Back to Journals » International Journal of General Medicine » Volume 14
Correlation of Serum Anti-Mullerian Hormone (AMH) Level on Ovarian Volume in Women with Endometrioma
Authors Suardi D, Permadi W, Djuwantono T, Hidayat YM , Bayuaji H , Edo Gautama GP
Received 7 September 2020
Accepted for publication 19 November 2020
Published 6 January 2021 Volume 2021:14 Pages 1—8
DOI https://doi.org/10.2147/IJGM.S272071
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Dodi Suardi, Wiryawan Permadi, Tono Djuwantono, Yudi Mulyana Hidayat, Hartanto Bayuaji, Gusti Putu Edo Gautama
Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin Hospital, Bandung, Indonesia
Correspondence: Gusti Putu Edo Gautama
Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin Hospital, Jl. Pasteur No. 38, Bandung, 40161 Jawa Barat, Indonesia
Tel +6222 203 2530
Fax +6222 203 9086
Email [email protected]
Background: The correlation between endometrioma and serum Anti-mullerian hormone (AMH) level is a benchmark in determining the prognosis and management of endometrioma. Endometrioma causes a decrease in ovarian reserve due to tissue damage that affects the formation of serum AMH. Serum AMH levels in daily practice are useful as a tool to determine ovarian reserve, markers for diagnosis and prognosis in infertility and reproductive abnormalities. The purpose of this study was to determine the relationship of serum AMH level in women with endometrioma and their correlation to ovarian volume.
Methods: This research was an analytical observational study with a cross-sectional design in women of reproductive age who were diagnosed with endometrioma within the period of August 2019–March 2020 at Hasan Sadikin Hospital, Bandung. Forty-four women who met the inclusion and exclusion criteria were then divided into endometrioma (n=22) and control (n=22) groups. In both groups, transvaginal ultrasound examination was performed to measure the volume of the ovary, then a laboratory examination of serum AMH level was carried out.
Results: Serum AMH levels in the endometrioma group were significantly lower than those in the control group (P< 0.001). Serum AMH level did not differ significantly based on laterality of the observation group (P=1.000). There was a negative correlation between serum AMH level and the volume of ovarian endometrioma, although not statistically significant (r=− 0.332; P=0.066).
Conclusion: There was a correlation between serum AMH level and endometrioma. Serum AMH levels were significantly lower in the endometrioma group but were not influenced by their laterality. We found a negative correlation between serum AMH level and ovarian volume containing endometrioma, but not statistically significant.
Keywords: anti-Mullerian hormone, AMH, endometrioma, ovarian volume
Introduction
Endometrioma is a cyst that forms when endometrial tissue grows in the ovarian epithelium. Endometriomas are also known as “brown cysts,” which are benign ovarian cysts containing thick, old blood that appear as brown liquid. Endometrioma is one form of manifestation of endometriosis.
Endometriosis affects 10–15% of all women of reproductive age and 70% of women with chronic pelvic pain. However, many women are often late in the diagnosis of endometriosis, which results in a decrease in quality-of-life. The prevalence of endometriosis in infertile women is very high (20–50%), and around 17–44% of women with endometriosis will experience endometrioma.1–3
Anti-Mullerian hormone (AMH) is a 140 kDa glycoprotein hormone linked by a disulfide chain whose genes are located on chromosome 19p13.3 and belong to the transforming growth factor-β (TGF- β) group. AMH women are involved in regulation of the number of primordial follicles which begin maturation and prevent the use of ovarian reserves earlier.4,5
Serum AMH levels in women with endometrioma tend to be low due to chronic inflammatory processes and massive reactive oxygen species (ROS) found in the ovaries. The greater the volume of endometrioma cysts, the greater the volume and damage to the ovaries. The mechanism underlying low AMH serum levels and decreased ovarian reserve is the presence of this inflammatory process and immunomodulating abnormalities associated with toxic endometrioma conditions. The inflammatory process causes follicular damage and dysfunction so that the quality and quantity of the follicle is also reduced. This leads to a decrease in serum AMH levels which results from the production of ovarian follicular granulosa cells.6–8
The purpose of this study was to determine differences in serum AMH levels between endometrioma patients and without endometrioma and determine the correlation between ovarian volume and serum AMH levels in the endometrioma group.
Materials and Methods
This research was an analytical observational study with a cross-sectional design in women of reproductive age who were diagnosed with endometrioma at Hasan Sadikin hospital, Bandung. The study was performed in accordance with the Declaration of Helsinki statement for medical research involving human subjects. Ethics approval was obtained from the Padjadjaran University Ethics Committee, with ethical approval number LB.02.01/X.6.5/35/2020. Informed consent was obtained from the study participants prior to study commencement. Using sample size method for correlation coefficient, the total sample size and actual power were calculated to be 44 (22 vs 22) and 0.80, respectively, when α err=0.05, β err=0.20, and expected correlation coefficient 0.60. Women who visited Aster and gynecology clinic within the period of August 2019–March 2020 and met the inclusion and exclusion criteria were recruited consecutively. Subjects were then divided into endometrioma (n=22) and control (n=22) groups.
The inclusion criteria were women of reproductive age (20–35 years), BMI <27 kg/m2, transvaginal ultrasound imaging with endometrioma for the case group, and imaging of normal uterus and ovaries for the control group. The identification of non-endometriotic ovarian cysts was by transvaginal ultrasound. Endometrioma diagnosis was made if the patient complains of abdominal mass and the presence of cyst with diffuse homogenous hypoechoic (ground-glass appearance) in transvaginal ultrasound. Patients who had undergone surgery for endometrioma, had disease or pelvic organ abnormalities, had a recent history of infection or malignancy, polycystic ovarian syndrome (PCOS), incomplete data, and were not willing to participate in the study were excluded from this study.
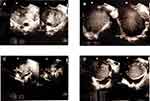
In both groups, the transvaginal ultrasound examination was performed to measure the volume of the ovary, then a laboratory examination of serum AMH level was carried out. Transvaginal ultrasound examination was performed at the initial follicular phase (Day 2–3 of menstruation cycle) using HD11XW 2D Transvaginal Philips ultrasound (Figure 1). For the control group, ovarian volume measurement was carried out, while for the endometrioma group, the ovarian and endometrioma volume, endometrium-ovary, and endometrioma were measured. Ovarian volume was obtained by calculating the three diameters (d1: longitudinal, d2: anteroposterior, d3: transversal) using the ellipsoid prolate formula V=0.523xd1xd2xd3, then adding up the left and right volumes. Furthermore, the examination of serum AMH levels was done by taking venous blood as much as 3 cc and measured using the Human AMH enzyme-linked immunosorbent assay (ELISA) kit (Elabscience, USA). The detection range of this assay was 93.75–6000 pg/mL. The sensitivity and coefficient of variation were 56.26 pg/mL and <10%, respectively. According to the manufacturer, the kit recognizes Human AMH in samples. No significant cross-reactivity or interference between Human AMH and analogs was observed.
The potential confounders in this study were endometrioma laterality, age, and body mass index (BMI). To avoid biases, we calculate the significance of these characteristics in both groups, so that homogeneity of both groups could be obtained.
Quantitative variables (age, volume of endometrioma, and serum AMH level) were presented by mean, standard deviation, and range. Normality test were performed on this data to measure the distribution. Age of patient were analysed using independent t-test (because it was normally distributed), while volume of endometrioma and serum AMH level were analyzed using Mann–Whitney test (because of not-normally distributed).
Results
A total of 44 subjects was recruited in this study. These subjects were divided into two groups: 22 of control and 22 of the endometrioma group. Data shows that there were no significant differences in the characteristics of the two study groups. These characteristics include age (P=0.678) and body mass index (P=0.351). The mean patient age was 31 (20–35) years in the endometrioma group and 30 (21–35) years in the control group. The mean of BMI was 23.2 (19.5–27.0) kg/m2 in the endometrioma group and 22.5 (18.3–26.6) kg/m2 in the non-endometrioma group. The average ovarian volume in the endometrioma group (106.83 cm3) was larger than the control group (16.58 cm3). Most of the endometrioma subjects presented with chief complaints of an abdominal mass (45.5%), while lower abdominal pain and infertility were the second and third most common complaints, with percentages of 31.8% and 22.7%, respectively. From the statistical analysis, the average of both baseline characteristics were homogeneous (P-value>0.05), so it was feasible to compare and further statistical tests are performed to assess a relationship between variables (Table 1).
 |
Table 1 Subjects Characteristics |
Comparison of serum AMH levels in the endometrioma and non-endometrioma groups is presented in Table 2.
 |
Table 2 Comparison of Serum AMH Level in Endometrioma and Non-Endometrioma Group |
Table 2 shows the median serum AMH level in the endometrioma group (0.59 ng/mL) is lower than the non-endometrioma group (3.45 ng/mL) with a significant difference (P<0.001).
Table 3 shows that AMH serum levels were quite similar in the unilateral endometrioma group (median=0.60 ng/mL) and bilateral group (median=0.59 ng/mL), but both groups were lower than the non-endometrioma group (median=3.45 ng/mL). There was no significant difference in serum AMH levels based on the laterality of the endometrioma (P=1.000).
 |
Table 3 Comparison of Serum AMH Level Based on Endometrioma Laterality of the Observation Group |
Research data on the correlation of ovarian volume with serum AMH levels in the endometrioma group can be seen in Table 4 and Figure 2.
 |
Figure 2 Scatterplot correlation of ovarian volume containing endometrioma with serum AMH level. |
 |
Table 4 Correlation of Ovary Volume Containing Endometrioma to Serum AMH Level in Endometrioma Group |
Table 4 and Figure 2 show no correlation of endometrioma volume with serum AMH levels (P=0.066). This table also illustrates the strength value of a weak correlation coefficient, r (0.2–0.4), with a negative direction. It can be concluded that the greater the ovarian volume, the smaller the serum AMH level, but the correlation is weak and insignificant.
Discussions
The characteristics of the endometrioma and non-endometrioma groups in this study were homogenous due to its non-significant difference of age and body mass index. The biases from these characteristics could be excluded.
We found that the chief complaint of women presenting with endometrioma were lower abdominal pain, abdominal mass, and infertility. This data is supported by research by a previous study which shows that the most common complaints experienced by women with endometriosis or endometrioma are chronic pelvic pain and infertility.9 Laterality images in the subject show that unilateral masses (n=15) were more frequently found compared to bilateral masses (n=7). This was in accordance with another previous study which detected unilateral endometrioma as the most common endometrioma mass with the ground-glass appearance in transvaginal ultrasonography.10
The present study was attempted to investigate the relationship of serum AMH level in women with endometrioma and their correlation to ovarian volume. Our results indicate that serum AMH levels significantly lower in endometrioma than non-endometrioma, with the lowest level found in the bilateral endometrioma group. For the correlation between serum AMH levels and ovarian volume in women with endometrioma, we found a non-statistically significant correlation.
The results of the study showed a significant difference in serum AMH levels between the endometrioma and non-endometrioma groups. Serum AMH levels in the endometrioma group were lower than the non-endometrioma group. Several previous studies showed that endometrioma is one of the factors that influence serum AMH levels, but most of the changes were due to iatrogenic damage to the ovarian reserve after surgical procedures.2,–11–13 In this study, all subjects were women who had not undergone any surgical procedures related to endometrioma. The results obtained remain consistent with previous studies with similar subject characteristics.
The mechanism underlying low serum AMH levels and decreased ovarian reserve are the presence of chronic inflammatory processes and immunomodulatory disorders associated with endometriosis. The inflammatory process causes follicular damage and dysfunction so that the quality and quantity of the follicle is reduced. This leads to a decrease in serum AMH levels which is a result of the production of ovarian follicular granulosa cells.2,12,13
An earlier study also observed that the group of women with endometrioma experienced more rapid reduction in serum AMH levels compared to the control group, but this was also strongly influenced by the therapy that had been undertaken by the subjects. The study also showed a decrease in serum AMH levels, resulting in earlier menopause onset in women with endometriosis, and led to an increase in morbidity.12
The results of this study showed that low serum AMH levels are one of the pathogeneses of endometrioma. Serum AMH levels in women with endometrioma tend to be low due to chronic inflammatory processes and massive reactive oxygen species (ROS) found in the ovaries. Toxic factors, such as proteolytic enzymes, iron, and ROS are able to penetrate the tissue around endometrioma cysts causing fibrosis, smooth muscle metaplasia, and reduced follicles in the ovarian tissue. The mechanism underlying the low serum AMH levels and decreased ovarian reserve are the inflammatory process and immunomodulation abnormalities associated with toxic endometrioma. The inflammatory process causes follicular damage and dysfunction so that the quality and quantity of the follicle are also reduced. The lower serum AMH level measured, the higher morbidity possessed by a woman due to endometrioma.14,15
Our result indicated that serum AMH levels are lower in the bilateral endometrioma group when compared to other groups. Serum AMH levels in the unilateral endometrioma group were higher than the bilateral group, but not statistically significant. It was similar to an earlier study that found subjects with bilateral endometriomas had lower serum AMH levels compared to unilateral.16 Subjects were assessed in preoperative conditions but were also statistically insignificant. Based on these research data it can be concluded that the endometrioma laterality can affect serum AMH levels, but it cannot be used to assess the severity of endometriosis.
The more endometrioma masses that people with endometriosis have, the higher the imbalance between oxidative and anti-oxidative agents, inflammatory reactions, and immunomodulation of the ovaries. Damage caused by the process has an impact on decreasing ovarian reserves. The lower the ovarian reserve, the lower the serum AMH level in patients.7 A significant decrease in serum AMH levels in a group of subjects with bilateral endometrioma were found in the previous study. The group was compared with a group of subjects with unilateral endometrioma that showed normal serum AMH levels.7 Another study also showed statistically significant differences in serum AMH levels, but the study was conducted on postoperative subjects, so the characteristics of the study group tended to differ based on surgery undertaken by research subjects.16 In this study, although the characteristics of the research subjects have homogeneous values and biases from the influence of endometrioma mass laterality can be removed statistically, further research needs to be done on the effect of endometrioma mass laterality in patients on serum AMH levels which can increase morbidity and complications related to their reproductive health.
The results of the study showed a not statistically significant correlation between serum AMH levels and ovarian volume in women with endometrioma. Ovarian volume in the subject of this study is the volume of ovaries containing endometrioma cysts. Volume is measured using transvaginal ultrasonography by measuring three diameters in endometrioma. The measurement value obtained was then calculated using the ellipsoid formula.17 Statistical test results showed a weak negative correlation. The results illustrated that the large volume of endometrioma has an impact on serum AMH levels even though the correlation is weak.
A study on preoperative serum AMH levels in women with endometrioma was carried out retrospectively. The study showed a correlation between serum AMH levels and endometrioma cyst size. A total of 97 women were divided into two groups (endometrioma cyst mass and non-endometrioma ovarian cyst mass). The results also indicated the impact of the large volume size on the serum AMH level.18 A meta-analysis previously conducted also showed the greater the mass of endometrioma, the greater the damage to ovarian reserves which leads to a decrease in serum AMH levels.19
This study illustrates the impact of endometrioma cysts on ovarian reserves which are assessed using serum AMH levels. It was supported by another previous study.18,19 The meta-analysis found higher morbidity and positive correlation with the magnitude of endometrioma cyst mass. The other study with similar subject characteristics as this study showed a correlation between endometrioma mass and serum AMH levels. These data indicated the greater the mass of endometrioma cysts, the greater the damage to the ovaries. This excessive damage to the ovaries results in low levels of serum AMH produced by ovarian follicular granulosa cells.18,19
Endometrioma causes ovarian damage due to mechanical stretching. Damage to normal ovarian function by endometrioma originates from the cyst content which could be potential sources of “toxicity” to the surrounding healthy tissue. Endometrioma cysts contain cellular damage-mediating factors, proteolytic enzymes, inflammatory molecules, reactive oxygen species (ROS), and iron. This causes changes in the surrounding cells, including modifications in gene expression and genetic changes that could potentially trigger tumor genesis. The larger the endometrioma cyst found in the ovary, the greater the metabolism of oxidative stress.2,–18–20
Changes in oxidative stress metabolism are associated with adverse effects on the development of oocytes and embryos, and ultimately the pregnancy outcomes. In addition, an imbalance of oxidative stress has also been identified as a potential cause of oocyte apoptosis and necrosis in early development of the follicle. AMH serum is mostly produced by granulosa cells from the preantral and early antral follicles obtained from primordial follicles until they become sensitive to gonadotropins. This imbalance in toxicity affects the level of serum AMH which decreases with decreasing ovarian reserve.14,20
We suggest that the examination of AMH levels can picture the severity of endometrioma patients when compared with the volume of cysts found in the ovaries. The use of a comparison of these two variables in daily clinical practice is expected to be a reference for selection of therapy in dealing with cases of endometriosis, especially cases with stages III or higher that have formed endometrioma cysts. But the correlation obtained in this study is weak. This was also related to the unrepresentative distribution of data, and there are samples with very different data distributions.
There is still insufficient data to consider smoking history, environmental condition, and subjects’ activities as another potential bias for serum AMH level measured. A limit of this study was the small number of subjects. The non-significant findings in this study could also be caused by the distribution of subject data that was not representative enough compared to other previous studies. Further larger studies are required to address this limitation.
Conclusions
It can be concluded that serum AMH levels in the endometrioma group were lower than in the non-endometrioma group. We found a negative correlation between serum AMH level and ovarian volume containing endometrioma, but not statistically significant.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34–41. doi:10.1007/s13669-017-0187-1
2. Sanchez A, Viganò P, Somigliana E, Panina-Bordignon P, Vercellini P, Candiani M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update. 2013;20(2):217–230. doi:10.1093/humupd/dmt053
3. Vercellini P, Somigliana E, Vigano P, Abbiati A, Barbara G, Fedele L. ‘Blood on the tracks’ from corpora lutea to endometriomas. BJOG. 2009;116(3):366–371. doi:10.1111/j.1471-0528.2008.02055.x
4. Lehmann P, Vélez MP, Saumet J, et al. Anti-Müllerian hormone (AMH): a reliable biomarker of oocyte quality in IVF. J Assist Reprod Genet. 2014;31(4):493–498. doi:10.1007/s10815-014-0193-4
5. Jamil Z, Fatima SS, Ahmed K, Malik R. Anti-Mullerian hormone: above and beyond conventional ovarian reserve markers. Dis Markers. 2016;2016:1–9. doi:10.1155/2016/5246217
6. Kovačević VM, Anđelić LM, Jovanović AM. Changes in serum antimüllerian hormone levels in patients 6 and 12 months after endometrioma stripping surgery. Fertil Steril. 2018;110(6):1173–1180. doi:10.1016/j.fertnstert.2018.07.019
7. Iwase A, Nakamura T, Nakahara T, Goto M, Kikkawa F. Anti-Müllerian hormone and assessment of ovarian reserve after ovarian toxic treatment: a systematic narrative review. Reprod Sci. 2015;22(5):519–526. doi:10.1177/1933719114549856
8. Lemos NA, Arbo E, Scalco R, Weiler E, Rosa V, Cunha-Filho JS. Decreased anti-Müllerian hormone and altered ovarian follicular cohort in infertile patients with mild/minimal endometriosis. Fertil Steril. 2008;89(5):1064–1068. doi:10.1016/j.fertnstert.2007.04.048
9. Uncu G, Kasapoglu I, Ozerkan K, Seyhan A, Yilmaztepe AO, Ata B. Prospective assessment of the impact of endometriomas and their removal on ovarian reserve and determinants of the rate of decline in ovarian reserve. Hum Reprod. 2013;28(8):2140–2145. doi:10.1093/humrep/det123
10. Wu M-H, Cheng Y-C, Chang F-M. Ultrasonographic assessment of ovarian endometrioma. J Med Ultrasound. 2008;16(4):241–248. doi:10.1016/S0929-6441(09)60001-1
11. Shebl O, Ebner T, Sommergruber M, Sir A, Tews G. Anti muellerian hormone serum levels in women with endometriosis: a case–control study. Gynecol Endocrinol. 2009;25(11):713–716. doi:10.3109/09513590903159615
12. Romanski PA, Brady PC, Farland LV, Thomas AM, Hornstein MD. The effect of endometriosis on the antimüllerian hormone level in the infertile population. J Assist Reprod Genet. 2019:1–6.
13. Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Müllerian hormone in women. Hum Reprod Update. 2014;20(3):370–385.
14. Carrillo L, Seidman D, Cittadini E, Meirow D. The role of fertility preservation in patients with endometriosis. J Assist Reprod Genet. 2016;33(3):317–323. doi:10.1007/s10815-016-0646-z
15. Marca AL, Sighinolfi G, Radi D, et al. Anti-Müllerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update. 2009;16(2):113–130. doi:10.1093/humupd/dmp036
16. Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril. 2010;94(1):343–349. doi:10.1016/j.fertnstert.2009.02.022
17. Lass A, Brinsden P. The role of ovarian volume in reproductive medicine. Hum Reprod Update. 1999;5(3):256–266. doi:10.1093/humupd/5.3.256
18. Jeon JH, Park SY, Lee SR, Jeong K, Chung HW. Serum anti-Müllerian hormone levels before surgery in patients with ovarian endometriomas compared to other benign ovarian cysts. J Menopausal Med. 2015;21(3):142–148. doi:10.6118/jmm.2015.21.3.142
19. Muzii L, Tucci CD, Feliciantonio MD, et al. Antimüllerian hormone is reduced in the presence of ovarian endometriomas: a systematic review and meta-analysis. Fertil Steril. 2018;110(5):932–40 e1. doi:10.1016/j.fertnstert.2018.06.025
20. Mavrelos D, Saridogan E. Treatment of endometriosis in women desiring fertility. JOGI. 2015;65(1):11–16.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.