Back to Journals » Cancer Management and Research » Volume 11
Correlation between pelvic bone marrow radiation dose and acute hematological toxicity in cervical cancer patients treated with concurrent chemoradiation
Authors Kumar T , Schernberg A, Busato F, Laurans M , Fumagalli I, Dumas I, Deutsch E, Haie-Meder C, Chargari C
Received 26 November 2018
Accepted for publication 25 March 2019
Published 8 July 2019 Volume 2019:11 Pages 6285—6297
DOI https://doi.org/10.2147/CMAR.S195989
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
T Kumar,1–3 A Schernberg,1,2 F Busato,1,2 M Laurans,1,2 I Fumagalli,1,2 I Dumas,1,2 E Deutsch,1,2 C Haie-Meder,1,2 C Chargari1–2,4–5
1Brachytherapy Unit, Gustave Roussy, Cancer Campus, Villejuif, France; 2Radiotherapy Department, Gustave Roussy, Villejuif, France; 3Radiotherapy Department, University Hospital of Grenoble, Grenoble, France; 4Departement ’Effets Biologiques des Radiations’, Institut de Recherche Biomédicale des Armées, Brétigny sur Orge, France; 5French Military Health Academy, Ecole du Val-de-Grâce, Paris, France
Purpose: To evaluate the association between pelvic bone marrow (BM) dose volume parameters and probability of acute hematological toxicity (HT), a cohort of cervical cancer patients receiving definitive chemoradiation (CRT) was assessed.
Materials and methods: Medical records of patients treated by CRT (45 Gy in 25 fractions, without dose constraints applied to the BM) were reviewed. Baseline and weekly hematological parameters were collected. BM was retrospectively delineated and divided into sub-sites: iliac crests, lower pelvis, lumbosacral region. BM volumes (V) receiving 5, 10, 20, 30, 40 Gy (V5, V10, V20, V30, V40, respectively) and mean dose (Dm) were calculated. Logistic regression was used to analyze associations between HT and dose-volume histograms parameters.
Results: 114 patients were included. 75.4% were treated with 3D radiation therapy and 24.6% were receiving intensity modulated radiation therapy (IMRT). Neither age, chemotherapy regimen (cisplatin vs carboplatin), number of chemotherapy cycles, performance status, body mass index, or para-aortic irradiation were associated with HT. In univariate analysis, more frequent grade 3+ leukopenia was found in the IMRT group (odds ratio [OR]: 3.5; 95% CI, 1.4–9.1; p=0.007). In multivariate analysis, grade 4 HT was associated with lower pelvis V5>95% (OR 4.1; 95% CI, 1.6–14. p=0.02), lower pelvis V20>45% (OR 3.5; 95% CI, 1.1–13.4; p=0.05), total pelvic bone V20>65%, and iliac crests Dm >31 Gy (OR 4.5; 95% CI, 1.4–14.7; p=0.02).
Conclusion: The following dose constraints could be proposed to decrease acute HT risk: lower pelvis V5<95%, lower pelvis V20≤45%, total pelvic bone V20<65%, and iliac crests Dm <31 Gy.
Keywords: bone marrow, dosimetric parameters, acute hematological toxicity, cervical cancer
Introduction
Concurrent chemoradiation followed by brachytherapy is the standard of care for locally advanced cervical cancer.1,2 However, this treatment is associated with a significant risk of hematological toxicity (HT), which favors infections, canceled chemotherapy cycles and may increase overall treatment time.3 Nugent et al, reported that patients receiving less chemotherapy cycles had a poorer prognosis and therefore, acute HT leading to a suboptimal chemotherapy dose intensity, may be associated with a higher probability of treatment failure.4
In adults, pelvic bone is the primary site of hematopoiesis. It is estimated that >50% of proliferating bone marrow is located in the pelvic region, including lumbar spine.5 Hematopoietic stem cells are highly radiosensitive, and bone marrow is a potential organ at risk (OAR) in the pelvic region.6 More than 60% of the patients treated by intensity modulated radiotherapy (IMRT) for a cervical cancer experience grade 2 neutropenia.7 Few data are available on the optimal dose constraints to be followed to limit HT probability. A correlation between the volume of whole pelvic bone receiving 20 Gy (V20) and grade 2+ HT has been reported.8 For patients treated by IMRT, other authors have shown a correlation between pelvic bone dose/volume parameters (V10Gy >95% and V20>76%) and grade 3 leukopenia probability.9 Measurements of 18F-fluoro-L-deoxythymidine (FLT) uptake have shown a decrease activity in the bone marrow receiving >35 Gy, lasting until one year after treatment completion.10
Dosimetric studies suggest that IMRT could decrease the dose to the pelvic bone marrow without increasing the dose to other OARs.7,11,12 Phase II trial (Radiation Therapy Oncology Group [RTOG] study 0418) confirmed these data in postoperative setting and highlighted that V40 and mean bone marrow dose were both correlated with grade 2+ HT.13 Mahantshetty et al, proposed to contour the inner cavity of the bone, as it could be a better surrogate of active bone marrow.14 Although there is consensus that HT should be prevented, the dosimetric planning constraints to be followed are not well established as dosimetric data are still scarce.15
We examined the correlations between pelvic bone marrow dose/volume parameters and acute HT incidence, in a cohort of cervical cancer patients, receiving definitive chemoradiation plus image-guided adaptive brachytherapy.
Materials and methods
Inclusion criteria
We retrospectively included patients with previously untreated FIGO (International Federation of Gynecology and Obstetrics) stage Ib-IVb cervical cancer treated with upfront concurrent chemoradiation and brachytherapy in our institution from 2009 to 2016. All patients were treated with a curative intent. Patients who could not receive at least one cycle of chemotherapy because of poor general health status or biological contra-indication were excluded. No patient received neoadjuvant chemotherapy. This retrospective study was conducted in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments and approved by the institutional review board for gynecological cancers from Gustave Roussy Cancer campus. Patients confidentiality was respected in data collection and analysis. Therefore, patient consent was not required.
Radiation therapy and brachytherapy
Patients were treated either by 3D conformal radiation therapy (3DRT) or IMRT. Simulation was performed on a spiral computed tomography scanner with a section thickness of 3 mm. Target volumes and organs at risk delineation were done using Isogray® treatment planning system.
The clinical target volume (CTV) included: the gross tumor, cervix, whole uterus, parametria, 2 cm below the lower vaginal extent and regional lymph nodes (internal iliac, external iliac, obturator, presacral, and common iliac lymph nodes). Groins were included in patients with involvement of lower third of vagina. Patients without any para-aortic lymph node uptake at PET-CT (Positrons Emission Tomography-Computed Tomography) underwent primary laparoscopic para-aortic lymph node dissection to guide radiotherapy volumes. Only patients with para-aortic node detected in PET-CT or with histological evidence of para-aortic metastases, received para-aortic lymph node irradiation. In case of para-aortic irradiation, the upper CTV limit was raised to T12-L1 border. The small bowel, bladder, rectum, and femoral heads were contoured as organs at risk.
The planning target volume (PTV) was defined as an expansion of 7 mm around the lymph node volume and 10–15 mm around the gross tumor and the uterus volume in 3DRT and IMRT.
Patients received 45 Gy in 25 daily fractions in the whole pelvis. Nodal boost was delivered if PET-CT was positive, sequentially after the brachytherapy in patients receiving 3DRT or as simultaneous integrated boost (SIB) in patients receiving IMRT, to reach a total physical dose of 60 Gy, taking into account the calculated (for sequential boosts) or expected (SIB) contribution of brachytherapy.16,17
The dose constraints used to optimize and validate the treatment followed current guidelines in terms of PTV and organs at risk dose constraints.18 The planning goal was to deliver 95% of the dose to at least 95% of the PTV without exceeding OARs dose constraints. Bone marrow was not used as an avoidance organ and especially not included in the reverse planning in IMRT. During external beam radiotherapy, daily set up was done according to bony structures.
Pulse dose rate brachytherapy was performed within 2 weeks after external beam completion in order to keep the overall treatment duration less than 55 days.
Chemotherapy
Patients received weekly cisplatin 40 mg/m2 or carboplatin AUC2 in case of renal impairment. Patients were planned to receive five cycles of chemotherapy during the external radiation therapy. Common guidelines were followed before each cycle, based on physical examination, tolerance to the previous cycle, and biological assays, including the following criteria: absolute neutrophil count >1000G/L, platelet count >100G/L, and creatinine clearance >60 mL/min.
Bone marrow delineation
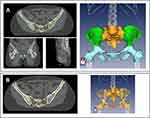
All bone marrow contours were retrospectively delineated manually, blinded of blood cell counts results, in each patient individually, according to Mell et al.19 The external contours of pelvic bones were contoured in bone window and sub-divided into three parts, as not all parts of the pelvic bone have the same importance regarding hematopoiesis (Figure 1):
- lumbosacral (LS) region including the fifth lumbar vertebrae and the entire sacrum,
- iliac crests extending from iliac crests to the upper border of the femoral heads,
- lower pelvis (LP) including pubes, ischia, acetabula, and femoral heads extending to the inferior border of the ischial tuberosities.Two additional volumes were defined:
- A total pelvic bone (TPB) volume was generated by adding (1), (2), and (3).
- The inner cavity of the TPB (IC-TPB) was delineated by excluding manually the cortical bone from the TPB. The IC-TPB corresponds to the lower density trabecular bone, following Mahantshetty et al.14 The inner cavity of the pelvic bone was used as a surrogate of bone marrow.
To limit inter-observer variations, all contours were done by a single radiation oncologist.
Hematological toxicity
All patients had complete blood cells count before any treatment, then weekly during the radiotherapy and at least once within 2 weeks following external radiation therapy completion. HT incidence was defined from analysis of all weekly blood cell counts, and the last blood cell count taken into consideration was performed the day prior to brachytherapy implantation. HT (including absolute neutrophil count, white blood cells, hemoglobin, platelets count and lymphocytes count) was graded based on each cell line nadir and according to common terminology criteria for adverse events version 4, from grade (Gr) 2 to Gr 4.
Statistical analysis
Based on the bone marrow delineation, the relative dose volume histograms (DVHs) were calculated. Bone marrow volumes receiving 5, 10, 15, 20, 30, 40 Gy (V5, V10, V15, V20, V30, V40, respectively) and the mean dose (Dm) were calculated for each volume. Bone marrow is considered as a parallel organ implying that mean dose could be a relevant parameter.20 Brachytherapy dose to the pelvic bone was not taken into account in our study, as the last HT assessment was done prior to brachytherapy. For each patient, incidence of HT was examined for each blood cell line separately and scored from Gr2 to Gr4, then also by merging all HT with focus on the toxicity of highest grade.
χ2 test was used to compare rates of HT with bone marrow dose metrics V5, V10, V15,V20, V30,V40, and Dm. Receiver operating characteristic curve analysis was performed for determining the optimal cut-off values for the dosimetric parameters. Logistic regression was used to identify potential associations between HT and DVHs parameters.
Dosimetric parameters with a p-value <0.1 in univariate analysis were included in the multivariate model. A p-value <0.05 was considered as statistically significant. The differences between the baseline blood cell counts and nadirs were calculated using Wilcoxon signed-rank test. Statistical analysis was conducted using R Studio software version 1.1.442.
Results
Patients, tumors, treatments
One hundred and fourteen patients fulfilled inclusion criteria. Median follow-up was 3.87 years (ranging from 0.56 to 7.72). A total of 75.4% of the patients were treated with 3DRT and 24.6% with IMRT. Eighty-seven (76.3%) patients received five cycles of concurrent chemotherapy. Twenty-two (19.3%) patients received four cycles and only five (4.4%) patients received less than four cycles. Fifty (43.9%) patients had a node positive disease. Lymph node boost was delivered as SIB in four patients and all the remaining patients with macroscopic lymph nodes received a sequential boost after the brachytherapy. Fifteen patients (13.2%) with IVB disease received a para-aortic lymph node irradiation. Patients, tumors, and treatments characteristics are reported in Table 1. The median protraction was 47 days in our study and G3+ HT were associated with a protraction of more than 55 days (OR 82.7; p=0.03).
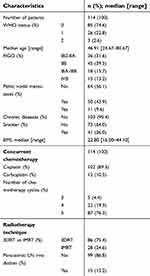 |
Table 1. Patients and treatments characteristics |
Univariate factors for acute HT
All blood cell lines merged (including absolute neutrophil count, white blood cells, hemoglobin, platelets count, and lymphocytes count) Gr 2, 3, and 4 HTs were reported in 27(23.7%), 87(76.3%), and 17(14.9%) patients, respectively (Table 2). No Gr5 toxicity occurred. The median percentage volumes of bone marrow receiving 5, 10, 15, 20, 30, and 40 Gy were 95.5%, 88.8%, 84.1%, 67.7%, 53.8%, and 33.6%, respectively.
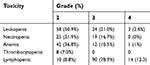 |
Table 2. Acute hematologic toxicity |
There was a highly significant and clinically relevant decrease in all types of blood cells between the baseline blood counts and the nadirs (Table 3).
 |
Table 3. Descriptive statistics of hematological baselines and nadirs |
Univariate analysis
In univariate analysis, age, chemotherapy regimen (cisplatin vs carboplatin), number of chemotherapy cycles, performance status, body mass index or delivery of para-aortic irradiation were not found significantly associated with HT. The incidence and severity of HT in patients receiving 3DRT or IMRT are reported in Table 4. More frequent Gr 3–4 (G3+) leukopenia was observed in the IMRT group with an odds ratio (OR) of 3.5 (95% CI, 1.4–9.1; p=0.007) and with lower pelvis V20>50% with OR of 2.8 (95% CI, 1.1–7.2; p=0.03). G3+ neutropenia correlated with lower pelvis V10>75% (OR 8.7; 95% CI, 1.7–160; p=0.04) and lower pelvis V20>42% (OR 3.2; 95% CI, 1.1–12; p=0.05). All blood lines merged Gr4 HT was associated with lower pelvis V5>95% (OR 4.6; 95% CI, 1.6–14; p=0.005), lower pelvis V20>45% (OR 4.1; 95% CI, 1.3–14.3; p=0.02), iliac crests Dm >31 Gy (OR 4.6; 95% CI, 1.6–14; p=0.005), and IC-TPB V5>98% (OR 4.3; 95% CI, 1.5–13.5; p=0.008). G4 HT also correlated with FIGO stage (< IIB vs IIB vs >IIB). The median volume of bone marrow receiving less than 20 Gy was higher in the IMRT group (p<0.001), while the volume of bone marrow receiving more than 40 Gy was lower (p<0.001) than with 3DRT. Detailed univariate analyses are presented in Table S1.
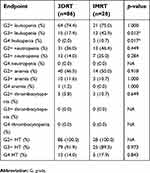 |
Table 4. Incidence and severity of hematologic toxicity (HT) in patients receiving 3D conformal radiotherapy (3DRT) or intensity modulated radiotherapy (IMRT) |
Sub-group univariate analysis: 3DRT and IMRT
In patients treated by IMRT, G3+ leukopenia correlated with LS bone V30>91% (OR 21.7; p=0.02), lower pelvis V15>65% (OR 18.3; p=0.03), lower pelvis V20>48% (OR 11; p=0.02), lower pelvis Dm >21.7 Gy (OR 14.1; p=0.01). Grade 3+ neutropenia correlated with LS bone V30>94% (OR 19.2; p=0.01), iliac crest V20>84% (OR 8; p=0.02), lower pelvis V15>65% (OR 87; p=0.01). Grade 4 HT was associated with lower pelvis V5>95% (OR 138; p=0.02) and lower pelvis V15>65% (OR 138; p=0.02) (Table S2).
In patients treated by 3DRT, grade 3+ anemia correlated with total pelvis V20>69% (OR 138; p=0.02). Grade 3+ lymphopenia was associated with iliac crests V20>52% (OR 20; p=0.01), iliac crests V30>37% (OR 19; p=0.01), iliac crests V40>27% (OR 14; p=0.01), and iliac crests Dm >26 Gy (OR 17; p=0.01) (Table S3).
Multivariate analysis
In multivariate analysis including other parameters with p<0.10 at univariate analysis (FIGO stage for G4 HT). G4 HT remained associated with lower pelvis V5>95% (OR 4.1; 95% CI, 1.6–14. p=0.02). lower pelvis V20>45% (OR 3.5; 95% CI, 1.1–13.4; p=0.05), iliac crests Dm >31 Gy (OR 4.5; 95% CI, 1.4–14.7; p=0.02) and TPB V20>65% (OR 5.0; 95% CI, 1.2–33.3; p=0.04) (Table S4). The DVH parameters calculated from the delineation of the inner cavity of the bone were not anymore significant in multivariate analysis.
Discussion
Bone marrow is a radiosensitive tissue and there is a strong link between the dose and the volume irradiated and the risk of HT. The decline in bone marrow hematopoietic cell is associated with an increased level of adipocytes and a chronic inhibition of hematopoiesis,21 as confirmed by experimental studies.10
In this study, we found correlations between various dosimetric parameters and probability of acute HT. We found that Gr3+ leukopenia incidence was significantly higher in the IMRT group. It is important to highlight that, in our study, the bone marrow had not been initially contoured and used as an avoidance organ in the optimization process. Similarly, Erpolat et al, found that Gr2+ anemia, neutropenia, leukopenia, and thrombocytopenia were higher in the IMRT group.22 This could be explained by the larger volume of bone marrow receiving lower doses in the IMRT group, and these doses are clinically relevant in the context of a high radiosensitivity of bone marrow (Table 5). The volume of the irradiated bone marrow is a key factor. It was previously shown that when small radiation therapy fields are used, unirradiated sub-regions could compensate by increasing the progenitor cell population.6 Several studies, however, suggest that if dose constraints are applied to the bone, IMRT could reduce significantly the dose to the bone marrow and result in lower HT during chemoradiation.23,24 Dosimetric studies have shown that bone marrow dose could be decreased without increasing the dose to other OARs (bladder, rectum, bowels) in IMRT and VMAT plans.25,26 In the prospective RTOG 0418 study, a correlation was found between pelvic bone marrow V40>37% and HT Gr2+. The mean bone marrow dose >34.2 Gy was also associated with higher rates of HT Gr2+.13 Thus, it can be concluded that if IMRT is the preferred radiotherapy modality, the bone marrow should ideally be contoured and taken into consideration for treatment planning optimization.
 |
Table 5. Patients and treatments characteristics |
To date, optimal bone marrow dose/volume constraints to be followed are not well defined and it is also uncertain whether specific anatomic sub-sites should be identified and more specifically spared at the time of treatment planning. We found that HT was more frequently associated with lower pelvis dose than with iliac crests dose, consistent with the findings of Mell et al.19 A possible explanation for these results may come from functional imaging of the pelvic bone marrow. In fact, the central and the lower region of the pelvis bone has higher FLT uptake than the peripheral regions, implying that bone marrow is especially active in these regions.10 This confirmation that lower pelvis dose is particularly relevant for predicting HT may guide treatment planning.
We did not find any associations between the LS bone dose metrics and HT in this study whereas previous studies stated the importance of that particular region.19 The median V20, V30, and Dm to the LS bone were 99.9%, 97.4%, and 40.9 Gy, respectively, in our study. This higher dose delivered to that region in almost the entire cohort could explain our inability to detect such associations and potentially limit the extrapolation of our study results.
The DVH parameters calculated from the delineation of the inner cavity of the bone were not significant in multivariate analysis in our study which is counterintuitive. These results could be explained by two factors: the delineation of these volumes is more difficult, and these volumes disappear in areas where the bone is thin (like the center part of the sacrum and iliac crests) while these areas are located near the high dose volumes (center of the pelvis, pelvic lymph nodes … ).
Li et al, showed that the chemotherapy increases HT while delivered concomitantly or prior to irradiation. As concurrent chemotherapy is a major part of the treatment, which cannot be ignored, the role of this study is to minimize the role played by un-necessary irradiation of the bone marrow and give some dose constraints to be followed. In our study, all patients received concomitant chemotherapy. We did not find an association between the number of chemotherapy cycles and HT but, the vast majority of the patients received five cycles (76.3%). The absence of a statistically significant difference could be explained by the lack of patients receiving less than five chemotherapy cycles.27
Four patients received SIB for lymph nodes, which could potentially affect the study results. Among these four patients, two had leukopenia G3 and one patient had neutropenia G3. These results are similar to the toxicity distribution in the whole population. These four patients were treated in IMRT and their dose distribution in the bone marrow are similar to those treated by IMRT.
One of the limitations of our study is that we did not differentiate proliferating active bone marrow from the inactive sub-regions. Rose et al, showed that functional imaging, using the 18-FDG-PET scanner, might help identifying active bone marrow sub-regions.28 Moreover, there is a correlation between the irradiation of these active sub-regions and acute HT. The international multi-center phase II study (INTERTECC-2) confirmed that PET-guided IMRT could reduce the incidence of Gr 3+ neutropenia by a factor of approximately 3.11 Nevertheless, defining the optimal SUV threshold remains challenging, and most radiotherapy facilities worldwide have not access to PET-CT to define these functional areas. Mahantshetty et al, found that the IC-TPB could be a better surrogate of the active bone marrow and therefore be a better OAR to spare.14 They reported that IC-TPM V40>40% correlated with Gr 2+ HT. In our study, a new finding was that Gr4 HT was associated with IC-TPM V5>98%.
Manual delineation of the different bone marrow volumes, as performed in our study, is time-consuming. The use of a semi-automated delineation may increase the acceptance by the radiation oncologists. Andreychenko et al, developed a semi-automatic segmentation of the active red bone marrow using magnetic resonance (MR) imaging of the pelvis, based on water and fat MR images.29 Another method is to use an atlas-based auto-segmentation with very promising results.30
There are promising biological and technological research perspectives to better understand the biological background of HT, as well as for mitigating this frequent adverse event. Irradiation of the blood vessels and the circulating progenitors could also participate in the acute HT during chemoradiation.31 Our current approach could not evaluate their contribution and further investigations are needed. To further reduce HT, intensity modulated proton therapy, could be an alternative to IMRT in bone marrow sparing pelvic radiation therapy.32 In this context of highly precise radiotherapy, identification of more active sub-regions involved in HT may be promising. The retrospective nature of our study may induce some bias, limited by the objective criteria of outcomes examined there. We also were not able to correlate bone dose volume parameters with the persistence of long-term changes in blood cell counts, such as anemia, which may have an impact on the quality of life. Further prospective studies are however needed to explore this issue, as well as to confirm our findings in an independent cohort.
Conclusion
Bone marrow dosimetric parameters were associated with Gr3+ leukopenia, Gr3+ neutropenia and G4+ HT. Bone marrow delineation should be advised and particularly recommended while treating patients with IMRT. Based on our findings from multivariate analysis using logistic regression, in our cohort of patients treated by 3DRT or IMRT without optimization on the pelvic bone marrow, the following dose constraints could be proposed:
- Lower pelvis V5<95%; lower pelvis V20<45%
- Iliac crests Dm ≤31 Gy
- Total pelvic bone V20<65%.
Acknowledgments
We would like to thank all our colleagues from the Radiotherapy Department, Gustave Roussy, and especially Dr Kissel for her comments and valuable feedback.
Disclosure
E Deutsch report grants, personal fees, and nonfinancial support from Roche, grants from Servier, grants and personal fees from Medimmune, Boerhinger, and Bristol-Myers Squibb, and personal fees from Amgen and Accuray, outside the submitted work, and reports no other conflicts of interest in this work. The other authors report no conflicts of interest in this work.
References
1. Morris M, Levenback C, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi:10.1056/NEJM199904153401501
2. Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of Radiation Therapy Oncology Group Trial (RTOG) 90-01. J Clin Oncol. 2004;22(5):872–880. doi:10.1200/JCO.2004.07.197
3. Kirwan JM, Symonds P, Green JA, Tierney J, Collingwood M, Williams CJ. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol. 2003;68(3):217–226.
4. Nugent EK, Case AS, Hoff JT, et al. Chemoradiation in locally advanced cervical carcinoma: an analysis of cisplatin dosing and other clinical prognostic factors. Gynecol Oncol. 2010;116(3):438–441. doi:10.1016/j.ygyno.2009.09.045
5. Hayman JA, Callahan JW, Herschtal A, et al. Distribution of proliferating bone marrow in adult cancer patients determined using FLT-PET imaging. Int J Radiat Oncol Biol Phys. 2011;79(3):847–852. doi:10.1016/j.ijrobp.2009.11.040
6. Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment. Int J Radiat Oncol Biol Phys. 1995;31(5):1319–1339. doi:10.1016/0360-3016(94)00430-S
7. Brixey C, Roeske J, Lujan A, Yamada SD, Rotmensch J, Mundt AJ. Impact of intensity modulated radiotherapy on haemotolgic toxicity in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;54(5):1388. doi:10.1016/S0360-3016(02)03801-4
8. Albuquerque K, Giangreco D, Morrison C, et al. Radiation-related predictors of hematologic toxicity after concurrent chemoradiation for cervical cancer and implications for bone marrow-sparing pelvic IMRT. Int J Radiat Oncol Biol Phys. 2011;79(4):1043–1047. doi:10.1016/j.ijrobp.2009.12.025
9. Rose BS, Aydogan B, Liang Y, et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(3):800–807. doi:10.1016/j.ijrobp.2009.11.010
10. McGuire SM, Bhatia SK, Sun W, et al. Using [18F]fluorothymidine imaged with positron emission tomography to quantify and reduce hematologic toxicity due to chemoradiation therapy for pelvic cancer patients. Int J Radiat Oncol Biol Phys. 2016;96(1):228–239. doi:10.1016/j.ijrobp.2016.04.009
11. Mell LK, Sirák I, Wei L, et al. Bone marrow-sparing intensity modulated radiation therapy with concurrent cisplatin for stage IB-IVA cervical cancer: an international multicenter phase II clinical trial (INTERTECC-2). Int J Radiat Oncol Biol Phys. 2017;97(3):536–545. doi:10.1016/j.ijrobp.2016.11.027
12. Lujan AE, Mundt AJ, Yamada SD, Rotmensch J, Roeske JC. Intensity-modulated radiotherapy as a means of reducing dose to bone marrow in gynecologic patients receiving whole pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57(2):516–521. doi:10.1016/S0360-3016(03)00521-2
13. Klopp AH, Moughan J, Portelance L, et al. Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys. 2013;86(1):83–90. doi:10.1016/j.ijrobp.2013.01.017
14. Mahantshetty U, Krishnatry R, Chaudhari S, et al. Comparison of 2 contouring methods of bone marrow on CT and correlation with hematological toxicities in non–bone marrow–sparing pelvic intensity-modulated radiotherapy with concurrent cisplatin for cervical cancer. Int J Gynecological Cancer. 2012;22(8):1427–1434. doi:10.1097/IGC.0b013e3182664b46
15. Schernberg A, Hennequin C. Doses dans les organes à risque en radiothérapie conformationnelle et en radiothérapie en conditions stéréotaxiques : os et moelle osseuse. Cancer/Radiothérapie. 2017;21:619–625. doi:10.1016/j.canrad.2017.06.005
16. Bacorro W, Dumas I, Levy A, et al. Contribution of image-guided adaptive brachytherapy to pelvic nodes treatment in locally advanced cervical cancer. Brachytherapy. 2017;16(2):366–372. doi:10.1016/j.brachy.2016.11.016
17. Bacorro W, Dumas I, Escande A, et al. Dose-volume effects in pathologic lymph nodes in locally advanced cervical cancer. Gynecol Oncol. 2018;148(3):461–467. doi:10.1016/j.ygyno.2017.12.028
18. Noël G, Antoni D, Barillot I, Chauvet B. Délinéation des organes à risque et contraintes dosimétriques. Cancer/Radiothérapie. 2016;20:S36–S60. doi:10.1016/j.canrad.2016.07.032
19. Mell LK, Kochanski JD, Roeske JC, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(5):1356–1365. doi:10.1016/j.ijrobp.2006.03.018
20. Bazan JG, Luxton G, Mok EC, Koong AC, Chang DT. Normal tissue complication probability modeling of acute hematologic toxicity in patients treated with intensity-modulated radiation therapy for squamous cell carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2012;84(3):700–706. doi:10.1016/j.ijrobp.2011.12.072
21. Green DE, Rubin CT. Consequences of irradiation on bone and marrow phenotypes, and its relation to disruption of hematopoietic precursors. Bone. 2014;63:87–94. doi:10.1016/j.bone.2014.02.018
22. Erpolat OP, Alco G, Caglar HB, et al. Comparison of hematologic toxicity between 3DCRT and IMRT planning in cervical cancer patients after concurrent chemoradiotherapy: a national multi-center study. Eur J Gynaecol Oncol. 2014;35(1):62–66.
23. Liang Y, Bydder M, Yashar CM, et al. Prospective study of functional bone marrow-sparing intensity modulated radiation therapy with concurrent chemotherapy for pelvic malignancies. Int J Radiat Oncol Biol Physs. 2013;85(2):406–414. doi:10.1016/j.ijrobp.2012.04.044
24. Chang Y, Yang Z-Y, Li G-L, et al. Correlations between radiation dose in bone marrow and hematological toxicity in patients with cervical cancer. Int J Gynecological Cancer. 2016;26(4):770–776. doi:10.1097/IGC.0000000000000660
25. Jodda A, Urbański B, Piotrowski T, et al. Relations between doses cumulated in bone marrow and dose delivery techniques during radiation therapy of cervical and endometrial cancer. Phys Med. 2017;36:54–59. doi:10.1016/j.ejmp.2017.03.006
26. Platta CS, Bayliss A, McHaffie D, Tomé WA, Straub MR, Bradley KA. A dosimetric analysis of tomotherapy based intensity modulated radiation therapy with and without bone marrow sparing in gynecologic malignancies. Technol Cancer Res Treat. 2013;12(1):19–29. doi:10.7785/tcrt.2012.500300
27. Li Q, Jiang M-H, Chen J, Liu W, Zhu B-Q, Lu E-M. Correlation between bone marrow dose volumes and acute hematological toxicity in postoperative gynecological cancer patients. Pak J Med Sci. 2016;32(6):1547–1552. doi:10.12669/pjms.326.11489
28. Rose BS, Liang Y, Lau SK, et al. Correlation between radiation dose to 18F-FDG-PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(4):1185–1191. doi:10.1016/j.ijrobp.2011.09.048
29. Andreychenko A, Kroon PS, Maspero M, et al. The feasibility of semi-automatically generated red bone marrow segmentations based on MR-only for patients with gynecologic cancer. Radiother Oncol. 2017;123(1):164–168. doi:10.1016/j.radonc.2017.01.020
30. Li N, Noticewala SS, Williamson CW, et al. Feasibility of atlas-based active bone marrow sparing intensity modulated radiation therapy for cervical cancer. Radiother Oncol. 2017;123(2):325–330. doi:10.1016/j.radonc.2017.02.017
31. Fliedner TM, Steinbach KH. Repopulating potential of hematopoietic precursor cells. Blood Cells. 1988;14(2–3):393–410.
32. Dinges E, Felderman N, McGuire S, et al. Bone marrow sparing in intensity modulated proton therapy for cervical cancer: efficacy and robustness under range and setup uncertainties. Radiother Oncol. 2015;115(3):373–378. doi:10.1016/j.radonc.2015.05.005
Supplementary materials
 |
Table S1 Univariate analysis of the associations between pelvic bone dose metrics, clinical parameters, and hematological toxicity. OR is calculated when p-value <0.05 |
 |
Table S2 Univariate analysis of the correlation between bone marrow dose metrics and hematological toxicity in patients treated by IMRT |
 |
Table S3 Univariate analysis of the correlation between bone marrow dose metrics and hematological toxicity in patients treated by 3DRT |
 |
Table S4 Multivariate analysis of the associations between pelvic bone dose metrics and grade 4 hematological toxicity (adjusted for FIGO stage) |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.