Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Correlation Between Plasma High Mobility Group Protein N1 Level and the Prognosis of Patients with Acute Cerebral Infarction: Preliminary Findings
Authors Lin Y, Wang K, Ji D, Gong Z , Wang Z
Received 25 January 2022
Accepted for publication 1 April 2022
Published 19 April 2022 Volume 2022:18 Pages 907—913
DOI https://doi.org/10.2147/NDT.S359879
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yufeng Lin,1 Kaiyuan Wang,2 Daowen Ji,1 Zhongying Gong,1 Zhiyun Wang1
1Department of Neurology, Tianjin First Central Hospital, School of Medicine, Nankai University, Tianjin, People’s Republic of China; 2Department of Anesthesiology, Tianjin Medical University Cancer Institute and Hospital, Tianjin, People’s Republic of China
Correspondence: Zhiyun Wang; Zhongying Gong, Department of Neurology, Tianjin First Central Hospital, School of Medicine, Nankai University, Tianjin, 300192, People’s Republic of China, Tel +86 22-23626600, Fax +86 22-23626600, Email [email protected]; [email protected]
Purpose: To investigate the correlation between plasma levels of high mobility group protein N1 (HMGN1) and the severity of neurological deficits and prognosis in patients with acute cerebral infarction (ACI).
Patients and Methods: The plasma HMGN1 levels of 108 patients with ACI were detected by ELISA. The National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale (mRS) were used to assess the neurological impairment and outcomes of these patients, respectively. The correlation between HMGN1 levels and clinical parameters was analyzed.
Results: The plasma HMGN1 levels of patients with ACI were positively correlated with their NIHSS and mRS scores. Patients with the large artery atherosclerosis (LAA) subtype in the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification had higher plasma HMGN1 levels than patients with other subtypes.
Conclusion: HMGN1 levels are positively correlated with the severity of ACI and could be used to predict the prognosis of these patients. HMGN1 can be used as a biological marker and potential target for clinical assessment and therapy of ACI.
Keywords: high mobility group protein N1, alarmins, cerebral infarction, neurologic examination
Introduction
Acute cerebral infarction (ACI) is the most common type of stroke, accounting for 69.6–70.8% of all stroke cases.1 ACI causes neurological deficits and even death due to different degrees of neuronal ischemia and necrosis, which poses a great risk to human health. Studies have shown that a series of inflammatory responses occur in the brain after ACI and that the imbalance in the secretion of cellular inflammatory factors can further promote the occurrence and development of cerebral infarction.2
Alarmins are a class of endogenous mediators that are rapidly released extracellularly when the body is stimulated by danger signals; they further activate the natural immune system.3,4 The high-mobility group (HMG) proteins consist of three families, HMGA, HMGB, and HMGN.5,6 HMGB1 and HMGN1 have been shown to function as alarmins.4,7 HMGB1 is a highly abundant intracellular non-histone chromosome-binding protein that is expressed in various tumor tissues and is involved in tumor invasion and metastasis;8 it is reported to correlate with the severity of acute cerebral infarction.9 HMGN1 is a highly conserved non-histone protein that regulates gene expression and affects chromosome structure by acting directly on nucleosomes in the nucleus, thus participating in various critical biological processes.10 Studies have demonstrated that the plasma HMGN1 level is associated with metastasis and the prognosis of patients with non-small cell lung cancer (NSCLC),11 while there are no studies on the relationship between HMGN1 and the progression of ACI. In this study, we investigated the correlation between plasma HMGN1 levels and the National Institutes of Health Stroke Scale (NIHSS) and the 3-month modified Rankin Scale (mRS) in ACI patients, providing theoretical support for the further assessment and treatment of ACI.
Materials and Methods
Participants
One hundred eight patients with ACI who were hospitalized in the Department of Neurology of Tianjin First Central Hospital from March 2019 to March 2021 were enrolled, with 56 men and 52 women aged 38 to 75 years. The clinical manifestations and head CT and/or MRI findings met the diagnostic criteria for ACI. There were 42 cases of basal nucleus infarction, 18 cases of thalamic infarction, 25 cases of lobar infarction, 12 cases of cerebellar infarction, and 11 cases of brainstem infarction. According to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification, there were 35 cases of small artery occlusions (SAO), 30 cases of large artery atherosclerosis (LAA), 20 cases of cardioembolism (CE), 11 cases of stroke of other determined etiologies (SOE), and 12 cases of stroke of undetermined etiology (SUE). The inclusion criteria were as follows: (1) acute onset, admitted within 72 hours of symptoms onset; (2) focal neurological deficits (weakness or numbness of one side of the face or limb, language disorder, and so on), and rarely, generalized neurological deficits; (4) definite vascular causes; (5) brain CT/MRI to rule out cerebral hemorrhage. The exclusion criteria were as follows: (1) history of surgery or trauma in the last 3 months; (2) heart disease other than coronary artery disease; (3) hepatic or renal insufficiency; (4) coexisting tumors (5) severe hypertension, diabetes, respiratory disease, peripheral vascular disease, or embolic disease. This study was approved by the ethics committee of Tianjin First Central Hospital (Review No.: 2019N195KY), and informed consent was obtained from each study participant or their legal custodian.
Plasma HMGN1 Levels by Enzyme-Linked Immunosorbent Assay (ELISA)
Five milliliters of venous blood was obtained from patients when fasting before any treatment and placed in a sterile heparin anticoagulation tube for 30 min. It was then centrifuged at 3000 rpm for 10 min; it separated and the supernatant was collected and stored at −80°C in a refrigerator until the tests were to be performed. The plasma HMGN1 level was assayed by ELISA (kit) according to the manufacturer’s instructions.
Assessment of Neurological Deficits and Outcomes
The cerebral impairments of patients with ACI were evaluated using the NIHSS score as follows: <5, mild neurological deficits and symptoms that do not affect daily activities; 5–15, moderate neurological deficits leading to partial activity restriction, limb, speech, or sensory impairment; >15, severe neurological deficits and patients completely unable to take care of themselves.
mRS scores were used to assess the patients’ outcomes at 3 months after the diagnosis of ACI as follows: a score of 0, no residual symptoms; 1, no significant disability (able to carry out all usual activities despite some symptoms); 2, slight disability (able to look after themselves without assistance, but unable to carry out all previous activities); 3, moderate disability (requires some help, but able to walk unassisted); 4, moderately severe disability (unable to attend to bodily functions without assistance and unable to walk unassisted); 5, Severe disability (bedridden, incontinent, and requires continuous care).
Statistics
GraphPad Prism 5.0 (GraphPad, USA) was used to perform all the statistical analyses. Data are presented as the mean and standard deviation (SD). The Kolmogorov–Smirnov test was used to assess the normality of the data. Numerical data were compared using two-tailed Student’s t-tests, paired t-test, or one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparisons test. Fisher’s exact test was used to compare categorical variables. The correlations between HMGN1 levels and NIHSS or mRS scores were assessed using Spearman correlation analysis. A two-tailed P value < 0.05 was considered statistically significant.
Results
Plasma HMGN1 Level and General Clinical Characteristics
The correlation between HMGN1 levels and the patients’ clinical characteristics is presented in Table 1. No significant differences were found in the HMGN1 levels in patients with different ages, genders, body mass index (BMI), and smoking or drinking history (all P > 0.05).
 |
Table 1 Comparison of General Information of ACI Patients |
Correlation Between Plasma HMGN1 Levels and NIHSS Scores
Plasma HMGN1 levels were significantly higher in patients with NIHSS scores >15 than in those with NIHSS scores <5 (P = 0.0476), as shown in Figure 1A. Correlation analysis showed a significant positive correlation between plasma HMGN1 levels and NIHSS scores (R2 = 0.0516; P = 0.0181), as shown in Figure 1B.
HMGN1 Levels are Positively Correlated with mRS Scores in ACI Patients
Plasma HMGN1 levels were significantly higher in patients with mRS scores of 3–5 than in those with mRS scores of 0–2 (P = 0.0376), as shown in Figure 2A. Correlation analysis indicated that plasma HMGN1 levels were also significantly associated with mRS scores (R2 = 0.0581; P = 0.012), and patients with higher HMGN1 levels often had high mRS scores, as shown in Figure 2B.
Increased Plasma HMGN1 Levels in Patients with the LAA Subtype
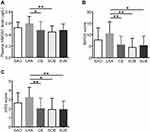
On comparing the HMGN1 expression levels in patients with various TOAST subtypes, we found that patients with the LAA subtype had increased plasma HMGN1 levels than those with SAO, CE, SOE, and SUE, with statistical significance (P < 0.05, Figure 3A). Moreover, the NIHSS and mRS scores of patients with LAA were also apparently higher than those of patients with other TOAST subtypes (P < 0.05, Figure 3B and C).
Discussion
Owing to the establishment of a prevention and control system, the prognosis of stroke patients appears to have improved.12 Despite this, ACI is still one of the main causes of mortality, disability, and long-term cognitive impairment in China.13 A convenient and rapid method to assess the severity of cerebral infarction at the early stage would be beneficial for prompt intervention and treatment. Studies on the mechanism of ACI have revealed that inflammatory responses occur in the infarcted area at an early stage and that the level of inflammatory factors is closely related to the severity of ACI.14,15
Alarmins are a class of multifunctional endogenous molecules that are released extracellularly upon cell injury or necrosis; they serve as endogenous danger signals to recruit and activate antigen-presenting cells (APC) and induce the release of inflammatory factors to generate immune and inflammatory responses.16,17 Various alarmin families have been identified, such as the HMG family, defensin α, and heat shock proteins. HMGB1 is a non-histone chromatin-binding protein that is expressed in normal neuronal cells and is an important cytokine that regulates neurological impairment in ACI.9,14 Animal experiments demonstrate that HMGB1 can migrate from the nucleus to the cytoplasm of neuronal cells 2 h after cerebral infarction.18 In patients with cerebral injury, plasma HMGB1 levels could predict a poor prognosis, suggesting that HMGB1 may be involved in the development of cerebral disease.19
HMGN1 also functions as alarmin and is mainly involved in lipopolysaccharide-induced immune responses, promoting dendritic cells phenotypic and functional maturation through the TLR4-MyD88-TRIF pathway.7,20 A previous study demonstrated that HMGN proteins regulate astrocyte expression and that mice lacking HMGN1 exhibit demyelination, leading to abnormal neurotransmission mediator production.21 It is also reported that the plasma HMGN1 level is associated with metastasis and the prognosis of patients with NSCLC,11 while HMGN1 target therapy exhibits anti-tumor effects in pre-clinical animal experiments.22–24 In this study, we tested the expression of HMGN1 in patients with ACI. We found that plasma HMGN1 levels were significantly higher in patients with NIHSS scores greater than 15. Further analysis revealed that HMGN1 levels are positively correlated with the NIHSS score in patients with ACI. Since the NIHSS score is widely used to evaluate the severity of cerebral infarction, the plasma HMGN1 level could determine the degree of cerebral impairment in patients with ACI.
The mRS is a clinician-reported assessment of global disability that is commonly used for the assessment of functional outcomes in stroke studies.25 It was designed to assess recovery from stroke. It is also used in more than 30 other neurological conditions, including Parkinson’s disease, and as a primary endpoint in randomized clinical trials (RCTs) of emerging acute stroke treatments.26 In this study, the plasma HMGN1 level was negatively correlated with the mRS of patients with ACI, which demonstrated that HMGN1 could be a novel marker to evaluate the prognosis of patients with ACI.
TOAST typing is commonly used in the international classification of cerebrovascular diseases and in diagnostic and therapeutic guidelines for acute ischemic stroke.27 It focuses on the analysis of the pathogenesis of cerebral infarction. In this study, HMGN1 levels were significantly higher in patients with the LAA subtype of TOAST than in those with the other four types. What’s more, the NIHSS and mRS scores of patients with the LAA subtype were also high, when compared with those of other patients with ACI. This suggests more severe neurological impairment and a poorer prognosis of patients with LAA than those with SAO, CE, SOE, and SUE. HMGN1 level could thus be a sensitive and direct biomarker to identify patients with LAA and further evaluate the development and outcomes of ischemic injury.
This study had two limitations. First, the sample size of the enrolled patients with ACI was relatively small, so these preliminary findings should be verified in a study involving a larger number of patients from multiple centers. Second, the underlying mechanisms of how HMGN1 regulates the development of ischemic cerebral impairment should be further investigated in preclinical studies.
Conclusions
The HMGN1 level is positively correlated with the severity of ACI and predicts a poor prognosis for these patients. HMGN1 can be used as a biological marker for the clinical detection of ACI progression, as well as a potential therapeutic target for ACI.
Data Sharing Statement
The original data supporting the findings of this article are available from the corresponding authors on reasonable request.
Ethics Statement
This study was approved by the Ethical Committee of Tianjin First Central Hospital (Review No.: 2019N195KY), and a written, informed consent was obtained from each participant or their legal custodian, in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang T, Zhao J, Li X, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of stroke rehabilitation. Stroke Vasc Neurol. 2020;5(3):250–259. doi:10.1136/svn-2019-000321
2. Jafarinaveh HR, Allahtavakoli M, Rezazadeh H, et al. Proinflammatory cytokines in the embolic model of cerebral ischemia in rat. Iran J Allergy Asthma Immunol. 2014;13(2):125–130.
3. Viemann D. S100-alarmins are essential pilots of postnatal innate immune adaptation. Front Immunol. 2020;11:688. doi:10.3389/fimmu.2020.00688
4. Yang D, Han Z, Oppenheim JJ. Alarmins and immunity. Immunol Rev. 2017;280(1):41–56. doi:10.1111/imr.12577
5. Furusawa T, Cherukuri S. Developmental function of HMGN proteins. Biochim Biophys Acta. 2010;1799(1–2):69–73. doi:10.1016/j.bbagrm.2009.11.011
6. Nanduri R, Furusawa T, Bustin M. Biological functions of HMGN chromosomal proteins. Int J Mol Sci. 2020;21(2):449. doi:10.3390/ijms21020449
7. Yang D, Han Z, Alam MM, Oppenheim JJ. High-mobility group nucleosome binding domain 1 (HMGN1) functions as a Th1-polarizing alarmin. Semin Immunol. 2018;38:49–53. doi:10.1016/j.smim.2018.02.012
8. Wang S, Zhang Y. HMGB1 in inflammation and cancer. J Hematol Oncol. 2020;13(1):116. doi:10.1186/s13045-020-00950-x
9. Umahara T, Uchihara T, Hirokawa K, et al. Time-dependent and lesion-dependent HMGB1-selective localization in brains of patients with cerebrovascular diseases. Histol Histopathol. 2018;33(2):215–222. doi:10.14670/HH-11-914
10. He B, Deng T, Zhu I, et al. Binding of HMGN proteins to cell specific enhancers stabilizes cell identity. Nat Commun. 2018;9(1):5240. doi:10.1038/s41467-018-07687-9
11. Wei F, Yang F, Jiang X, Yu W, Ren X. High-mobility group nucleosome-binding protein 1 is a novel clinical biomarker in non-small cell lung cancer. Tumour Biol. 2015;36(12):9405–9410. doi:10.1007/s13277-015-3693-7
12. Tu WJ, Chao BH, Ma L, et al. Case-fatality, disability and recurrence rates after first-ever stroke: a study from bigdata observatory platform for stroke of China. Brain Res Bull. 2021;175:130–135. doi:10.1016/j.brainresbull.2021.07.020
13. Chao BH, Yan F, Hua Y, et al. Stroke prevention and control system in China: CSPPC-Stroke Program. Intl j Stroke. 2021;16(3):265–272. doi:10.1177/1747493020913557
14. Kim ID, Lee H, Kim SW, et al. Alarmin HMGB1 induces systemic and brain inflammatory exacerbation in post-stroke infection rat model. Cell Death Dis. 2018;9(4):426. doi:10.1038/s41419-018-0438-8
15. Zhang XG, Xue J, Yang WH, et al. Inflammatory markers as independent predictors for stroke outcomes. Brain Behav. 2021;11(1):e01922. doi:10.1002/brb3.1922
16. Giovannetti A, Straface E, Rosato E, Casciaro M, Pioggia G, Gangemi S. Role of alarmins in the pathogenesis of systemic sclerosis. Int J Mol Sci. 2020;21:14. doi:10.3390/ijms21144985
17. Rider P, Voronov E, Dinarello CA, Apte RN, Cohen I. Alarmins: feel the Stress. J Immunol. 2017;198(4):1395–1402. doi:10.4049/jimmunol.1601342
18. Kim SW, Lee JK. Role of HMGB1 in the interplay between NETosis and Thrombosis in ischemic stroke: a review. Cells. 2020;9(8):1794. doi:10.3390/cells9081794
19. Tsukagawa T, Katsumata R, Fujita M, et al. Elevated serum high-mobility group box-1 protein level is associated with poor functional outcome in ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26(10):2404–2411. doi:10.1016/j.jstrokecerebrovasdis.2017.05.033
20. Yu J, Da J, Dong R, et al. IGF-1R inhibitor ameliorates diabetic nephropathy with suppressed HMGN1/TLR4 pathway. Endocr Metab Immune Disord Drug Targets. 2018;18(3):241–250. doi:10.2174/1871530318666180131102707
21. Deng T, Postnikov Y, Zhang S, et al. Interplay between H1 and HMGN epigenetically regulates OLIG1&2 expression and oligodendrocyte differentiation. Nucleic Acids Res. 2017;45(6):3031–3045. doi:10.1093/nar/gkw1222
22. Chen CY, Ueha S, Ishiwata Y, et al. Combined treatment with HMGN1 and anti-CD4 depleting antibody reverses T cell exhaustion and exerts robust anti-tumor effects in mice. J Immunother Cancer. 2019;7(1):21. doi:10.1186/s40425-019-0503-6
23. Wei F, Yang D, Tewary P, et al. The Alarmin HMGN1 contributes to antitumor immunity and is a potent immunoadjuvant. Cancer Res. 2014;74(21):5989–5998. doi:10.1158/0008-5472.CAN-13-2042
24. Zuo B, Qi H, Lu Z, et al. Alarmin-painted exosomes elicit persistent antitumor immunity in large established tumors in mice. Nat Commun. 2020;11(1):1790. doi:10.1038/s41467-020-15569-2
25. Yi K, Inatomi Y, Nakajima M, Yonehara T, Ueda M. Reliability of the modified rankin scale assessment using a simplified questionnaire in Japanese. J Stroke Cerebrovasc Dis. 2021;30(2):105517. doi:10.1016/j.jstrokecerebrovasdis.2020.105517
26. Broderick JP, Adeoye O, Elm J. Evolution of the modified rankin scale and its use in future stroke trials. Stroke. 2017;48(7):2007–2012. doi:10.1161/STROKEAHA.117.017866
27. Hui C, Tadi P, Patti L. Ischemic stroke. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.