Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Correlates of Uptake of Antiretroviral Therapy in HIV-Positive Orphans and Vulnerable Children Aged 0–14 Years in Tanzania
Authors Exavery A , Charles J, Kuhlik E, Barankena A, Ally A, Mbwambo T , Kyaruzi C, Mubyazi GM, Kikoyo L, Jere E
Received 20 April 2020
Accepted for publication 25 June 2020
Published 13 July 2020 Volume 2020:12 Pages 233—241
DOI https://doi.org/10.2147/HIV.S259074
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Amon Exavery,1 John Charles,1 Erica Kuhlik,2 Asheri Barankena,1 Amal Ally,1 Tumainiel Mbwambo,1 Christina Kyaruzi,1 Godfrey Martin Mubyazi,3 Levina Kikoyo,1 Elizabeth Jere1
1Pact, Dar es Salaam, Tanzania; 2Pact, Inc., Washington, DC, WA 20036, USA; 3National Institute for Medical Research (NIMR), Dar es Salaam, Tanzania
Correspondence: Amon Exavery Email [email protected]
Background: In 2018, only 65% of Tanzanian children aged 0– 14 years living with human immunodeficiency virus (HIV) were on treatment, suggesting that challenges exist. This study explores factors associated with uptake of antiretroviral therapy (ART) among HIV-positive orphans and vulnerable children (OVC).
Methods: Data are from the USAID Kizazi Kipya project that aims to increase the uptake of HIV/AIDS and other health and social services by OVC and their caregivers. HIV-positive OVC aged 0– 14 years who were enrolled in the project from January 2017 to September 2018 were analyzed. ART status (off ART or on ART) was the outcome variable. Multivariate analysis was performed using multilevel logistic regression.
Results: Of the 10,047 HIV-positive OVC aged 0– 14 years analyzed, 93.5% were currently on ART at enrollment. In the multivariate analysis, OVC with male caregivers were 4-times more likely than those with female caregivers to be on ART (OR=4.03, 95% CI=1.49– 10.90). OVC with HIV-positive caregivers were 12-times more likely than those with HIV-negative caregivers to be on ART (OR=12.0, 95% CI=3.81– 37.70). OVC with caregivers who did not disclose their HIV status were 74% less likely to be on ART than OVC of HIV-negative caregivers (OR=0.26, 95% CI=0.08– 0.90). OVC living in urban areas were more than 5-times as likely as their rural counterparts to be on ART (OR=5.55, 95% CI=2.21– 14.0).
Conclusion: The majority of the OVCLHIV in the current study were currently on ART (93.5%) at enrollment. However, uptake of ART by the OVC was dependent on factors external to themselves. Advancing ART uptake may require targeting OVC of female caregivers, OVC of HIV-negative caregivers, as well as OVC of caregivers of undisclosed HIV statu, and rural areas.
Keywords: antiretroviral therapy, uptake, 0– 14 years, orphans, vulnerable children, HIV, AIDS, Tanzania
Background
Human immunodeficiency virus (HIV) remains a major threat to public health for years to come because of the growing number of people living with HIV attributable to the unchanging incidence of HIV.1 Given this, accelerated access to antiretroviral therapy (ART) has been recommended.1,2 Sustained ART use leads to viral load suppression (VLS), a state which prevents further spread of HIV, thus reducing HIV incidence among others.3 In 2016, the World Health Organization (WHO) removed all barriers for ART eligibility by recommending that all people living with HIV (PLHIV) should start ART early, regardless of WHO clinical stage or CD4 count.4 Early use of ART keeps PLHIV alive and healthier. ART improves health and prolongs the lives of children living with HIV (CLHIV). It reduces viral load, increases CD4 count, reduces incidences of opportunistic infections, and consequently improves growth and overall development of the child.5,6 Early use of ART reduces early infant mortality by up to 75%.7
Factors associated with ART uptake in HIV-positive children have been identified in a few studies in sub-Saharan Africa (SSA). The frequency of visiting a clinic, distance to the nearest clinic, and caregiver relationship with their health provider were observed in Swaziland.8 Another study in Kahama district in north-western Tanzania found a lack of transport fare and long distance to the health facility being barriers to attending clinics for ART uptake in children.9 The same study also observed that being divorced or widowed and primary education level of the caregivers were associated with poor uptake of ART in children.9 While these exemplar studies in the two mentioned countries were all based on the general child population, similar evidences for HIV-related orphans or vulnerable children are lacking, suggesting a need for further investigations to be carried out. OVC represent a significant population worldwide enduring poor health and living conditions.10 For example, while HIV prevalence among the general child population in Tanzania has been estimated at 0.4%,11 a recent study estimated it at 7.1% among OVC.12 Therefore, research evidence is greatly needed to inform appropriate programming interventions for OVC better health outcomes and living conditions.
Unfortunately, global records show that not all PLHV are on ART, even those knowing their HIV-positive status.13,16 The UNAIDS estimates that, in 2018, up to 92,000 children aged 0–14 years were living with HIV in Tanzania.17 Of these, only 65% were on treatment,17 suggesting that more HIV-positive children are more likely to die from AIDS-related causes.18 Coverage of care and treatment services for CLHIV remains a big challenge in Tanzania, largely due to difficulties in identification and linkage.18 Given this coverage gap, advancing research and programming efforts to understand the barriers is crucial to achieve universal treatment coverage. Considering that without ART, up to 52% of children infected with HIV die before their second birthday,19 timely ART uptake should be the main focus. In view of this, this study explored factors associated with uptake of ART among orphans and vulnerable children (OVC) living with HIV (OVCLHIV) in Tanzania. Knowledge of these factors is essential to reveal contexts that require implementational attention towards universal ART coverage in children, particularly those who are orphaned and vulnerable.
Methods
Data Source
The current study is based on data from a community-based program that aims at increasing the uptake of age-appropriate HIV-related, and other health and social services by OVC and their families in Tanzania. The program is known as the USAID Kizazi Kipya Project. It is a 5-year project (2016–2021), with coverage of almost all regions of Tanzania. The project links OVC and their caregivers to service delivery points in areas of health, nutrition, education, child protection, social protection, and economic strengthening. The project directly provides psychosocial support, nutrition assessments, counseling and support, referrals and linkages, and care plan monitoring. Further details about the USAID Kizazi Kipya project are available.20
For the current study, screening and enrollment, and family and child asset assessment (FCAA) datasets collected from January 2017 to September 2018 by trained project lay volunteers known as Community Case Workers (CCWs) and Lead Case Workers (LCWs) were used. The USAID Kizazi Kipya project enrolls beneficiaries whose household meets one or more of the following 14 household vulnerabilities related to HIV: household is headed by child (under 18 years old); household is headed by an elderly caregiver (60 years or older); household cares for one or more single or double orphan; caregiver is chronically ill and unable to meet the basic needs of children; caregiver is a drug user; caregiver or adolescent age 10–19 in household is a sex worker; one or more adolescent girls aged 10–19 who are sexually active; adolescent girl age 10–19 in the household is pregnant or has a child of her own; one or more household members are HIV positive; one or more children in the household have tuberculosis; one or more children in the household are severely malnourished; one or more children in the household have been or are abused or at risk for abuse; one or more children are living and or working on the streets; and one or more children in the household are working in mines.12 For each of the enrolled households, the data were collected largely based on caregivers’ self-reports.
Study Area
Eligible OVC analyzed for the current study are from 70 councils in 19 regions of Tanzania where the USAID Kizazi Kipya project had conducted beneficiary screening and enrollment activities from January 2017 to September 2018. The regions were: Arusha, Dodoma, Geita, Kagera, Katavi, Kigoma, Kilimanjaro, Mjini Magharibi, Morogoro, Mtwara, Mwanza, Njombe, Pwani, Rukwa, Shinyanga, Simiyu, Songwe, Tabora, and Tanga.
Study Design
This was a cross-sectional secondary analysis of existing data of the USAID Kizazi Kipya project described. Screening and enrollment of beneficiaries into the project is the first activity, after which the enrolled beneficiaries are regularly followed up by the CCWs with various health and social services.
Study Population
The current analysis was based on 10,047 HIV-positive OVC aged 0–14 years who were enrolled in the USAID Kizazi Kipya project during January 2017–September 2018. These were OVC whose ART status was reported to the program. An OVC was defined as a child, adolescent, or a young person orphaned (ie, has one or both biological parents dead) and made vulnerable by HIV and other adversities. A caregiver is a guardian, not necessarily a biological parent, with the greatest responsibility for the daily care and rearing of one or more OVC in a household.
Variables
ART uptake was the dependent (outcome) variable for the current study. The variable was in two categories: “off ART” if the OVC was reported HIV positive but currently not on ART, and “on ART” if the OVC was reported HIV positive and currently on ART. For computational purposes, the codes “0” and “1” were used for the former and the latter, respectively. The variable was thus organized as follows.
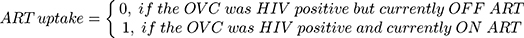
The independent (explanatory) variables were in four groups, namely, individual OVC characteristics, caregiver characteristics, household characteristics, and geographical characteristics. The individual OVC characteristics were sex, age, and school enrollment status; caregiver characteristics were sex, age, marital status, HIV status, and education attained; household characteristics were wealth quintile, and health insurance status; and the geographical characteristics were represented by place of residence.
Wealth quintile was constructed using principal component analysis (PCA) of household assets to determine household socio–economic status.21 Five wealth quintiles were formed, ranging from the lowest quintile (Q1) for the poorest households, to the highest quintile (Q5) for the most well-off households. Household assets included in the PCA were: dwelling materials (brick, concrete, cement, aluminium, and/or other material), livestock (chicken, goats, cows, and others), transportation assets (bicycle, motorcycle/moped, tractor, motor vehicle, and others), and productive assets (sewing machine, television, couch or sofa, cooking gas, hair dryer, radio, refrigerator, blender, oven, and others).
Data Analysis
Beginning with exploratory analysis, one-way tabulations were conducted to obtain distributional features of the OVC in each variable. Cross-tabulations of OVC ART status by each of the independent variables were conducted to assess the variability in OVC ART uptake. Chi-square (χ2) test was used to test the significance of the associations between OVC ART status and the independent variables. Multivariate analysis to identify factors associated with ART uptake among the OVCLHIV was conducted using multilevel logistic regression model. This choice was based on the clustered structure of the data,22 thus considered individual- and household-levels during model specification. The assumption of independence of observations did not hold because OVC who reside in the same household or those of the same caregiver may be correlated with respect to ART use. Therefore, households or caregivers (ie, there was one caregiver per household) served as clusters. A multilevel model was appropriate because it recognizes these data hierarchies.23 All statistical inferences were made at a significance level of 5% (α=0.05). Data analysis was conducted using Stata (version 14.0) statistical software.
Results
OVC Profile
As presented in Table 1, the analysis was based on 10,047 HIV-positive OVC from 8485 households (ie, clusters), with mean age of 8.2, ranging from 0–14 years. The OVC were evenly distributed by sex (51.5% female). However, the majority of them (70.8%) had female caregivers. Two-thirds (67.2%) of the OVC were enrolled in school. Rural residences accounted for 58.9% of the OVC, and almost half of them (49.3%) had caregivers who were married or living together with their spouses. The majority of the OVC (64.7%) were living with HIV-positive caregivers.
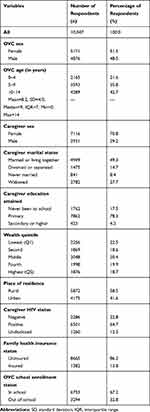 |
Table 1 Profile of Respondents (n = 10,047) |
ART Uptake by Background Characteristics
Results in Table 2 show that 93.5% (n=9394) of the OVC were currently on ART at enrollment. However, this level of ART uptake varied by some background characteristics. ART uptake was higher among OVC with male than female caregivers (P=0.023). The uptake was also found to be significantly higher in urban than in rural areas (P<0.001). With respect to caregiver HIV status, ART uptake was highest among OVC of HIV-positive caregivers and lowest among OVC whose caregivers did not disclose their HIV status to the program (P<0.001). ART uptake was lowest among OVC whose caregivers were married or living together and highest among those with divorced or separated caregivers (P=0.009). Wealth quintile was associated with ART uptake, but there was no clear pattern of the association (P<0.001).
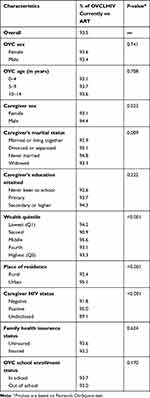 |
Table 2 Percentage of OVCLHIV Currently on ART at Enrollment in the USAID Kizazi Kipya Program by Background Characteristics (n=10,047) |
Multivariate Analysis
Multivariate analysis in Table 3 showed that OVC with male caregivers were four times as likely as those with female caregivers to be on ART (OR=4.03, 95% CI=1.49–10.90). OVC with HIV-positive caregivers were 12-times more likely than those with HIV-negative caregivers to be on ART (OR=12.00, 95% CI=3.81–37.70). OVC with caregivers who did not disclose their HIV status to the program were 74% less likely to be on ART than OVC with HIV-negative caregivers (OR=0.26, 95% CI=0.08–0.90). OVC living in urban areas were more than 5-times as likely as their rural counterparts to be on ART (OR=5.55, 95% CI=2.21–14.0). Intraclass correlation coefficient (ICC) was as high as 96.0%, implying a very correlated pattern of ART uptake among OVC of the same caregiver. These observations were adjusted for OVC characteristics (sex, age, co-residence, and school enrolment), caregiver characteristics (age, education, marital status), and wealth quintile.
 |
Table 3 Multilevel Logistic Regression Analyses of the Factors Associated with ART Uptake Among Orphans and Vulnerable Children Living with HIV in Tanzania, 2018 (n=10,047) |
Discussion
This study explored the uptake of ART among OVCLHIV, based on screening and enrollment data from a community-based program to enhance the uptake of HIV, health, and other social services by OVC and their caregivers in Tanzania. Findings revealed that 93.5% of the 10,047 OVCLHIV analyzed were currently on ART at enrollment. This proportion was high, reflecting efforts and investments of the Government of Tanzania (GoT) and partners in scaling up care and treatment services for PLHIV, including children. Another possibility for this high ART uptake is that, before the USAID Kizazi Kipya project, some of these OVC were from families previously exposed to another program known as Pamoja Tuwalee whose focus was to improve the lives of vulnerable children and their caregivers. Despite this high ART uptake, additional efforts and strategies are required to ensure that the small unreached segment of the OVCLHIV (ie, 6.5% OVC not on ART in this study) are eventually reached with and sustained on ART, and this is imperative not only to enhance their health outcomes, but also to prevent further spread of the virus.24 It is also crucial because the future of the HIV epidemic may be driven by small segments of different populations unreached or underserved.1
The current study identified several factors with significant association with ART uptake among the OVC. OVC with HIV-positive caregivers were more likely to be on ART than OVC with HIV-negative caregivers. In a separate analysis involving the caregivers of the studied OVC, it was observed that 96% of the HIV-positive caregivers were on ART. This suggested that HIV-positive caregivers may be better experienced with the value of ART, thus more likely to have their HIV-positive OVC on it to improve their health. This is supported by one study conducted in Kahama district of Tanzania, that observed that most caregivers perceived ART to be beneficial to CLHIV, as it prolongs child survival and decreases illness episodes.9 Another mechanism could be that if the caregiver is HIV positive and on ART, factors which were barriers to the caregiver’s uptake of ART are no longer present for that family, hence the child is also more likely to be on ART.
On the other hand, OVC of caregivers who refrained from disclosing their HIV status to the program were less likely to be on ART than OVC who had caregivers who disclosed their HIV status as negative. It has been emphasized that better public knowledge about HIV would facilitate uptake of ART.25 Therefore, while it remains crucial to enhance public knowledge about the epidemic (an act which will also encourage disclosure and uptake of services), the findings suggest a need to target HIV-negative and HIV status unknown caregivers with HIV-positive OVC with additional support to ensure that their OVC are started and sustained on ART.
This study also observed that ART uptake among the OVC was better in urban than in rural areas. Although other reasons behind this situation may not be obvious, there is vast literature suggesting that some health programs perform better in urban areas where service centers, infrastructures, and distance to clinics are generally more favorable than in rural areas.26,27 Poor ART uptake due to distance in rural areas has been reported in other studies,9,28 besides the limitations relating to healthcare infrastructures, eg, where there is no privacy and or a shortage of voluntary counselling and testing services.29,31 Availability of alternative care providers, especially in rural settings, is another limiting factor for ART uptake in many countries in SSA.29 For instance, one study done in rural Tanzania reported the preference for traditional medicine as a major barrier to ART in children.32 Cultural values have also been found to be stronger in rural areas, adversely affecting uptake of modern care and treatment services.29 These field observations suggest the need for programs to target rural areas with additional support in order to enhance ART uptake among the vulnerable populations including the OVCLHIV in Tanzania.
Moreover, the present study revealed that OVC living with male caregivers were more likely to be on ART than those living with female caregivers. Although it was not clear how differences between men and women might have contributed to this, it is unknown whether men as the main decision-makers in most households in traditional African settings33 and other developing countries34 can facilitate ART uptake for their OVCLHIV. Further research evidence to establish how male caregivers are more likely to have their OVCLHIV on ART than their female counterparts is required.
Finally, the intra-class correlation coefficient (ICC) of 0.96 observed in this study indicated that there was a significant clustering of the OVCLHIV at household-level in relation to ART use. Up to 96.0% of the variability in ART use among the OVC was due to residence in the same household. This level of ICC indicated excellent reliability.35 The possibility of this is that two or more OVC who live in the same household or of the same caregiver are sharing the same resources as well as risks available at the household level. They are also more likely to receive the same quality and quantity of care, thus the highly correlated pattern of ART use among them. This suggested a need for research, programs, and policies to target caregivers who have multiple OVC with additional health, social, and economic empowerment to enhance ART use among the OVC they care for.
Limitations
Self-reported data may be affected by recall bias. However, findings of this study suggest a minimal effect because results are comparable with extant biomedical and clinical studies. Since this study was cross-sectional in design, temporality was not possible to establish, thus unlikely to conclude causal inferences.
Conclusions
The level of ART uptake among OVCLHIV as revealed by the current study was high (93.5%), a credible achievement in view of the 90–90–90 global targets to end the AIDS epidemic.36 The observed difference in ART uptake between rural and urban settings deserves attention from a program intervention viewpoint. Since the future of the HIV epidemic has so much to do with small segments of the populations unreached, unserved, or underserved, we suggest that it is equally important to invest so much more towards 100% coverage of ART among OVCLHIV.
As individual OVC characteristics were not seen to be associated with ART uptake, the results from this study suggest that ART uptake among OVCLHIV depends on factors that are external to the OVC themselves. Therefore, to improve ART uptake in OVCLHIV in both rural and urban areas, there may be a need to target OVC of female caregivers, OVC of HIV-negative caregivers, as well as OVC of caregivers of undisclosed HIV status. Also, additional support is greatly needed for caregivers with multiple OVC to enhance ART use among OVCLHV they care for.
Abbreviations
AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; CD4, cluster of differentiation 4; CI, confidence interval; FCAA, Family and Child Asset Assessment; HIV, human immunodeficiency virus; ICC, Intra-class correlation coefficient; IQR, interquartile range; LHIV, living with HIV; MRCC, Medical Research Coordinating Committee; NIMR, National Institute for Medical Research; OR, odds ratio; OVC, orphans and vulnerable children; OVCLHIV, orphans and vulnerable children living with HIV; PCA, principal component analysis; PLHIV, people living with HIV; SD, standard deviation; SSA, sub-Saharan Africa; UNAIDS, Joint United Nations Programme on HIV/AIDS; USAID, United States Agency for International Development.
Ethics Approval
Ethics approval was received from the Medical Research Coordinating Committee (MRCC) of the National Institute for Medical Research (NIMR) in Tanzania (NIMR/HQ/R.8a/Vol.IX/3024). Screening and enrollment of beneficiaries into the USAID Kizazi Kipya Project was entirely voluntary. The FCAA tool was completed only after each screened caregiver of the OVC had voluntarily agreed by signing a statement of an informed consent. Datasets used for the current study were anonymous, with no name, phone numbers or any beneficiary identifiers. Unique identification numbers were used instead. Storage of the datasets was very secure and confidential, with access limited only to the monitoring and evaluation team.
Acknowledgments
A version of this manuscript was presented as a poster at the 20th International Conference on AIDS and STIs in Africa (ICASA 2019), which took place at the Kigali Convention Centre in Kigali, Rwanda, December 2-7, 2019. Comments received are acknowledged. We also acknowledge the project staff, the consortium partners implementing the USAID Kizazi Kipya Project, Civil Society Organizations (CSOs), Community Case Workers (CCW), and District Social Welfare Officers (DSWO) for their commitment and tireless efforts to make the program a success.
Disclosure
The authors have no competing interests regarding this publication.
References
1. Frank TD, Carter A, Jahagirdar D, et al. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV. 2019;6(12):e831–59. doi:10.1016/S2352-3018(19)30196-1
2. Desmonde S, Tanser F, Vreeman R, et al. Access to antiretroviral therapy in HIV-infected children aged 0–19 years in the International Epidemiology Databases to Evaluate AIDS (IeDEA) Global Cohort Consortium, 2004–2015: a prospective cohort study. PLoS Med. 2018;15(5):e1002565. doi:10.1371/journal.pmed.1002565
3. Enane LA, Vreeman RC, Foster C. Retention and adherence: global challenges for the long-term care of adolescents and young adults living with HIV. Curr Opin HIV AIDS. 2018;13(3):212–219. doi:10.1097/COH.0000000000000459
4. WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach — 2nd ed; 2016. Available from: http://www.deslibris.ca/ID/10089566.
5. Patel K, Hernán MA, Williams PL, et al. Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clin Infect Dis. 2008;46(4):507–515. doi:10.1086/526524
6. Resino S, Resino R, Maria Bellón J, et al. Clinical outcomes improve with highly active antiretroviral therapy in vertically HIV type-1-infected children. Clin Infect Dis. 2006;43(2):243–252. doi:10.1086/505213
7. Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–2244. doi:10.1056/NEJMoa0800971
8. Jolly P, Padilla L, Ahmed C, et al. Barriers to antiretroviral therapy initiation for HIV-positive children aged 2–18 months in Swaziland. Afr J AIDS Res. 2018;17:193–202. doi:10.2989/16085906.2018.1488266
9. Urassa DP, Matemu S, Sunguya BF. Antiretroviral therapy clinic attendance among children aged 0–14 years in Kahama district, Tanzania: a cross-sectional study. Tanzan J Health Res. 2018;20(1).
10. Kelley MC, Brazg T, Wilfond BS, et al. Ethical challenges in research with orphans and vulnerable children: a qualitative study of researcher experiences. Int Health. 2016;8(3):187–196. doi:10.1093/inthealth/ihw020
11. Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC). Tanzania HIV impact survey (THIS) 2016–2017: final report. Dar es Salaam, Tanzania; 2018. Available from: http://www.nbs.go.tz/nbs/takwimu/this2016-17/Tanzania_SummarySheet_English.pdf.
12. Exavery A, Charles J, Kuhlik E, et al. Understanding the association between caregiver sex and HIV infection among orphans and vulnerable children in Tanzania: learning from the USAID Kizazi Kipya project. BMC Health Serv Res. 2020;20(1):1–14. doi:10.1186/s12913-020-05102-y
13. UNAIDS. Global HIV & AIDS statistics — 2019 fact sheet; 2019. Available from: https://www.unaids.org/en/resources/fact-sheet.
14. UNAIDS. Fact sheet - Global AIDS update 2019; 2019. Available from: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf.
15. UNAIDS. Country factsheets. United Republic of Tanzania; 2018. Available from: https://www.unaids.org/en/regionscountries/countries/unitedrepublicoftanzania.
16. Ahmed S, Autrey J, Katz IT, et al. Why do people living with HIV not initiate treatment? A systematic review of qualitative evidence from low- and middle-income countries. Soc Sci Med. 2018;213:72–84. doi:10.1016/j.socscimed.2018.05.048
17. UNAIDS. United Republic of Tanzania; 2018. Available from: https://www.unaids.org/en/regionscountries/countries/unitedrepublicoftanzania.
18. Tanzania Commission for AIDS (TACAIDS). National HIV and AIDS response report for 2017 - Tanzania Mainland; 2018. Available from: http://library.tacaids.go.tz/bitstream/handle/123456789/134/National%20HIV%20and%20AIDS%20Response%20Report%20for%202017%20-%20Tanzania%20Mainland.pdf?sequence=1&isAllowed=y.
19. Newell M-L, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet London Engl. 2004;364(9441):1236–1243. doi:10.1016/S0140-6736(04)17140-7
20. Pact. Kizazi Kipya: new generation. Pact; 2019. Available from: http://www.pactworld.org/country/tanzania/project.
21. Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21(6):459–468. doi:10.1093/heapol/czl029
22. Rodrıguez G, Elo I. Intra-class correlation in random-effects models for binary data. Stata J. 2003;3(1):32–46. doi:10.1177/1536867X0300300102
23. University of Bristol. What are multilevel models and why should I use them? 2018. Available from: http://www.bristol.ac.uk/cmm/learning/multilevel-models/what-why.html.
24. Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830–839. doi:10.1056/NEJMoa1600693
25. Yeap A, Hamilton R, Charalambous S, et al. Factors influencing uptake of HIV care and treatment among children in South Africa-A qualitative study of caregivers and clinic staff. AIDS Care. 2010;22:1101–1107. doi:10.1080/09540121003602218
26. Kwesigabo G, Mwangu MA, Kakoko DC, et al. Tanzania’s health system and workforce crisis. J Public Health Policy. 2012;33:s35–44. doi:10.1057/jphp.2012.55
27. Mujinja PGM, Kida TM. Implications of Health Sector Reforms in Tanzania: Policies, Indicators and Accessibility to Health Services. Economic and Social Research Foundation (Tanzania); 2014.
28. Williams M, Van Rooyen DRM, Ricks EJ. Accessing antiretroviral therapy for children: caregivers’ voices. Health SA Gesondheid. 2016;21:331–338. doi:10.1016/j.hsag.2016.03.001
29. Gourlay A, Birdthistle I, Mburu G, Iorpenda K, Wringe A. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16:18588. doi:10.7448/IAS.16.1.18588
30. Meremo A, Mboya B, Ngilangwa D, et al. Barriers to accessibility and utilization of HIV testing and counseling services in Tanzania: experience from Angaza Zaidi programme. Pan Afr Med J. 2016;23(189). doi:10.11604/pamj.2016.23.189.5683.
31. Nyato D, Nnko S, Komba A, et al. Facilitators and barriers to linkage to HIV care and treatment among female sex workers in a community-based HIV prevention intervention in Tanzania: a qualitative study. PLoS One. 2019;14(11):e0219032. doi:10.1371/journal.pone.0219032
32. Nyogea D, Mtenga S, Henning L, et al. Determinants of antiretroviral adherence among HIV positive children and teenagers in rural Tanzania: a mixed methods study. BMC Infect Dis. 2015;15(1):28. doi:10.1186/s12879-015-0753-y
33. Ganle JK, Obeng B, Segbefia AY, Mwinyuri V, Yeboah JY, Baatiema L. How intra-familial decision-making affects women’s access to, and use of maternal healthcare services in Ghana: a qualitative study. BMC Pregnancy Childbirth. 2015;15. doi:10.1186/s12884-015-0590-4
34. Colfer CJP, Achdiawan R, Roshetko JM, et al. The balance of power in household decision-making: encouraging news on gender in Southern Sulawesi. World Dev. 2015;76:147–164. doi:10.1016/j.worlddev.2015.06.008
35. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi:10.1016/j.jcm.2016.02.012
36. UNAIDS. Ending AIDS: progress towards the 90–90–90 targets. Geneva, Switzerland: UNAIDS; 2017. Available from: http://www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
