (1:500; cat. No. YT4737; ImmunoWay Biotechnology) overnight at 4°C. After incubation with the primary antibody, sections were washed with TBS and then incubated with HRP-conjugated goat anti-rabbit secondary antibody (1:500; cat. No. RS0002; ImmunoWay Biotechnology) for 1 hour at 37°C.9 The sections were first stained with DAB (3,3ʹ-diaminobenzidine) (Dako; Agilent Technologies, Inc.) at 37°C for 10 minutes, then weakly counter-stained with hematoxylin at 37°C for 1 minute, and finally dehydrated and covered with coverslips. Images were gained with a light microscope (Nikon; magnification, x200). Expression of SIDT1 in breast cancer tissue was used as a positive control.
Immunohistochemistry Evaluation
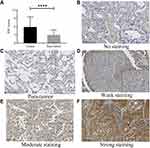
The expression level of SIDT1 in NSCLC tissue was quantified by staining intensity and staining area of positive tumor cells in each section.10 Two experienced pathologists independently evaluated the immunohistochemical score. According to the protocol developed by Xu et al,11 the score was calculated using the product of the staining intensity and staining ratio of positive tumor cells in the sections. Scoring of cytoplasmic staining intensity: a) 0 (no staining); b) 1 (weak staining, yellowish); c) 2 (moderate staining, yellowish-brown); d) 3 (strong staining, brown). The percentage of cytoplasmic positive cells was defined as 0–100%, with a classification score: a) 1 (0–25% positive cells); b) 2 (2–50% positive cells); c) 3 (51–75% positive cells); d) 4 (76–100% positive cells).12 The higher the immunohistochemical score, the higher the expression of SIDT1. The median score was taken as the cut-off value, ≥ the median was the high expression group, and < the median was the low expression group.13
Immune Cell Infiltration
The proportion of 22 immune cell infiltrates in each patient in the training and validation sets was calculated using the CIBERSORT (HTTP://cibersort.Stanford.Edu/) based deconvolution algorithm. Differences in the infiltration of immune cells with high and low SIDT1 expression in the above dataset were assessed using the Wilcoxon test, with P < 0.05 being a significant difference in infiltration.9 Follow-up analyses were performed on the above cells to assess the impact of their infiltration levels on patient prognosis.
Pathway Enrichment Analysis
Visualization was performed by Cytoscape (version 3.7.2). To elucidate the relationship between SIDT1 and signalling pathways in the development of NSCLC, enrichment of Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) was performed using GSEA (version 4.0.1) Analysis.14
Statistical Analysis
All results are expressed as mean ± standard deviation (SD). The time from the first surgery to the patient’s death or last follow-up was defined as the overall survival (OS) time.7 Paired t-tests, χ2 tests, Log rank tests, Kaplan-Meier survival analysis, univariate and multivariate Wald tests, and Cox regression analysis were performed.15 Analyzes were completed using SPSS software (version 26.0; IBM Corporation) and GraphPad Prism software (version 6.0; GraphPad Software, Inc.), and p <0.05 were considered to indicate a statistically significant difference.16
Results
Characteristics of 140 Patients
It shows the clinical data of 140 patients with NSCLC in the TMA in Table 1. The mean age of the 140 patients was 60.1 ± 6.4 years (range, 26–79 years), most of whom were male (80.0%). The pathological composition was 51 LUAD (36%) (grade 1–3, LUAD including bronchoalveolar and mucoepidermoid carcinomas), 80 LUSC (grade 1–3), 5 adenosquamous cell carcinomas, 3 large cell neuroendocrine carcinomas, and 1 sarcomatoid carcinoma. Clinical follow-up records are available up to July 2013. Finally, only 51 LUAD and 80 LUSC were included in this study.
 |
Table 1 Clinicopathologic Features of 140 Patients with Non-Small Cell Lung Cancer |
Expression of SIDT1 in NSCLC Tissues
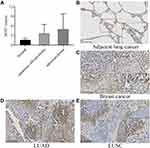
SIDT1 expression was detected and scored in 60 paired tissues (tumor and para-tumor). As shown in Figure 1A, SIDT1 was highly expressed in tumor cells compared with para-tumor cells (5.850 ± 3.677 vs 3.000 ± 1.776, p <0.0001). SIDT1 was expressed mainly in the cell membrane and cytoplasm of tumor cells and para-tumor cells (Figure 1B-F). The median immunohistochemical score for SIDT1 in 120 tissues was 4. With a cut-off value of 4, 33 cases (55.0%) in the tumor group and 5 cases (8.3%) in the para-tumor group were classified as high SIDT1 expression. In the quantitative scores of the TMA immunohistochemistry, the mean score of lung cancer tissues in the TMA was 2.7-fold higher than normal lung tissues (Figure 2A). The median immunohistochemical score for SIDT1 in TMA was 4. SIDT1 was highly expressed in breast cancer as a positive control, while it was lowly expressed in adjacent lung tissue as a negative control (Figure 2B-E). The LUSC was 3.66 ± 2.87 and LUAD was 5.16 ± 4.64, while the adjacent lung tissue was 1.60 ± 0.55.
SIDT1 Expression and Clinicopathological Characteristics
The 131 NSCLC patients were divided into 2 groups according to cut-off value (score=4): high SIDT1 expression group (70 patients, 53.4%) and low SIDT1 expression group (61 patients, 46.6%), and the clinicopathological data of the patients were compared. Patients with high SIDT1 expression had a significant association with the later pathological stage (p = 0.047), as shown in Table 2. However, there was no statistically significant association between SIDT1 expression and any other clinicopathological features, such as gender (P=0.055), tumor size (P=0.577), lymph node status (P=0.067), tumor differentiation (P=0.909), pathological tumor type (P=0.788), tumor location (P=0. 166), and patient age (P=0.058).
 |
Table 2 The Correlation Between SIDT1 Expression and Clinicopathologic Features in Patients with NSCLC |
Prognostic Value of SIDT1 in NSCLC
In addition, Kaplan-Meier survival curves were plotted to assess the prognostic value of SIDT1. Survival analysis showed that high expression of SIDT1 in NSCLC patients was significantly associated with poorer OS (P=0.0029, Figure 3A). The curves showed that the high expression of SIDT1 suggested a shorter OS in stage I patients (P = 0.0017, Figure 3B). We further explored the correlation between SIDT1 expression and clinicopathological features in LUAD and LUSC. Survival analysis showed that high expression of SIDT1 in LUAD patients was not associated with poorer OS (P = 0.0923, Figure 3C). However, SIDT1 expression was found to be significantly correlated with OS in LUSC patients (P = 0. 0124, Figure 3D). Finally, high expression of SIDT1 was found to be an independent prognostic factor for worsening OS in LUSC by univariate and multivariate analysis (P = 0.040, Table 3).
 |
Table 3 Multivariate Cox Regression Analysis for Potential Factors Influencing Overall Survival in LUAD |
SIDT1-Expressing Tumor Immune Microenvironment
Using the CIBERSORT algorithm to observe the clustering of 22 tumor immune cells in NSCLC tissues (Figure 4A), there were significant differences in the proportion and subpopulation distribution of the above tumor immune cells in the high and low SIDT1 expression groups (Figure 4B). In addition, resting memory CD4+ T cells, regulatory T cells, macrophage M0, macrophage M1, activated mast cell follicular helper T cells, and high and low expression of SIDT1 were significantly different in the TCGA data.
SIDT1 Expression Correlates with the Level of Immune Infiltration and Cumulative Survival in NSCLC
Based on TIMER database analysis, the results showed that SIDT1 expression correlated significantly with B-cell, CD8T cell, CD4T cell, macrophage, neutrophil, and dendritic cell infiltration (all p<0.001) (Figure 5A). In addition, we identified an essential role for SIDT1 in the immune infiltration of B cells and neutrophils and dendritic cells in LUAD, with B cells and neutrophils and dendritic cells being factors associated with cumulative survival over time in LUAD (Figure 5B).
SIDT1 Expression-Rich Gene Set
Bioinformatics screening of SIDT1 co-expressed genes and their functions yielded 981 genes co-expressed with SIDT1 (Figure 6A-C), and GO analysis showed that these genes were involved in the following biological processes: cell mitosis, cell cycle regulation, cell cycle checkpoint, DNA damage checkpoint, tumor necrosis factor production, regulation of MAP kinase activity, leukocyte migration, lymphocyte migration, lymphocyte aggregation, neutrophil migration, etc. (Figure 6D-I).
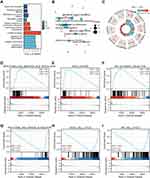 |
Figure 6 (A–C) Enrichment analysis of co-expressed genes. (D–I) GSEA results show differential enrichment between high SIDT1 expression and low SIDT1 expression. |
Prognostic Model
We further constructed a nomogram based on the results of univariate and multivariate cox regression analyses, combining two independent prognostic factors, tumor stage, and SIDT1, for risk scoring. The column line plot shows that each corresponds to a score and the final score is obtained by adding up each score, with a higher total score implying a worse prognosis for that patient (Figure 7A). To verify the predictive accuracy of the line graphs, calibration plots were also drawn. The calibration plots showed that the predictions at 1, 3, and 5 years were close to the actual picture, indicating that the SIDT1 prognostic model has good accuracy in predicting the prognosis of NSCLC patients (Figure 7B-D).
 |
Figure 7 (A) Line graph of NSCLC prognostic model. (B–D) NSCLC prognostic model 1-year and 3-year 5-year calibration curves. |
Discussion
NSCLC is a tumor with a high degree of malignancy and aggressiveness with a meager 5-year OS rate, and there have been many studies on NSCLC diagnosis, treatment, and prognosis in recent decades, but it is still difficult to detect and intervene in the early stage of lung cancer.17 This may also be the primary reason for the low 5-year survival rate and high mortality rate of lung cancer.18 Therefore, it is urgent to find biomarkers that can effectively detect and diagnose early lung cancer. SIDT1, a transporter protein of siRNA, has rarely been studied in tumor cells. Here, we used two TMAs made from 60 pairs of tissues (tumor and para-tumor) and 131 NSCLC patients to investigate preliminarily the expression profile and prognostic value of SIDT1 in NSCLC.
To our knowledge, this is the first study to investigate the expression level and prognostic significance of SIDT1 in NSCLC. By analyzing the immunohistochemical results of 60 pairs of tissues, we found that the expression of SIDT1 was higher in lung tumor tissues than in para-tumor tissues. SIDT1 has been reported to belong to the SID family and to express a highly well-conserved transmembrane channel protein. SIDT1, a transmembrane channel for intercellular communication, has been found to fuel the uptake of siRNA or miRNA and it also mediates cholesterol transport in humans.19 SIDT1 is essential for the organization of normal tissues, and recent studies suggest it mediates the uptake of dietary miRNAs in the mammalian stomach, but it also has an important role in tumor progression and chemoresistance. Specifically, SIDT1 could act as a mediator of intercellular communication to enhance chemoresistance to miR21 driven gemcitabine in pancreatic cancer. In addition, Zhang et al20 found that SIDT1 could be regulated by IL-4 in breast cancer cells through Stat6-dependent and non-dependent pathways. Wang et al4 found that SIDT1 was associated with recurrence-free survival in triple-negative breast cancer and could even inhibit breast cancer cell proliferation. Although the specific mechanism of action of biological level systemic RNA interference (sysRNAi) in mammals has not been explained so far, SIDT1-dependent intercellular communication-mediated RNA transfer may have multiple functional genomic and therapeutic applications in cancer development.21 We have gained some insight into this throughout biology through studies of miRNAs in cancer genesis, metastasis, and drug resistance. miRNA levels can be increased through contact-mediated SIDT1-dependent mechanisms, enhancing the outcome of post-transcriptional gene regulation and allowing for broader adaptive changes within the tumor microenvironment.22
In the present study, we found that SIDT1 was highly expressed in NSCLC and that upregulation of SIDT1 expression was associated with more advanced pathological stages. Survival analysis suggested that high expression of SIDT1 was closely associated with the prognosis of NSCLC patients, especially in stage I NSCLC patients. High expression of SIDT1 in LUAD patients was statistically significantly correlated with age, lymph node metastasis, and advanced pathological stage, but not with poorer OS. In LUSC, high expression of SIDT1 did not correlate with any clinicopathological features, and high expression of SIDT1 was significantly associated with poorer OS. Based on previous studies and our results, we cautiously believe that SIDT1 affects biological phenomena such as proliferation and metastasis of NSCLC, and the specific mechanism may be that SIDT1 is involved in the transport of some miRNAs.
In the lung cancer microenvironment, tumor-infiltrating immune cells not only attacked and killed lung cancer cells to inhibit tumor progression, but also SIDT1 expression levels were found to correlate with the level of immune infiltration in NSCLC by CIBERSORT and analysis.23 The results showed that SIDT1 expression levels correlated with CD4+ T cells, activated CD4+ T cells, dendritic cells, activated dendritic cells, neutrophils granulocytes, macrophages, regulatory T cells, and natural killer T cells showed moderate or strong correlations (all p<0.01).SIDT1 is necessary for immune cell infiltration in tumor pathology.24 Furthermore, we used the timer database for survival analysis by Kaplan-Meier curves, where B cells (p<0.001) neutrophils (p<0.05) and dendritic cells (p<0.05) were significantly correlated with NCAPG survival in LUAD. Thus, it is reasonable to affirm that the regulation of immune cell infiltration by SIDT1 affects the prognosis of NSCLC patients. T cells predominate in the NSCLC tumor microenvironment, followed by B cells, macrophages, DCs, and natural killer cells as the primary immune cell types.25 Dendritic cells play an essential role in activating anti-tumor T lymphocytes to express specific antigens.26 The impaired function of tumor infiltration may severely affect the body’s anti-tumor immune response, further affecting the development and progression of NSCLC and consequently the survival of patients.27 Therefore, NCAPG affects the prognosis of NSCLC by influencing the immune status of patients. The results of GSEA further confirm that NCAPG may be involved in the immunosuppression of NSCLC. Our results suggest that NCAPG can suppress immune infiltrating cells in NSCLC. Enrichment analysis revealed various biological processes associated with the SIDT1 gene, such as cell mitosis, cell cycle regulation, and cell cycle detection sites.28 The enrichment analysis also revealed that SIDT1 might be associated with tumor immune infiltration, consistent with our previous studies. The prognostic model we made has good predictive power and is expected to help enhance the prognostic prediction of NSCLC.
Conclusion
In conclusion, this study aimed to explore the expression trend of SIDT1 in NSCLC and the correlation between its expression level and prognosis. Finally, according to the results of our study, the expression of SIDT1 was up-regulated in NSCLC, suggesting that the high expression of SIDT1 was associated with poor prognosis. In particular, high SIDT1 expression in LUSC is an independent risk factor associated with poor prognosis. Therefore, SIDT1 may be considered a potential biomarker of poor prognosis for identifying NSCLC patients with poor clinical prognosis and may play a specific role in immune infiltration, providing a reference for further development of subsequent immunotherapy and targeted targeting therapy in NSCLC.
Abbreviations
LUAD, lung adenocarcinoma; LUSC, Lung squamous cell carcinoma; TCGA, The Cancer Genome Atlas; OS, Overall Survival; CIBERSORT, Cell type Identification by Estimating Relative Subsets of RNA Transcripts; TMA, Tissue Microarray; AOD, Average Optical Density; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Ethical Conduct of Research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Informed Consent
All patients or their family members provided written informed consent.
Research Involving Human Participants and/or Animals
All procedures performed in studies involving human participants were by the ethical standards of the institutional and national research.
Funding
The following funds support this work:1. “333 Project” of Jiangsu Province (Grant number: BRA2020157)2. “Six One Project,” Research Projects of High-level Medical Personnel of Jiangsu Province (Grant number: LGY2019025)3. High-level Talent Selection and Training Project of the 16th Batch of “Six Talent Peak” in Jiangsu Province (Grant number: WSN-245)4. Medical Scientific Research Foundation of Jiangsu Commission of Health (Grant number: H2018083)5. Jiangsu Provincial Medical Youth Talent (Jiangsu Health Scientific Education 2017 no.3)6. 333 High-Level Talent Training Project (Grant number: 2016, III-0719)7. High-Level Medical Talents Training Project (Grant number: 2016CZBJ042).
Disclosure
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest. Yubin Tian and Yong Zhou are co-first authors for this study.
References
1. Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer. 2019;10(1):3–7. doi:10.1111/1759-7714.12916
2. Chen WQ, Li H, Sun KX, et al. [Report of Cancer Incidence and Mortality in China, 2014]. Zhonghua Zhong Liu Za Zhi. 2018;40(1):5–13. Chinese. doi:10.3760/cma.j.issn.0253-3766.2018.01.002
3. Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science. 2002;295(5564):2456–2459. doi:10.1126/science.1068836
4. Wang Y, Li H, Ma J, et al. Integrated bioinformatics data analysis reveals prognostic significance of SIDT1 in triple-negative breast cancer. Onco Targets Ther. 2019;12:8401–8410. doi:10.2147/OTT.S215898
5. Chen Q, Zhang F, Dong L, et al. SIDT1-dependent absorption in the stomach mediates host uptake of dietary and orally administered microRNAs. Cell Res. 2021;31(3):247–258. doi:10.1038/s41422-020-0389-3
6. Gao ZJ, Wang Y, Yuan WD, Yuan JQ, Yuan K. HIF-2alpha not HIF-1alpha overexpression confers poor prognosis in non-small cell lung cancer. Tumour Biol. 2017;39(6):1010428317709637. doi:10.1177/1010428317709637
7. Lee KM, Guerrero-Zotano AL, Servetto A, et al. Proline rich 11 (PRR11) overexpression amplifies PI3K signaling and promotes antiestrogen resistance in breast cancer. Nat Commun. 2020;11(1):5488. doi:10.1038/s41467-020-19291-x
8. Shen H, Wang Z, Ren S, et al. Prognostic biomarker MITD1 and its correlation with immune infiltrates in hepatocellular carcinoma (HCC). Int Immunopharmacol. 2020;81:106222. doi:10.1016/j.intimp.2020.106222
9. Zhao R, Peng C, Song C, et al. BICC1 as a novel prognostic biomarker in gastric cancer correlating with immune infiltrates. Int Immunopharmacol. 2020;87:106828. doi:10.1016/j.intimp.2020.106828
10. Gu Y, Li X, Bi Y, et al. CCL14 is a prognostic biomarker and correlates with immune infiltrates in hepatocellular carcinoma. Aging (Albany NY). 2020;12(1):784–807. doi:10.18632/aging.102656
11. Xu H, Yu S, Yuan X, et al. DACH1 suppresses breast cancer as a negative regulator of CD44. Sci Rep. 2017;7(1):4361. doi:10.1038/s41598-017-04709-2
12. Sun J, Xie T, Jamal M, et al. CLEC3B as a potential diagnostic and prognostic biomarker in lung cancer and association with the immune microenvironment. Cancer Cell Int. 2020;20:106. doi:10.1186/s12935-020-01183-1
13. Wu Y, Lin Y, Pan J, et al. NCAPG promotes the progression of lung adenocarcinoma via the TGF-β signaling pathway. Cancer Cell Int. 2021;21(1):443. doi:10.1186/s12935-021-02138-w
14. Chen J, Wang Z, Wang W, et al. SYT16 is a prognostic biomarker and correlated with immune infiltrates in glioma: a study based on TCGA data. Int Immunopharmacol. 2020;84:106490. doi:10.1016/j.intimp.2020.106490
15. Chen Q, Pu N, Yin H, et al. CD73 acts as a prognostic biomarker and promotes progression and immune escape in pancreatic cancer. J Cell Mol Med. 2020;24(15):8674–8686. doi:10.1111/jcmm.15500
16. Geng Q, Shen Z, Li L, Zhao J. COL1A1 is a prognostic biomarker and correlated with immune infiltrates in lung cancer. PeerJ. 2021;9:e11145. doi:10.7717/peerj.11145
17. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
18. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi:10.1097/JTO.0000000000000630
19. Elhassan MO, Christie J, Duxbury MS. Homo sapiens systemic RNA interference-defective-1 transmembrane family member 1 (SIDT1) protein mediates contact-dependent small RNA transfer and microRNA-21-driven chemoresistance. J Biol Chem. 2012;287(8):5267–5277. doi:10.1074/jbc.M111.318865
20. Zhang WJ, Li BH, Yang XZ, et al. IL-4-induced Stat6 activities affect apoptosis and gene expression in breast cancer cells. Cytokine. 2008;42(1):39–47. doi:10.1016/j.cyto.2008.01.016
21. Wolfrum C, Shi S, Jayaprakash KN, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25(10):1149–1157. doi:10.1038/nbt1339
22. Mendez-Acevedo KM, Valdes VJ, Asanov A, Vaca L. A novel family of mammalian transmembrane proteins involved in cholesterol transport. Sci Rep. 2017;7(1):7450. doi:10.1038/s41598-017-07077-z
23. Liu J, Han X, Chen L, et al. is a distinct prognostic biomarker that worsens the tumor immune microenvironment in lung adenocarcinoma. Aging (Albany NY). 2020;12(20):20308–20331. doi:10.18632/aging.103804
24. Sui S, An X, Xu C, et al. An immune cell infiltration-based immune score model predicts prognosis and chemotherapy effects in breast cancer. Theranostics. 2020;10(26):11938–11949. doi:10.7150/thno.49451
25. Vahl JC, Drees C, Heger K, et al. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41(5):722–736. doi:10.1016/j.immuni.2014.10.012
26. Li Y, Zhao X, Xiao H, et al. APE1 may influence CD4+ naïve T cells on recurrence free survival in early stage NSCLC. BMC Cancer. 2021;21(1):233. doi:10.1186/s12885-021-07950-1
27. Donnem T, Hald S, Paulsen E, et al. Stromal CD8+ T-cell density—a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res. 2015;21(11):2635–2643. doi:10.1158/1078-0432.Ccr-14-1905
28. Xiao Z, Hu L, Yang L, et al. TGFβ2 is a prognostic-related biomarker and correlated with immune infiltrates in gastric cancer. J Cell Mol Med. 2020;24(13):7151–7162. doi:10.1111/jcmm.15164
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.