Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Cornel Iridoid Glycoside Protects Against White Matter Lesions Induced by Cerebral Ischemia in Rats via Activation of the Brain-Derived Neurotrophic Factor/Neuregulin-1 Pathway
Authors Wang M, Hua X , Niu H, Sun Z, Zhang L, Li Y, Zhang L, Li L
Received 24 August 2019
Accepted for publication 13 November 2019
Published 2 December 2019 Volume 2019:15 Pages 3327—3340
DOI https://doi.org/10.2147/NDT.S228417
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Mingyang Wang,1 Xuesi Hua,2 Hongmei Niu,1 Zhengyu Sun,1 Li Zhang,1 Yali Li,1 Lan Zhang,1 Lin Li1
1Department of Pharmacy, Xuanwu Hospital of Capital Medical University, National Clinical Research Center for Geriatric Diseases, Key Laboratory for Neurodegenerative Diseases of Ministry of Education, Beijing Institute for Brain Disorders, Beijing Engineering Research Center for Nerve System Drugs, Beijing, People’s Republic of China; 2University of Michigan, Ann Arbor, MI, USA
Correspondence: Lin Li; Lan Zhang
Department of Pharmacy, Xuanwu Hospital of Capital Medical University, 45 Changchun Street, Beijing 100053, People’s Republic of China
Tel +86-10-83198886
; +86-10-83199443
Fax +86-10-63042809
Email [email protected]; [email protected]
Background: Ischemic stroke often induces profound white matter lesions, resulting in poor neurological outcomes and impaired post-stroke recovery. The present study aimed to investigate the effects of cornel iridoid glycoside (CIG), a major active component extracted from Cornus officinalis, on the white matter injury induced by ischemic stroke and further investigate its neuroprotective mechanisms.
Methods: Adult male Sprague-Dawley rats underwent middle cerebral artery occlusion (MCAO) surgery for 2 h, followed by reperfusion. Rats were intragastrically administered CIG (60 mg/kg and 120 mg/kg) beginning 6 h afters reperfusion, once daily for seven days. A series of behavioral tests (modified neurological severity scores test, object recognition test, adhesive removal test, and beam walking test) were performed to evaluate the neurological functioning in MCAO rats. Histology of the white matter was studied using luxol fast blue staining and transmission electron microscopy. Immunohistochemical staining was performed to assess myelin loss, oligodendrocyte maturation, and glial activation. Activation of the brain-derived neurotrophic factor (BDNF)/neuregulin-1 (NRG1) pathway was evaluated by Western blotting.
Results: CIG treatment remarkably decreased the neurological deficit score, accelerated the recovery of somatosensory and motor functions, and ameliorated the memory deficit in MCAO rats. Furthermore, CIG alleviated white matter lesions and demyelination, increased myelin basic protein expression and the number of mature oligodendrocytes, and decreased the number of activated microglia and astrocytes in the corpus callosum of MCAO rats. In addition, Western blot analysis indicated that CIG increased the expression of BDNF/p-TrkB, NRG1/ErbB4 proteins, which further elevated PI3K p110α/p-Akt/p-mTOR signaling in the corpus callosum of MCAO rats.
Conclusion: We demonstrated that CIG protects against white matter lesions induced by cerebral ischemia partially by decreasing the number of activated microglia and astrocytes, increasing BDNF level, and activating NRG1/ErbB4 and its downstream PI3K/Akt/mTOR pathways in the white matter. CIG might be used as a potential neuroprotective agent for the treatment of ischemic stroke.
Keywords: cornel iridoid glycoside, white matter lesion, cerebral ischemia, brain-derived neurotrophic factor, neuregulin-1 pathway
Introduction
Over the last 30 years or more, both stroke incidence and mortality have decreased; however, stroke is still the second leading cause of death globally.1 Approximately 85% of strokes are ischemic, exerting a profoundly negative impact on both patients and society.2 Despite the emergence of promising preclinical results, there are currently no drugs available that demonstrate consistent clinical improvements,3,4 possibly due to the highly complex pathophysiologic responses occurring in the brain during a stroke. Apart from gray matter injury, stroke also elicits profound white matter injury, a risk factor for higher stroke incidence and poor neurological outcomes.5 Owing to the histological characteristics of the white matter, which has little collateral circulation and limited blood supply, the white matter is extremely vulnerable to ischemic stress and is often injured even more severely than the gray matter after stroke.5 However, white matter integrity is reported to play an important role in long-term recovery after stroke and small vessel disease.6,7 As the connection between white matter injury and ischemic stroke is critically related to clinical outcomes, neuroprotection of the white matter should be considered during the design of therapeutic strategies.8 While previous studies on stroke have mostly emphasized on gray matter injury over white matter injury, it is important to find new drugs that benefit both gray and white matter in ischemic stroke.
Cornus officinalis Sieb. et Zucc, known in Chinese as “Shanzhuyu”, is a traditional Chinese herb, clinically used in liver and kidney deficiency therapy, and for the treatment of stroke in combination with other herbs in traditional Chinese medicine. Cornel iridoid glycoside (CIG) is the main active component extracted from Cornus officinalis, with a purity of 71.19%. In our previous studies, we observed that CIG treatment exerted neuroprotective effects in ischemic rats in both acute and chronic stages. The effects of CIG were partially due to its anti-neuroinflammatory and anti-apoptotic effects,9 and its promotion of neurogenesis and angiogenesis in the gray matter.10 However, the potential protective role of CIG against white matter injury induced by cerebral ischemia remains unknown.
In this study, we investigated the effect of CIG on white matter injury induced by ischemic stroke using a rat model of transient middle cerebral artery occlusion (MCAO). Our novel results revealed that CIG alleviated white matter lesions after focal ischemic stroke. We further explored the underlying mechanisms related to anti-inflammation and BDNF/NRG1/ErbB and PI3K/Akt/mTOR signaling pathways in the white matter.
Materials and Methods
Drugs
CIG was extracted from Cornus officinalis at the Department of Pharmacy of Xuanwu Hospital as described previously.10 Cornus officinalis was purchased from Tong-Ren-Tang Company (Beijing, China). The purity of CIG was 71.19% as determined by reversed phase high performance liquid chromatography; CIG contained 67% morroniside and 33% loganin. The extract of Ginkgo biloba (EGB) was used as the positive control drug,11 and purchased from Yangtze River Pharmaceutical (Group) Co., Ltd. (Jiangsu, China).
Animals
The animal study was conducted in accordance with the guidelines established by the National Institutes of Health for the care and use of laboratory animals and was approved by the Bioethics Committee of Xuanwu Hospital of Capital Medical University. Adult male Sprague-Dawley rats weighing 260–280 g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Prior to surgery, rats were acclimatized for one week and housed in a temperature-controlled environment (22 ± 2°C) under a 12 h light/dark cycle and with ad libitum access to food and water.
Preparation of Cerebral Ischemia Model
The MCAO surgery was conducted as previously described with several modifications.9,12 Following a 12 h fast, rats were deep anesthetized. Next, an intraluminal suture was inserted from the external carotid artery stump into the internal carotid artery to block the blood supply to the middle cerebral artery. Two hours later, the suture was removed to recover blood circulation. During and after the surgery, all animals were kept warm prior to regaining consciousness. The rats in the sham group underwent the same surgery without artery occlusion.
Rats were randomly assigned into six groups according to the method of random number table (n = 10): sham, sham + CIG 60 mg/kg, model, model + CIG 60 mg/kg (low dose), model + CIG 120 mg/kg (high dose), and model + EGB 12 mg/kg groups. Drugs were dissolved in 0.9% saline and administered intragastrically starting 6 h after reperfusion, administered once daily for seven days until the animals were sacrificed. Rats in sham and model groups received an equivalent volume of saline. The volume of the gastrointestinal treatment was 10 mL/kg.
Neurological Function Assessment
In all animals, the neurological functional assessment was performed seven days after MCAO by an investigator blinded to the experimental design. Neurological deficits were evaluated by a set of modified neurological severity scores (mNSS) tests as described previously,13 involving a series of measurements of motor and sensory function, reflex, and balance.14 Here, neurological functions were graded from 0 to 18 (normal score, 0; maximal deficit score, 18).
Object Recognition Test
The object recognition test (ORT) was performed to evaluate non-spatial memory as described previously.15,16 Briefly, the test lasted for three days: habituation day, training day, and testing day. On the first day of habituation: rats were placed in the middle of an empty arena and allowed to explore the arena freely for 5 min. On the training day (day 2): two identical objects were placed at opposite sides of the arena. Rats were placed in the center of the arena and equidistant from the two objects. Each rat was allowed free exploration of the objects for 5 min. One the testing day (third day): one familiar (previously observed) object was replaced with one new object at the same position in the arena. Rats were placed in the middle of each object to start a 5 min ORT session. The apparatus and objects were thoroughly cleaned after each individual trial using 75% vol/vol ethanol. The time spent exploring the familiar object and the new object on testing day were recorded, which were used to calculate a memory discrimination index (DI): DI = (N − F)/(N + F), where N is the time spent in exploring the new object and F is the time spent in exploring the familiar object.17 The lower the DI value, the worse the memory capacity of the rats.
Adhesive Removal Test
The adhesive removal test was carried out as previously reported with several modifications.13 In brief, two small pieces of adhesive-backed paper dots (of equal size, 100 mm2) were used as bilateral tactile stimuli, placed on the distal-radial region on the wrist of each forelimb. The rats were then returned to the hyaline cage for observation. The time taken by the rats to remove the stimulus from the forelimbs was recorded. All rats were familiarized with the test environment prior to testing. The maximal time allowed for each test was 2 min.
Beam Walking Test
The beam walking test is typically used to evaluate locomotion and motor coordination capacity.18 In our study, rats first received training to walk across a narrow wooden beam (4 cm in width and 105 cm in length), which was placed 80 cm above the ground. The initial 20 cm on the beam was considered as the starting area, with a horizontal line drawn at a distance of 20 cm from the staring zone. The rats were placed at the starting zone and a stopwatch started immediately on releasing the animal. The total time taken to cross the beam was recorded. All rats were pre-trained three times each day, for two days before the test. The maximal time allowed for the task was 2 min.
Immunohistochemical Staining
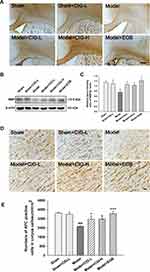
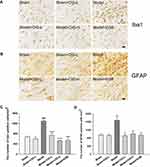
Following the performance of behavioral tests, rats were deep anesthetized and perfused using the intracardiac route with 0.01 mol/L phosphate buffered saline (PBS) and then 4% paraformaldehyde (pH 7.4). The brains were harvested and post-fixed in 4% paraformaldehyde solution, followed by the achievement of equilibrium with 0.01 mol/L PBS containing 15%, 20%, and 30% sucrose at 4°C, respectively. The whole brain tissues were sectioned into continuous coronary slices (30 μm thick) using a Leica cryostat. The chosen slices (Bregma 0.50 mm) were incubated in 3% H2O2 solution for 10 min to reduce the endogenous peroxidase activity at room temperature. After three washes with 0.01 mol/L PBS containing 0.3% Triton-X (PBST), the slices were blocked in 10% bovine serum for 1 h, and incubated with primary antibody at 4°C overnight. The following primary antibodies were used in this study: rat anti-myelin basic protein (MBP) (1:1000, Merck Millipore, Germany), mouse anti-APC (1:100, Merck Millipore, Germany), mouse anti-GFAP (1:200, Santa Cruz, CA, USA), rabbit anti-Iba1 (1:1000, Wako, Japan). After washing with PBS for three times, the slices were incubated with HRP-goat anti-rat/rabbit/mouse or HRP-rabbit anti-goat secondary antibody according to the manufacturer’s instructions (Beijing Zhong Shan Biotechnology Co., China). The slices were then visualized using a DAB substrate kit (Beijing Zhong Shan Biotechnology Co., China). All slices were washed in PBS, mounted on amino propyltriethoxysilane-coated slides, dried, dehydrated in a graded series of ethanol, cleared in xylene, and coverslipped. Images were captured using an Olympus microscope. Using the Image-Pro Plus software, the number of positively labeled cells were blindly counted for three fields of vision evenly distributed throughout the areas of interest.
Luxol Fast Blue Staining
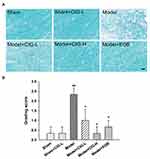
Luxol fast blue (LFB) staining was used to evaluate the white matter lesions. Briefly, 30 μm thick coronal sections were obtained as described in the immunohistochemical staining section and stained with LFB. The severity of the white matter lesions was graded from 0 to 3. The grade standard was in accordance with a previous study and listed as below: grade 0, normal; grade 1, disarrangement of the nerve fibers; grade 2, the formation of marked vacuoles; grade 3, the disappearance of myelinated fibers.19,20 Representative brain section images, stained with LFB in the corpus callosum, were obtained using an Olympus microscope.
Transmission Electron Microscopy
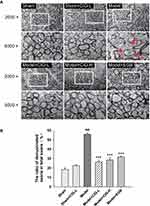
Following the behavioral tests, three rats from each group were deep anesthetized and perfused using the intracardiac route as described above. The brains were harvested and approximately 1 mm3 brain sections were cut from the right corpus callosum and post-fixed in 2.5% glutaraldehyde and 4% paraformaldehyde in PBS at 4°C for 2 h. After washing three times with PBS, the blocks were fixed in 1% osmium tetroxide for 35 min. The specimens were dehydrated using gradient alcohol from 50 to 100%, and an embedding reagent was used to replace the water within the tissue. After dehydration and embedment, semi-thin sections (1 μm) were observed under a light microscope for trimming. Serial ultra-thin sections (80 nm) were prepared, stained with lead citrate and uranyl acetate, and observed under a JEM-2100 transmission electron microscope (Tokyo, Japan). From each section, ten fields of vision were randomly photographed at a magnification of 2000× and 5000×. The ratio of demyelinated axons in the total axons (%) was calculated.
Western Blot
Following the behavioral tests, three rats in each group were euthanized and the brains were rapidly removed. The total proteins were extracted from the right corpus callosum in Radio Immunoprecipitation Assay lysis buffer, which contained the phosphatase inhibitor and protease inhibitor cocktail (Beyotime institute of biotechnology, Shanghai, China). The protein concentration was measured using the Enhanced BCA Protein Assay Kit in accordance with the manufacturer’s instructions (Beyotime institute of biotechnology, Shanghai, China). Equal amounts of protein from each rat were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred onto polyvinylidene fluoride (PVDF) membranes, blocked with 5% non-fat milk for 1 h at room temperature. Next, the membranes were probed with the primary antibodies overnight at 4°C. The following primary antibodies were used in this study: rat anti-MBP (1:1000, Merck Millipore, Germany), rabbit anti-BDNF, rabbit anti-TrkB, rabbit anti-phospho-TrkB-Y515 (1:1000, Abcam, Cambridge, UK), mouse anti-NRG1, rabbit anti-ErbB4, rabbit anti-PI3K p85, rabbit anti-PI3K p110α, rabbit anti-Akt, rabbit anti-phospho-Akt-Ser473, rabbit anti-mTOR, and rabbit anti-phospho-mTOR-Ser2448 (1:1000, CST, MA, USA). On the second day, membranes were incubated with the corresponding secondary antibodies conjugated with horseradish peroxidase for 1 h. The immunoblots were visualized with enhanced chemiluminescence and analyzed using the GelPro software. β-actin was used as an internal loading control for each blot.
Statistical Analyses
All data are presented as the mean ± SEM (standard error of the mean) and analyzed by One-way Analysis of Variance (ANOVA) using the SPSS 17.0 software. Multiple comparison post-hoc tests between groups were performed using the least-significant difference test or Dunnett’s post-hoc test depending on the homogeneity of variance. Differences between groups were considered significant at P < 0.05.
Results
CIG Improved Neurological Functions in MCAO Rats
Two hours after the induction of ischemia by MCAO and following 7 days of reperfusion, the neurological functions were evaluated by employing a variety of behavioral tests in rats. The results demonstrated that cerebral ischemia induced an obvious increase in the neurological deficit score in mNSS test in the model group (P<0.01); intragastric administration of CIG (60 and 120 mg/kg) for seven days starting 6 h after surgery significantly reduced the mNSS score (P<0.05; Figure 1A). In addition, in the beam walking test and adhesive removal test, the model rats exhibited longer time to walk across the whole beam (P<0.001) and to remove the stimulus (P<0.001). Notably, treatment with CIG shortened the time to walk across the whole beam (P<0.01, P<0.001; Figure 1B) and to remove stimulus (P<0.05; Figure 1C). The results indicated that CIG ameliorated the impairment of motor and somatosensory functions in the MCAO rats. In the ORT, the MCAO model rats showed a lower recognition index than rats in the sham control group (P<0.05); CIG treatment significantly increased the recognition index (P<0.05; Figure 1D), demonstrating that CIG improved the memory deficit in MCAO rats.
CIG Ameliorated White Matter Lesions in the Brain of MCAO Rats
LFB binds specifically to the myelin sheath in ethanol. In the present study, we evaluated white matter lesions using LFB myelin staining in the corpus callosum of MCAO rats. Figure 2A displays that no white matter lesion was observed in the sham rats; the MCAO model rats demonstrated sparse and disarranged myelin fibers, as well as obvious vacuoles in the corpus callosum; and the administration of CIG attenuated these lesions in MCAO rats. The quantification analysis indicated that the grading scores of white matter lesions following LFB staining were considerably increased in the model group (P<0.01), while CIG significantly decreased the grading score in MCAO rats (P<0.05; Figure 2B).
The ultrastructure of myelin sheaths and myelinated axons was further evaluated by transmission electron microscopy. The MCAO model rats exhibited severe damage in the corpus callosum as shown by the obvious loss of myelin sheaths, myelin swelling and a significant increment in the number of demyelinated axons (P<0.001). Treatment with CIG markedly alleviated the demyelination and myelin swelling, as well as decreased the number of demyelinated axons (P<0.001; Figure 3). These results demonstrated the protective effects of CIG against white matter damage induced by cerebral ischemia in rats.
CIG Increased MBP Expression and the Number of Mature Oligodendrocytes in the Corpus Callosum of MCAO Rats
Mature oligodendrocytes are myelin-forming cells in the central nervous system, while MBP is the main protein of the myelin sheath and synthesized by mature oligodendrocytes.21 In the present study, we examined the expression of MBP using immunohistochemical and Western blotting analyses (Figure 4A and B). We reported that the MBP level was remarkably decreased in the corpus callosum of MCAO model rats (P<0.05); CIG treatment significantly increased MBP expression (P<0.05; Figure 4C), indicating the recovery of the myelin sheath.
Oligodendrocytes are essential for the remyelination of damaged white matter after ischemic stroke.22 We detected mature oligodendrocytes labeled by APC using immunohistochemical analysis. As shown in Figure 4D and E, MCAO injury significantly decreased the number of mature oligodendrocytes in the corpus callosum (P<0.001), while treatment with CIG notably increased the number of mature oligodendrocytes (P<0.05, P<0.001).
CIG Inhibited the Activation of Microglia and Astrocytes in the Corpus Callosum of MCAO Rats
Immunohistochemical staining was used to detect microglial cells labeled by Iba-1 and astrocytes labeled by GFAP in the rat brains. Our results indicated that the number of microglia and astrocytes was obviously increased in the corpus callosum of MCAO model rats when compared to the sham control group (P<0.05, P<0.001). Treatment with CIG significantly decreased the number of microglia and astrocytes (P<0.05, P<0.001; Figure 5), implying the anti-inflammatory effect of CIG in the white matter of cerebral ischemic rats.
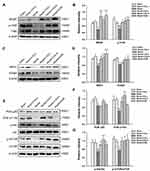
CIG Up-Regulated BDNF/TrkB, NRG/ErbB and PI3K/Akt/mTOR Pathways in the Corpus Callosum of MCAO Rats
To further explore the underlying mechanisms of CIG, we detected the expression of BDNF/TrkB and the key molecules in NRG/ErbB and PI3K/Akt/mTOR pathways using Western blot analysis. The results demonstrated that the expression of BDNF and its receptor, phosphorylated (p)-TrkB (active form), was markedly decreased in the corpus callosum of the MCAO model rats (P<0.05, P<0.001). CIG treatment significantly increased the levels of BDNF and p-TrkB in the ischemic rats (P<0.05, P<0.01, P<0.001; Figure 6A and B).
We also observed that MCAO induced a notable decline in the expression of neuregulin 1 (NRG1) and its receptor ErbB4 in the corpus callosum (P<0.05, P<0.001); the treatment with CIG markedly elevated the levels of NRG1 and ErbB4 (P<0.05; Figure 6C and D), indicating that CIG up-regulated NRG1/ErbB4 signal pathway in the brain of MCAO rats.
Furthermore, we detected PI3K/Akt/mTOR signaling, which is the downstream pathway of NRG1/ErbB. The results indicated that MCAO remarkably reduced the expression of PI3K catalytic subunit p110α, p-Akt, and p-mTOR in the corpus callosum compared with sham control group (P<0.05, P<0.01). The administration of CIG significantly increased the levels of PI3K p110α (P<0.01, P<0.001; Figure 6E and F), and promoted the phosphorylation of Akt and mTOR (P<0.05; Figure 6E and G), demonstrating that CIG activated the PI3K/Akt/mTOR signal pathway in the brain of ischemic rats.
Discussion
In the present study, we demonstrated that treatment with CIG for 7 days starting at 6 h after ischemia reperfusion improved neurological deficits, accelerated the recovery of somatosensory and motor function, and ameliorated the memory impairment in MCAO rats.
Ischemic stroke is a complex pathophysiological process influencing both gray matter and white matter. The principal components of gray matter include neuronal cell bodies, dendrites, and axons for local information processing, whereas white matter mainly contains long extensions of myelinated and unmyelinated axons that are organized into tracts and surrounding glial cells and blood vessels. Both gray matter and white matter are critically dependent on a continuous supply of oxygen and glucose. However, white matter receives less collateral circulation than gray matter and has a smaller blood supply, leading to more susceptible to ischemia.5 Cerebral white matter injury has been reported in 64–86% of stroke patients.23,24 It causes a loss of white matter bundle architecture and functional integrity,25 and finally damages cerebral plasticity and impairs post-stroke recovery.26 White matter is composed of nerve fiber tracts, while the corpus callosum is a bundle of nerve fibers connecting the left and right hemispheres of the brain. Besides, the corpus callosum region is the peri-infarct area where transient focal cerebral ischemia induces severe myelin loss and axonal damage.27 White matter injury results in profound cognitive impairment as well as motor and sensory dysfunction, which is the consequence of disrupted signal transmission between the cerebral cortex and subcortical structures.28 In the current study, we found that focal cerebral ischemia induced serious white matter injury, including demyelination, myelin swelling, and axon disruption in the corpus callosum. However, these lesions were dramatically ameliorated by CIG treatment. Thus, we believe that the preservation or restoration of white matter integrity may promote the recovery of sensorimotor and cognitive functions after ischemic stroke.
White matter integrity and connectivity are maintained by multiple cell types and intercellular signaling cascades, including axons, oligodendrocytes, astrocytes, and microglia.29 Oligodendrocytes are myelin-producing cells responsible for axonal myelination under normal conditions and for remyelination after axonal damage.5 Like neurons, oligodendrocytes are highly sensitive to oxidative stress, excitatory toxicity, neurotrophic factor deficiency, and activation of apoptotic pathway.30 In the white matter, because oligodendrocytes are selectively vulnerable to ischemia, there is an early loss of myelin sheath under ischemic stress.31,32 The results of our study showed that the number of mature oligodendrocytes in MCAO rats markedly decreased, while CIG treatment increased this number in the corpus callosum. It has been reported that MBP, a major protein located on the serosal surface of the myelin sheath, is synthesized by oligodendrocytes in the central nervous system (CNS).21 MBP maintains the stability of the structure and function of the myelin sheath in the CNS, and has the nerve tissue specificity.33 We revealed that MBP expression was markedly reduced in the corpus callosum of MCAO rats, whereas CIG treatment reversed this change, further confirming CIG’s effects of alleviating demyelination and promoting the recovery of white matter lesions after stroke.
Microglia and astrocytes are also reported to play important roles in maintaining homeostatic equilibrium and influence myelination in white matter after ischemic stroke.34–36 Ischemia evokes the activation of microglia and astrocytes to produce detrimental cytokine factors, which may induce bystander damage to neighboring glia and neurons.37,38 While oligodendrocytes are especially susceptible to these cytokine factors, astrocytes and even axons might also be affected. In such inflammatory conditions, oligodendrocytes typically respond by producing poor-quality myelin, which is susceptible to degradation and ultimately results in hypomyelination.39 Furthermore, neuroinflammation in the CNS has been shown to induce secondary damage after neural injury,40 which is also detrimental to white matter. Hence, prevention or amelioration of neural inflammatory conditions may be beneficial in maintaining white matter integrity after ischemic stroke. Our previous study revealed that CIG inhibited the activation of microglia and astrocytes, decreased the levels of pro-inflammatory cytokines IL-1β and TNF-α, and reduced the number of apoptotic cells in the ischemic cerebral cortex of MCAO model rats.9 These results suggest that the improvement of CIG on neuronal damages may be through inhibiting neuroinflammatory response. In the present study, we found that the number of microglia and astrocytes increased in the corpus callosum after cerebral ischemia, while CIG decreased the number of microglia and astrocytes, indicating that CIG also had anti-inflammatory effects in white matter, which may be a factor leading to the restoration of damaged white matter.
Upon further analysis of the alleviation of demyelination by CIG, we found that it elevated the levels of brain-derived neurotrophic factor (BDNF) and its receptor p-TrkB (active form) in the corpus callosum of MCAO rats. BDNF has a direct effect on oligodendroglia; it promotes the proliferation and differentiation of oligodendrocyte precursor cells (OPCs) and facilitates myelination,41,42 both of which are vital to white matter restoration after ischemic stroke. Except for OPCs, BDNF may also promote the differentiation of neural stem/progenitor cells into oligodendrocyte lineage cells.43 It has been reported that astrocytes could support the maturation of OPCs by secreting BDNF.44 However, in the present study, we found that the level of BDNF was negatively correlated with the number of astrocytes in the corpus callosum of MCAO rats, which implied that the BDNF contributed by astrocytes might not be sufficient to compensate for the BDNF loss due to cerebral ischemia. This suggests that the increase in BDNF level after CIG treatment is mainly due to the effect of CIG on oligodendrocytes.
BDNF can also promote the rapid release of soluble neuregulin-1 (NRG1) from axons,45,46 while NRG1 is a key signaling molecule that regulates the process of myelination.47 A positive feedback loop exists between axonal NRG1 and BDNF.48 Decreasing NRG/ErbB signaling has been reported to reduce myelination via oligodendrocytes in the CNS,49 indicating that myelination partially depends on NRG1/ErbB signaling. Binding of NRG1 to ErbBs (the transmembrane tyrosine kinase receptors) activates intracellular PI3K/Akt/mTOR signaling, a downstream pathway of the NRG1/ErbB signaling network, and finally promotes the oligodendrocyte differentiation and myelination.50 It has been reported that the activation of PI3K/Akt/mTOR pathway is required at the onset of myelination and for the myelin sheath wrapping by oligodendrocytes.51 BDNF also activates the PI3K/Akt/mTOR pathway.52,53 In the present study, CIG elevated the levels of BDNF/TrkB and activated NRG1/ErbB signaling, thereby activating the PI3K/Akt/mTOR pathway in the corpus callosum of MCAO rats. This might have triggered the differentiation of oligodendrocytes and the promotion of remyelination.
In conclusion, our results reveal that CIG treatment alleviates white matter lesions and improves neurological function outcomes in rats after ischemic stroke. CIG decreases the number of activated microglia and astrocytes, thereby inhibiting the inflammation after MCAO. CIG also elevates the levels of BDNF/p-TrkB, activates NRG1/ErbB signaling and its downstream PI3K/Akt/mTOR pathway, thereby increasing the number of mature oligodendrocytes, which promotes myelination in the white matter of cerebral ischemic rats. These findings, at least in part, elucidate the mechanisms involved in the protective effects of CIG against acute cerebral ischemic stroke. Based on our research, we highlight the protective effects of CIG against cerebral ischemia-induced injury to both white matter and gray matter, advocating its use as a potential neuroprotective agent for the treatment of ischemic stroke.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81473373, 81874351, 81673406), National Science and Technology Major Project of China (2015ZX09101-016), and Beijing Postdoctoral Research Foundation (2018-ZZ-112).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Guzik A, Bushnell C. Stroke epidemiology and risk factor management. Continuum (Minneap Minn). 2017;23(1, Cerebrovascular Disease):15–39. doi:10.1212/CON.0000000000000416
2. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi:10.1016/S0140-6736(19)30427-1
3. Moretti A, Ferrari F, Villa RF. Neuroprotection for ischaemic stroke: current status and challenges. Pharmacol Ther. 2015;146:23–34. doi:10.1016/j.pharmthera.2014.09.003
4. Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. Int J Stroke. 2012;7(5):378–385. doi:10.1111/j.1747-4949.2012.00839.x
5. Wang Y, Liu G, Hong D, Chen F, Ji X, Cao G. White matter injury in ischemic stroke. Prog Neurobiol. 2016;141:45–60. doi:10.1016/j.pneurobio.2016.04.005
6. Munoz MS, Chappell FM, Valdes HM, et al. Integrity of normal-appearing white matter: influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab. 2017;37(2):644–656. doi:10.1177/0271678X16635657
7. Ding G, Chen J, Chopp M, et al. White matter changes after stroke in type 2 diabetic rats measured by diffusion magnetic resonance imaging. J Cereb Blood Flow Metab. 2017;37(1):241–251. doi:10.1177/0271678X15622464
8. Ho PW, Reutens DC, Phan TG, et al. Is white matter involved in patients entered into typical trials of neuroprotection? Stroke. 2005;36(12):2742–2744. doi:10.1161/01.STR.0000189748.52500.a7
9. Ya B, Li C, Zhang L, Wang W, Li L. Cornel iridoid glycoside inhibits inflammation and apoptosis in brains of rats with focal cerebral ischemia. Neurochem Res. 2010;35(5):773–781. doi:10.1007/s11064-010-0134-2
10. Yao RQ, Zhang L, Wang W, Li L. Cornel iridoid glycoside promotes neurogenesis and angiogenesis and improves neurological function after focal cerebral ischemia in rats. Brain Res Bull. 2009;79(1):69–76. doi:10.1016/j.brainresbull.2008.12.010
11. Yin B, Xu Y, Wei R, Luo B. Ginkgo biloba on focal cerebral ischemia: a systematic review and meta-analysis. Am J Chin Med (Gard City N Y). 2014;42(04):769–783. doi:10.1142/S0192415X14500499
12. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi:10.1161/01.STR.20.1.84
13. Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi:10.1161/01.STR.32.4.1005
14. Germano AF, Dixon CE, D’Avella D, Hayes RL, Tomasello F. Behavioral deficits following experimental subarachnoid hemorrhage in the rat. J Neurotrauma. 1994;11(3):345–353. doi:10.1089/neu.1994.11.345
15. Ma D, Zhu Y, Li Y, et al. Beneficial effects of cornel iridoid glycoside on behavioral impairment and senescence status in SAMP8 mice at different ages. Behav Brain Res. 2016;312:20–29. doi:10.1016/j.bbr.2016.06.008
16. Akkerman S, Blokland A, Reneerkens O, et al. Object recognition testing: methodological considerations on exploration and discrimination measures. Behav Brain Res. 2012;232(2):335–347. doi:10.1016/j.bbr.2012.03.022
17. Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp. 2017;126:e55718.
18. Zhang R, Yang N, Ji C, et al. Neuroprotective effects of Aceglutamide on motor function in a rat model of cerebral ischemia and reperfusion. Restor Neurol Neurosci. 2015;33(5):741–759. doi:10.3233/RNN-150509
19. He XL, Wang YH, Bi MG, Du GH. Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur J Pharmacol. 2012;680(1–3):41–48. doi:10.1016/j.ejphar.2012.01.025
20. Wakita H, Tomimoto H, Akiguchi I, Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol. 1994;87(5):484–492. doi:10.1007/BF00294175
21. Sozmen EG, Kolekar A, Havton LA, Carmichael ST. A white matter stroke model in the mouse: axonal damage, progenitor responses and MRI correlates. J Neurosci Methods. 2009;180(2):261–272. doi:10.1016/j.jneumeth.2009.03.017
22. Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Front Cell Neurosci. 2013;7:201. doi:10.3389/fncel.2013.00201
23. Fu JH, Lu CZ, Hong Z, Dong Q, Luo Y, Wong KS. Extent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76(6):793–796. doi:10.1136/jnnp.2003.032771
24. Li L, Simoni M, Küker W, et al. Population-based case-control study of white matter changes on brain imaging in transient ischemic attack and ischemic stroke. Stroke. 2013;44(11):3063–3070. doi:10.1161/STROKEAHA.113.002775
25. Lawrence AJ, Chung AW, Morris RG, Markus HS, Barrick TR. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology. 2014;83(4):304–311. doi:10.1212/WNL.0000000000000612
26. Ruber T, Schlaug G, Lindenberg R. Compensatory role of the cortico-rubro-spinal tract in motor recovery after stroke. Neurology. 2012;79(6):515–522. doi:10.1212/WNL.0b013e31826356e8
27. Han L, Cai W, Mao L, et al. Rosiglitazone promotes white matter integrity and long-term functional recovery after focal cerebral ischemia. Stroke. 2015;46(9):2628–2636. doi:10.1161/STROKEAHA.115.010091
28. Mifsud G, Zammit C, Muscat R, Di Giovanni G, Valentino M. Oligodendrocyte pathophysiology and treatment strategies in cerebral ischemia. CNS Neurosci Ther. 2014;20(7):603–612. doi:10.1111/cns.2014.20.issue-7
29. Hayakawa K, Lo EH. Brain-peripheral cell crosstalk in white matter damage and repair. Biochim Biophys Acta. 2016;1862(5):901–908. doi:10.1016/j.bbadis.2015.08.006
30. Shindo A, Liang AC, Maki T, et al. Subcortical ischemic vascular disease: roles of oligodendrocyte function in experimental models of subcortical white-matter injury. J Cereb Blood Flow Metab. 2016;36(1):187–198. doi:10.1038/jcbfm.2015.80
31. Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23(3):263–274. doi:10.1097/01.WCB.0000053472.41007.F9
32. Petito CK, Olarte JP, Roberts B, Nowak TJ, Pulsinelli WA. Selective glial vulnerability following transient global ischemia in rat brain. J Neuropathol Exp Neurol. 1998;57(3):231–238. doi:10.1097/00005072-199803000-00004
33. Harauz G, Ladizhansky V, Boggs JM. Structural polymorphism and multifunctionality of myelin basic protein. Biochemistry-US. 2009;48(34):8094–8104. doi:10.1021/bi901005f
34. Lundgaard I, Osorio MJ, Kress BT, Sanggaard S, Nedergaard M. White matter astrocytes in health and disease. Neuroscience. 2014;276:161–173. doi:10.1016/j.neuroscience.2013.10.050
35. Wang G, Zhang J, Hu X, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33(12):1864–1874. doi:10.1038/jcbfm.2013.146
36. Wang G, Shi Y, Jiang X, et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis. Proc Natl Acad Sci U S A. 2015;112(9):2853–2858. doi:10.1073/pnas.1501441112
37. Wang X, Chen S, Ni J, Cheng J, Jia J, Zhen X. miRNA-3473b contributes to neuroinflammation following cerebral ischemia. Cell Death Dis. 2018;9(1):11. doi:10.1038/s41419-017-0014-7
38. Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32(9):1677–1698. doi:10.1038/jcbfm.2012.88
39. Peferoen L, Kipp M, van der Valk P, van Noort JM, Amor S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology. 2014;141(3):302–313. doi:10.1111/imm.12163
40. Truettner JS, Bramlett HM, Dietrich WD. Posttraumatic therapeutic hypothermia alters microglial and macrophage polarization toward a beneficial phenotype. J Cereb Blood Flow Metab. 2017;37(8):2952–2962. doi:10.1177/0271678X16680003
41. Ramos-Cejudo J, Gutierrez-Fernandez M, Otero-Ortega L, et al. Brain-derived neurotrophic factor administration mediated oligodendrocyte differentiation and myelin formation in subcortical ischemic stroke. Stroke. 2015;46(1):221–228. doi:10.1161/STROKEAHA.114.006692
42. Xiao J, Wong AW, Willingham MM, van den Buuse M, Kilpatrick TJ, Murray SS. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals. 2010;18(3):186–202. doi:10.1159/000323170
43. Chen BY, Wang X, Wang ZY, Wang YZ, Chen LW, Luo ZJ. Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/beta-catenin signaling pathway. J Neurosci Res. 2013;91(1):30–41. doi:10.1002/jnr.23138
44. Miyamoto N, Maki T, Shindo A, et al. Astrocytes promote oligodendrogenesis after white matter damage via brain-derived neurotrophic factor. J Neurosci. 2015;35(41):14002–14008. doi:10.1523/JNEUROSCI.1592-15.2015
45. Esper RM, Loeb JA. Rapid axoglial signaling mediated by neuregulin and neurotrophic factors. J Neurosci. 2004;24(27):6218–6227. doi:10.1523/JNEUROSCI.1692-04.2004
46. Wang J, Hmadcha A, Zakarian V, Song F, Loeb JA. Rapid transient isoform-specific neuregulin1 transcription in motor neurons is regulated by neurotrophic factors and axon-target interactions. Mol Cell Neurosci. 2015;68:73–81. doi:10.1016/j.mcn.2015.04.003
47. Kataria H, Alizadeh A, Shahriary GM, et al. Neuregulin-1 promotes remyelination and fosters a pro-regenerative inflammatory response in focal demyelinating lesions of the spinal cord. Glia. 2018;66(3):538–561. doi:10.1002/glia.v66.3
48. Ma Z, Wang J, Song F, Loeb JA. Critical period of axoglial signaling between neuregulin-1 and brain-derived neurotrophic factor required for early Schwann cell survival and differentiation. J Neurosci. 2011;31(26):9630–9640. doi:10.1523/JNEUROSCI.1659-11.2011
49. Taveggia C, Thaker P, Petrylak A, et al. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56(3):284–293. doi:10.1002/(ISSN)1098-1136
50. Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2014;83(1):27–49. doi:10.1016/j.neuron.2014.06.007
51. Guardiola-Diaz HM, Ishii A, Bansal R. Erk1/2 MAPK and mTOR signaling sequentially regulates progression through distinct stages of oligodendrocyte differentiation. Glia. 2012;60(3):476–486. doi:10.1002/glia.22281
52. Yang J, Yan H, Li S, Zhang M. Berberine ameliorates MCAO induced cerebral ischemia/reperfusion injury via activation of the BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem Res. 2018;43(3):702–710. doi:10.1007/s11064-018-2472-4
53. Khalaj AJ, Hasselmann J, Augello C, Moore S, Tiwari-Woodruff SK. Nudging oligodendrocyte intrinsic signaling to remyelinate and repair: estrogen receptor ligand effects. J Steroid Biochem Mol Biol. 2016;160:43–52. doi:10.1016/j.jsbmb.2016.01.006
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.