Back to Journals » International Journal of General Medicine » Volume 16
Correlation of Atherosclerotic Dyslipidemia with Long-Term Stroke Recurrence in Patients Undergoing Intravenous Thrombolysis for Acute Ischemic Stroke
Authors Cheng Y, Wang Q, Niu G, Luo C
Received 28 February 2023
Accepted for publication 21 April 2023
Published 2 May 2023 Volume 2023:16 Pages 1621—1629
DOI https://doi.org/10.2147/IJGM.S407971
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yuan Cheng,1,* Qingqing Wang,2,* Guihong Niu,1 Chun Luo3
1Medical School, Fuyang Normal University, Fuyang, Anhui, People’s Republic of China; 2Department of Neurology, The Affiliated Fuyang People’s Hospital of Anhui Medical University, Fuyang, Anhui, People’s Republic of China; 3Department of Pediatric Surgery, Fuyang Women and Children’s Hospital, Fuyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guihong Niu, Medical School, Fuyang Normal University, Fuyang, Anhui, 236300, People’s Republic of China, Email [email protected] Chun Luo, Department of Pediatric Surgery, Fuyang Women and Children’s Hospital, Fuyang, Anhui, 236300, People’s Republic of China, Email [email protected]
Background: Atherosclerotic dyslipidemia (AD) is associated with an increased risk of cardiovascular diseases and stroke events, but the effect of AD among acute ischemic stroke (AIS) patients undergoing intravenous thrombolysis is unclear. This study aimed to investigate the relationship between AD and long-term stroke recurrence in AIS patients undergoing intravenous thrombolysis.
Methods: This prospective cohort study included 499 AIS patients treated with intravenous thrombolysis. Stroke subtype was classified according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria, patients’ clinical characteristics, and results from multiple diagnostic tests. The primary endpoint event was ischemic stroke recurrence; the time to first AIS recurrence was estimated using Kaplan‒Meier analysis and compared using the two-sided Log rank test. Cox univariate and multivariate regression analyses were used to assess the association between AD and long-term stroke recurrence.
Results: Of the 499 patients with AIS treated with rt-PA intravenous thrombolysis, 80 (16.0%) had AD, and 60 (12.0%) had a stroke recurrence event. Kaplan‒Meier analysis showed that the stroke recurrence rate was significantly higher in patients with AD than in those without AD (p = 0.035, log rank test) and in the large-artery disease (LAD) subtype (p = 0.006, log rank test). Multivariate Cox regression analysis showed that AD (HR = 2.363, 95% CI: 1.294– 4.314, P = 0.005) and atrial fibrillation (HR = 2.325, 95% CI: 1.007– 5.366, P = 0.048) were associated with an increased risk of long-term stroke recurrence in AIS patients who underwent intravenous thrombolysis. Furthermore, AD was associated with an increased risk of stroke recurrence in patients undergoing intravenous thrombolysis in the LAD subtype (HR = 3.122, 95% CI: 1.304– 7.437, P = 0.011).
Conclusion: We found that AD increases the risk of long-term stroke recurrence in AIS patients undergoing intravenous thrombolysis. This association may be stronger in the LAD subtype.
Keywords: atherosclerotic dyslipidemia, ischemic stroke, stroke recurrence, thrombolysis
Introduction
Acute ischemic stroke (AIS) accounts for approximately 60–80% of all strokes and is characterized by high morbidity, disability and mortality.1–4 In the past two decades, significant advances have been made in treatment strategies, particularly the widespread clinical use of recombinant tissue plasminogen (rt-PA). The use of rt-PA for AIS results in the rapid recovery of cerebral blood flow by dissolving thrombosis through the activation of plasminogen. This benefits the improved clinical outcomes of patients with AIS, but it has a limited time window and a low recanalization rate for intravenous thrombolysis, leaving a proportion of patients with severe neurological and cognitive deficits.5,6 Therefore, a reliable and valid method for predicting the risk of long-term recurrence of stroke in patients who underwent intravenous thrombolysis for AIS is needed.
Atherosclerotic dyslipidemia (AD) is a lipid abnormality defined as elevated triglyceride (TG) levels and low high-density lipoprotein cholesterol (HDL-C) levels.7–9 Related research shows that AD increases the risk of coronary events in patients with and without coronary artery diseases.10–13 Hoshino et al studied 792 patients with AIS or transient ischemic attack (TIA) and found that AD was associated with intracranial atherosclerosis and a high residual vascular risk at 1 year after stroke or TIA.14 Previous studies on AIS have also focused on risk factors for first stroke and short-term prognosis, although there has been less research on factors influencing the long-term recurrence of stroke in people with intravenous thrombolysis for AIS. Moreover, the effect of AD on long-term stroke recurrence in patients who underwent intravenous thrombolysis for AIS has not been reported. This study is the first to examine the effect of AD on long-term stroke recurrence among patients who underwent intravenous thrombolysis for AIS.
Methods
Subjects
Patients with AIS who were hospitalized in the Department of Neurology of Affiliated Fuyang People’s Hospital of Anhui Medical University and received rt-PA intravenous thrombolytic therapy from July 2017 to December 2021 were consecutively enrolled in the study. The inclusion criteria were as follows: patients aged ≥18 years who met the indications for intravenous thrombolysis with AIS and underwent intravenous thrombolysis. The exclusion criteria were as follows: (1) contraindications for intravenous thrombolysis; (2) treated with bridging endovascular therapy; (3) severe liver and kidney failure; and (4) incomplete imaging and clinical data. All patients underwent diffusion-weighted imaging (DWI) and MRA or CTA angiography within 48 hours of admission. All imaging data were interpreted by experienced neurologists and neuroradiologists who were blinded to the patient’s clinical information. All patients in this study signed informed consent forms and were certified by the Ethics Committee of the Affiliated Fuyang People’s Hospital of Anhui Medical University.
Clinical Data Collection
Baseline information was collected from patients, including sex, age, body mass index, vascular risk factors (hypertension, diabetes, coronary artery disease, atrial fibrillation, history of stroke or TIA, etc.), lipid levels in the fasting state, and discharge medications (antiplatelet drugs, anticoagulants, statins, antihypertensive drugs, hypoglycemic drugs, etc.). The subtypes of AIS were classified as large artery disease (LAD), small artery disease (SAD), cardioembolism (CE), or other and undetermined etiologies by two specialized neurologists based on the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification and the clinical characteristics and the results of multiple diagnostic tests of the patients.15
Blood Lipid Determination
Five milliliters of venous blood was collected from the patient in a fasting state and sent to the biochemistry laboratory of the hospital. After centrifugation for 10 min at 3000 r/min, the supernatant was retained, and the lipid index was determined by using the Siemens 2400 Automatic Biochemical Analyzer. We used TG and HDL-C levels assessed at hospitalization to define AD. AD is defined as high TGs (≥150 mg/dL) and low HDL-C levels. Given the known sex differences in HDL-C levels at baseline, different HDL-C cutoffs were used for males (< 40 mg/dL) and females (< 50 mg/dL).16
Assessment of Stroke Recurrence Risk
The Essen Stroke Risk Score (ESRS) assesses each patient’s risk of stroke recurrence on a 10-point scale: 2 points for age >75 years and 1 point each for age ≥65–75 years, diabetes mellitus, arterial hypertension, peripheral arterial disease, previous myocardial infarction, other cardiovascular disease (except myocardial infarction and atrial fibrillation), smokers, and previous history of TIA or ischemic stroke. The risk of stroke recurrence was assessed for each patient according to the ESRS.17
Follow-Up of People with Long-Term Recurrence of Ischemic Stroke
Patients enrolled in this study who underwent intravenous thrombolysis for AIS were followed up by medical professionals every 6 months by telephone interviews or outpatient visits, primarily to document survival status. Once patients presented with new symptoms, they were immediately hospitalized and underwent a complete cranial MRI to determine if AIS recurrence had occurred. AIS recurrence was defined as a recently diagnosed stroke that manifests as a new neurological deficit or worsening of a previous neurological deficit. All instances of recurrence after thrombolysis were based on clear medical records. If the neurological deficit lasted longer than 24 hours, the lesion was confirmed by neuroimaging methods.
Statistical Analysis
SPSS 22.0 statistical analysis software was used for data analysis. Continuous variables were tested for normality using the Kolmogorov‒Smirnov test. Normally distributed measures were expressed as the mean±standard deviation, t tests were used for comparisons between two groups, and ANOVA was used for comparisons between multiple groups. The time to first recurrence in patients who underwent intravenous thrombolysis for AIS was analyzed by the Kaplan‒Meier method. Stroke recurrence rates were compared between patients with and without AD. The association between stroke recurrence and potential factors in patients who underwent intravenous thrombolysis for AIS was assessed by the Cox proportional risk model with and without adjustment for the prespecified factors mentioned above. Additionally, the hazard ratios (HRs) and 95% confidence intervals (CIs) of the associations were estimated. A two-sided P value of <0.05 was used as the threshold for statistical significance for all tests. Figures were generated using PowerPoint and GraphPad Prism software (version 8.0).
Results
Clinical and Demographic Data
A total of 670 patients receiving intravenous thrombolysis for AIS were admitted during the study period. Of these patients, 152 met the exclusion criteria, and 19 were lost to follow-up. Finally, 499 eligible patients were enrolled in our study (Figure 1), including 318 (63.7%) males and 181 (36.3%) females, aged 65.3±12.9 years; 80 (16.0%) patients had AD, and 419 (80.0%) did not. The baseline characteristics of the study population according to the presence or absence of AD are shown in Table 1. The atrial fibrillation prevalence and ages were lower while those with glucose-lowering medications and a history of diabetes were higher in the AD group compared to the non-AD group. The median follow-up was 26.3 months (IQR=24.8; range, 0.43–63.9 months), and a total of 60 (12.0%) patients had a recurrence of stroke during the study period. Of these, stroke recurrence was 15 (18.8%) in patients with acute ischemic stroke with AD and 45 (10.7%) in patients with ischemic stroke without AD. During the follow-up period, four patients experienced cerebral hemorrhage, excluding the endpoint event.
 |
Table 1 Clinical Characteristics of Patients |
 |
Figure 1 Patient flow diagram. |
Risk Analysis of Recurrence in Patients Who Underwent Intravenous Thrombolysis for AIS
Univariate Cox proportional risk analysis showed that AD was associated with an increased risk of long-term stroke recurrence in patients undergoing intravenous thrombolysis for AIS (HR=1.860, 95% CI: 1.036–3.339, P=0.038), using patients without AD as a reference. Factors that increased the risk of long-term recurrence of AIS among patients who underwent intravenous thrombolysis included age (HR=1.025, 95% CI: 1.004–1.047, P=0.019), atrial fibrillation (HR=2.883, 95% CI: 1.669–4.980, P<0.001), coronary artery disease (HR=2.222, 95% CI: 1.267–3.896, P=0.005), ESRS score (HR=1.288, 95% CI: 1.070–1.549, P=0.007), vascular stenosis (HR=1.794, 95% CI: 1.076–2.991, P=0.025), and cardioembolism (HR=3.419, 95% CI: 1.616–7.232, P=0.001), with small artery occlusion stroke as the reference. After correcting for relevant variables (P < 0.10), AD (HR = 2.363, 95% CI: 1.294–4.314, P = 0.005) and atrial fibrillation (HR = 2.325, 95% CI: 1.007–5.366, P = 0.048) were significantly associated with an increased risk of long-term stroke recurrence in patients undergoing intravenous thrombolysis for AIS (Table 2). Kaplan‒Meier curve analysis showed that the risk of long-term recurrence was significantly higher in AD patients who underwent intravenous thrombolysis for AIS than in patients without AD (P = 0.035, log rank test) (Figure 2).
 |
Table 2 Univariate and Multivariate Cox Risk Models for the Risk of Recurrence in Patients Undergoing Intravenous Thrombolysis for AIS |
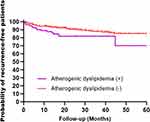 |
Figure 2 Kaplan‒Meier curves estimating the probability of recurrent survival in patients with AD, non-AD and intravenous thrombolysis for AIS (P=0.035, log rank test). +, Yes; -, No. |
Association Between AD and Stroke Recurrence in Patients Stratified by AIS Subtypes
Analysis of the risk of recurrence of AD in patients undergoing intravenous thrombolysis in different stroke subtypes showed that AD was associated with an increased risk of stroke recurrence in patients undergoing intravenous thrombolysis in LAD (HR = 3.122, 95% CI: 1.304–7.437, P = 0.011) and a higher risk of long-term recurrence than in the total stroke population, with no significant differences in other stroke subtypes, as shown in Table 3. Kaplan‒Meier curve analysis showed that the risk of long-term recurrence was also significantly higher in patients with AD than in non-AD patients with large artery atherosclerotic disease type stroke (P = 0.006, log rank test) (Figure 3).
 |
Table 3 Analysis of AD and the Risk of Recurrence in Patients with Different Subtypes of Ischemic Stroke with Intravenous Thrombolysis |
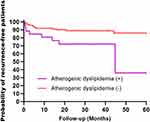 |
Figure 3 Kaplan‒Meier curves estimating the probability of recurrent survival in patients with AD, non-AD and intravenous thrombolysis in the LAD subtype (P=0.006, log rank test).+, Yes; -, No. |
Discussion
To our knowledge, this study is the first cohort study to confirm the effect of AD on the long-term recurrence of stroke in patients undergoing intravenous thrombolysis for AIS. This study not only provides a basis for the secondary prevention of stroke recurrence among patients who underwent intravenous thrombolysis of AIS but also provides relevant data to support such studies. This study showed two key findings: first, AD predicted long-term stroke recurrence in patients who underwent thrombolysis for AIS and was more pronounced in the LAD subtype; second, data from this study showed that atrial fibrillation was also significantly associated with the risk of long-term stroke recurrence in this target population. We recommend building a complete and effective secondary prevention system to reduce the risk of long-term recurrence in patients who underwent intravenous thrombolysis for AIS by establishing comorbidity management, promoting healthy lifestyles, and promoting safe medication use.
Both high TG and low HDL-C levels are predictors of cardiovascular events independent of LDL-C levels. AD combines information from TG and HDL-C and is now widely used in prognostic studies of cardiovascular disease.10–13,18 A study of 510 patients with AIS found that AD was significantly associated with the risk of stroke recurrence in patients with AIS.19 In this cohort study, we found that the prevalence of AD in the study population was 16.3%, and the risk of long-term stroke recurrence was significantly higher in patients with AD than in those without AD. Therefore, we hypothesize that the AD index is also applicable to assess the risk of long-term stroke recurrence in the thrombolysis population for AIS. The first possible mechanism is triglyceride-rich lipoproteins (residual cholesterol), which are small enough to enter the intima of the arteries. Once inside the intima, the residues cause low-grade inflammation and foam cell formation, leading to the development of atherosclerotic plaques, which can lead to atherosclerotic thrombosis. Second, atherosclerosis can accelerate the formation of fibrous lipid plaques in the intima of arteries, leading to wall thickening and lumen narrowing, further promoting the development of atherosclerotic plaques and thrombosis.20,21 The studies of Turan et al and Hoshino et al also confirmed that lipid abnormalities are strongly correlated with the severity of intracranial large artery stenosis and that large vessel stenosis can further increase the residual vascular risk in patients with AIS.14,22
Moreover, in the study of AD and stroke subtype, the role of AD on stroke recurrence in patients with LAD was obvious, and the same finding was obtained in our study population, whereby AD was associated with a higher risk of long-term stroke recurrence in the LAD subgroup.19,23 Thus, AD may be a new modifiable factor in this subgroup of patients. Atherosclerotic thrombosis is a good target when treating AD in clinical trials to prevent stroke recurrence.
Based on data from our study, atrial fibrillation is associated with the risk of long-term recurrence in the AIS intravenous thrombolysis population. Atrial fibrillation causes hemodynamic and structural changes in the heart that lead to the formation of appendage thrombi, which are dislodged and cause embolism, mostly blocking large intracranial vessels and involving multiple vascular divisions, resulting in large infarcts and severe neurological deficits. Studies have shown that atrial fibrillation increases stroke risk at all ages by 3 to 5 times and is associated with ischemic stroke severity, recurrence rates, and mortality after adjustment for stroke risk factors.24,25
Previous studies of AIS have focused on the effect of traditional risk factors such as sex, age, BMI, baseline NIHSS score, and diabetes mellitus on stroke recurrence in AIS.26–31 In this study, we also evaluated the effect of the ESRS score on the long-term recurrence of stroke in the study target population, as this score assesses the risk of stroke recurrence in each patient. Based on our study data, the validity of the ESRS score in predicting stroke recurrence was verified; stroke recurrence was significantly higher when the ESRS score was >2 than when the ESRS score was low (15.7% vs 8.4%, P=0.011). We suggest that for patients with high ESRS scores, efforts should focus on follow-up and re-evaluation of secondary prevention strategies.
This study is the first to confirm the effect of AD on the long-term recurrence of stroke in patients who underwent intravenous thrombolysis for AIS, but it also has some limitations. First, this study is a single-center study with a small sample size; thus, the risk of selection bias was high. In the future, we will conduct a multicenter cohort study and increase the sample size to overcome the limitations of the sample study. Second, as patients with a history of cardiovascular and cerebrovascular disease are likely to have taken lipid-lowering drugs before the study was started, we may have underestimated the prevalence of AD in this study.
Conclusion
In summary, our study supports that AD is associated with a higher risk of long-term recurrence among patients who underwent intravenous thrombolysis for AIS and that this association may be more pronounced in the LAD subtype, but our conclusions require larger, longer follow-up studies for validation.
Data Sharing Statement
All data generated for this study are included in the article. The datasets generated during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The study was performed according to the Declaration of Helsinki guidelines and was approved by the Institutional Review Board of the Affiliated Fuyang People’s Hospital of Anhui Medical University (approval number: [2019] 67). Written informed consent was obtained from the patient or from a family member.
Acknowledgments
We acknowledge the American Journal Experts (AJE) team for their help in language editing. This study was supported by the Natural Science Foundation Key Project of Fuyang Normal University (2020YXZX01ZD).
Disclosure
The authors have declared no conflicts of interest.
References
1. Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. doi:10.1016/S1474-4422(18)30500-3
2. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi:10.1161/STR.0000000000000211
3. Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in china: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759–771. doi:10.1161/CIRCULATIONAHA.116.025250
4. Tu WJ, Zhao Z, Yin P, et al. Estimated burden of stroke in China in 2020. JAMA Netw Open. 2023;6(3):e231455. doi:10.1001/jamanetworkopen.2023.1455
5. Mueller L, Pult F, Meisterernst J, et al. Impact of intravenous thrombolysis on recanalization rates in patients with stroke treated with bridging therapy. Eur J Neurol. 2017;24(8):1016–1021. doi:10.1111/ene.13330
6. Cui Y, Wang L, Clinical A. Observation of intravenous thrombolysis in acute ischemic stroke with minor trauma. Neuropsychiatr Dis Treat. 2021;17:1983–1987. doi:10.2147/NDT.S290443
7. Sirimarco G, Deplanque D, Lavallée PC, et al. Atherogenic dyslipidemia in patients with transient ischemic attack. Stroke. 2011;42(8):2131–2137. doi:10.1161/STROKEAHA.110.609727
8. López-Montoya P, Cerqueda-García D, Rodríguez-Flores M, et al. Association of gut microbiota with atherogenic dyslipidemia, and its impact on serum lipid levels after bariatric surgery. Nutrients. 2022;14(17):3545. doi:10.3390/nu14173545
9. Julián MT, Pera G, Soldevila B, et al. Atherogenic dyslipidemia, but not hyperglycemia, is an independent factor associated with liver fibrosis in subjects with type 2 diabetes and NAFLD: a population-based study. Eur J Endocrinol. 2021;184(4):587–596. doi:10.1530/EJE-20-1240
10. Kutkiene S, Petrulioniene Z, Laucevicius A, et al. Cardiovascular risk profile of patients with atherogenic dyslipidemia in middle age Lithuanian population. Lipids Health Dis. 2018;17(1):208. doi:10.1186/s12944-018-0851-0
11. Stahel P, Xiao C, Hegele RA, Lewis GF. The atherogenic dyslipidemia complex and novel approaches to cardiovascular disease prevention in diabetes. Can J Cardiol. 2018;34(5):595–604. doi:10.1016/j.cjca.2017.12.007
12. Manninen V, Tenkanen L, Koskinen P, et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation. 1992;85(1):37–45. doi:10.1161/01.cir.85.1.37
13. Arca M, Montali A, Valiante S, et al. Usefulness of atherogenic dyslipidemia for predicting cardiovascular risk in patients with angiographically defined coronary artery disease. Am J Cardiol. 2007;100(10):1511–1516. doi:10.1016/j.amjcard.2007.06.049
14. Hoshino T, Ishizuka K, Toi S, et al. Atherogenic dyslipidemia and residual vascular risk after stroke or transient ischemic attack. Stroke. 2022;53(1):79–86. doi:10.1161/STROKEAHA.121.034593
15. Adams HP
16. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. doi:10.1161/circ.106.25.3143
17. Diener HC, Ringleb PA, Savi P. Clopidogrel for the secondary prevention of stroke. Expert Opin Pharmacother. 2005;6(5):755–764. doi:10.1517/14656566.6.5.755
18. Sirimarco G, Labreuche J, Bruckert E, et al. Atherogenic dyslipidemia and residual cardiovascular risk in statin-treated patients. Stroke. 2014;45(5):1429–1436. doi:10.1161/STROKEAHA.113.004229
19. Zhao L, Wang R, Song B, et al. Association between atherogenic dyslipidemia and recurrent stroke risk in patients with different subtypes of ischemic stroke. Int J Stroke. 2015;10(5):752–758. doi:10.1111/ijs.12471
20. Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128(12):1298–1309. doi:10.1161/CIRCULATIONAHA.113.003008
21. Goldberg IJ, Eckel RH, McPherson R. Triglycerides and heart disease: still a hypothesis? Arterioscler Thromb Vasc Biol. 2011;31(8):1716–1725. doi:10.1161/ATVBAHA.111.226100
22. Turan TN, Makki AA, Tsappidi S, et al. Risk factors associated with severity and location of intracranial arterial stenosis. Stroke. 2010;41(8):1636–1640. doi:10.1161/STROKEAHA.110.584672
23. Kumral E, Evyapan D, Gökçay F, Karaman B, Orman M. Association of baseline dyslipidemia with stroke recurrence within five-years after ischemic stroke. Int J Stroke. 2014;9(Suppl A100):119–126. doi:10.1111/ijs.12341
24. Hu Y, Ji C. Efficacy and safety of thrombolysis for acute ischemic stroke with atrial fibrillation: a meta-analysis. BMC Neurol. 2021;21(1):66. doi:10.1186/s12883-021-02095-x
25. Findler M, Molad J, Bornstein NM, Auriel E. Worse outcome in patients with acute stroke and atrial fibrillation following thrombolysis. Isr Med Assoc J. 2017;19(5):293–295.
26. Zheng S, Yao B. Impact of risk factors for recurrence after the first ischemic stroke in adults: a systematic review and meta-analysis. J Clin Neurosci. 2019;60:24–30. doi:10.1016/j.jocn.2018.10.026
27. Horenstein RB, Smith DE, Mosca L. Cholesterol predicts stroke mortality in the women’s pooling project. Stroke. 2002;33(7):1863–1868. doi:10.1161/01.str.0000020093.67593.0b
28. Iso H, Jacobs DR
29. Sacco RL, Benson RT, Kargman DE, et al. High-density lipoprotein cholesterol and ischemic stroke in the elderly: the Northern Manhattan stroke study. JAMA. 2001;285(21):2729–2735. doi:10.1001/jama.285.21.2729
30. Chen XW, Nazri Shafei M, Abdul Aziz Z, Nazifah Sidek N, Imran Musa K. Modelling the prognostic effect of glucose and lipid profiles on stroke recurrence in Malaysia: an event-history analysis. PeerJ. 2020;8:e8378. doi:10.7717/peerj.8378
31. Barkas F, Elisaf M, Liberopoulos E, Liontos A, Rizos EC. High triglyceride levels alter the correlation of apolipoprotein B with low- and non-high-density lipoprotein cholesterol mostly in individuals with diabetes or metabolic syndrome. Atherosclerosis. 2016;247:58–63. doi:10.1016/j.atherosclerosis.2016.02.001
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
