Back to Journals » Clinical Ophthalmology » Volume 16
Corneal Epithelium Asymmetry in Children With Atopy: The Effect of Hand Dominance
Authors Loureiro T , Rodrigues-Barros S, Carreira AR, Gouveia-Moraes F , Carreira P , Vide Escada A, Campos P, Machado I, Campos N, Archer TJ, Reinstein DZ , Ambrósio R Jr
Received 31 May 2022
Accepted for publication 25 July 2022
Published 6 August 2022 Volume 2022:16 Pages 2453—2461
DOI https://doi.org/10.2147/OPTH.S375504
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Tomás Loureiro,1 Sandra Rodrigues-Barros,1 Ana Rita Carreira,1 Filipe Gouveia-Moraes,1 Pedro Carreira,1 Ana Vide Escada,1 Paul Campos,1 Inês Machado,1 Nuno Campos,1 Timothy J Archer,2 Dan Z Reinstein,2 Renato Ambrósio Jr3
1Ophthalmology Department, Hospital Garcia de Orta, Almada, Portugal; 2London Vision Clinic, London, UK; 3Federal University of the State of Rio de Janeiro, Rio de Janeiro, Brazil
Correspondence: Tomás Loureiro, Ophthalmology Department, Hospital Garcia de Orta, Avenida Torrado da Silva, Almada, 2805-267, Portugal, Tel +35 1 913 513 175, Email [email protected]
Purpose: To evaluate the effect of eye rubbing on the epithelial thickness profile in tomographically normal corneas by AS-OCT and to compare right and left eyes in right-handed children.
Methods: Thirty right-handed boys (mean age 11.2 years) with ocular allergy and history of eye rubbing were evaluated using Scheimpflug (Pentacam HR, Oculus Wetzlar, Germany) and anterior segment optical coherence tomography. Epithelial thickness (ET) and full corneal thickness (CT) parameters were compared between right and left eyes with a non-parametric Mann–Whitney test. A p-value lower than 0.05 was considered for statistical significance.
Results: No eyes had topometric nor tomographic criteria for keratoconus. The min-max ET was lower in right eyes (− 2.8 μm vs − 3.5; p = 0.02). The difference between inferior and superior (I-S) octants was lower in right eyes (1.1 μm vs 1.9 μm; p = 0.03) as a result of inferotemporal thinning. The highest ET difference was registered between nasal and temporal octants and was more pronounced in the right eyes (2 μm vs 3.1 μm; p < 0.001).
Conclusion: AS-OCT analyses reveal different epithelial thickness patterns between the eyes in young atopic patients, likely eye rubbers. Inferior and temporal epithelial thickness seem to be more affected by thinning in the eye on the side of the dominant hand.
Keywords: eye rubbing, keratoconus, epithelial thickness, AS-OCT, pediatrics
Introduction
Keratoconus is the most common corneal ectasia with a prevalence of 1.38 per 1000 in the world’s population.1 It is a progressive disease characterized by corneal steepening and thinning, causing irregular astigmatism. Onset is usually in early adolescence and progresses into the third decade of life. However, some cases can start earlier or later in life, and progression can go beyond the 30ʹs.
Although the exact etiology of KC remains unclear, it is recognized as a multifactorial disease influenced by genetic and environmental factors.2–4 There are well-known risk factors associated with KC, such as allergy, eye rubbing, asthma and eczema.1,5 Positive family history has been reported as the strongest risk factor in a recent meta-analysis, however eye rubbing was considered the most important risk factor according to some epidemiological studies, which reported an odds ratio from 3.35 to 10.31 for KC development.1,6,7
Gatinel described eye rubbing as a sine qua non condition for KC development and highlighted the association between repeated mechanical trauma and KC.8 Children who intensely rub their eyes, like those with atopic dermatitis, are at higher risk of developing keratoconus.9–11 Microscopically, eye rubbing leads to progressive distortion and disorganization of the collagen fibers, which would disturb the homogeneity of the fiber matrix and further impair corneal biomechanics.8 By creating localized weakened zones, the force exerted by intraocular pressure causes deformation of the corneal wall.8 On the other hand, eye rubbing has been shown to increase the level of the tear-film matrix metalloproteinases, interleukin 6, and tumor necrosis factor-alpha, which play a role in the pathogenesis of KC.12 In 2015, the Global Consensus on Keratoconus and Ectatic Diseases recognized “not rubbing the eyes” as one of the most important nonsurgical treatment measures in KC.13 Ambrosio created a public awareness campaign called Violet June that started in Rio de Janeiro about keratoconus, highlighting the perils of eye rubbing.14
Epithelial thickness changes may be the first morphological alterations to be detected in keratoconus.15 Corneal epithelial analyses, in particular its thickness, have raised interest in many ocular diseases. Models have been developed to detect KC with good accuracy based only on epithelial thickness (ET) analyses even from the early stages where tomographic examinations could be equivocal.16,17
Anterior segment optical coherence tomography (AS-OCT) is a non-contact imaging technique that provides high-resolution images of the cornea and enables epithelial thickness analysis. AS-OCT proved to be reliable and reproducible in ET measurements.18–20
This study aimed to evaluate the effect of eye rubbing on the epithelial thickness profile in tomographically normal corneas using AS-OCT and to compare right and left eyes of right-handed children.
Methods
This was a single-center study including atopic boys (8–12 years) with ocular allergy and a history of eye rubbing with the knuckle referred from the Pediatric Department in the acute stage. Previous history of ocular surgery or trauma, contact lens wearing, family history of KC, corrected distance visual acuity (CDVA) <0.1 logMAR, manifest refractive cylinder of more than 2.00 diopters (D) and history of treatment with eyedrops over the past six months excluded boys from the study. Abnormal tomographic parameters using Scheimpflug (Pentacam HR, Oculus Wetzlar, Germany), such as Belin-Ambrosio Deviation Value >1.22 were considered exclusion criteria. Boys who had a history of treatment with artificial tears or anti-histaminic eyedrops over the past six months were excluded. The study was conducted using the principles of the Declaration of Helsinki, the parents gave their written informed consent and approval was obtained from the institutional Research Committee of Hospital Garcia de Orta (Almada, Portugal).
Ophthalmological evaluation included CDVA (logMAR), spherical equivalent (SE), intraocular pressure (IOP) measured with Goldmann tonometer, biomicroscopy of anterior segment, and fundoscopy.
Corneal epithelial thickness (ET) and full corneal thickness (CT) were measured by anterior segment spectral-domain Zeiss Cirrus 5000 (Carl Zeiss Meditec, Dublin, CA, USA). The pachymetry map includes eight radial scans (1024 axial scans each) repeated five times, covering a 9-mm diameter area. Two scans were obtained for each eye by one examiner with a minute break, and average values were registered.
Epithelial thickness is measured as the distance between the middle of the first (tear film) and second (anterior surface of the Bowman layer) hyperreflective lines on the B-scan. CT was measured as the distance between the air-tear and cornea-aqueous interfaces.
Data was exported and processed with Cirrus HD-OCT review software (version 10.0) which provides average automated ET of three concentric ring-shaped zones centered at the center of the cornea (central (CET): 0–2 mm, paracentral: 2–5 mm, and mid-peripheral: 5–7 mm). ET and CT were calculated in eight specific regions of the cornea (octants): superior (S), inferior (I), temporal (T), nasal (N), superonasal (SN), superotemporal (ST), inferotemporal (IT), and inferonasal (IN) within the paracentral and midperipheral zones. Associations between the corresponding corneal regions were calculated. Children were asked about their dominant hand to confirm that they were right-handed to subsequently label the right and left eyes as hand-dominant and non-hand-dominant eyes correspondingly.
Qualitative variables are presented as numbers and percentages. Continuous variables were evaluated to meet the normality conditions of the Shapiro–Wilk test. The Mann–Whitney-U test was performed to compare ET and CT from the eyes of dominant and non-dominant hands. ET data from right-handed age-matched healthy boys (control group) were imported from our previously reported normative database to further compare with eye rubbers.21 Statistical significance was set at p < 0.05 (two-sided). IBM® SPSS® Statistics v23.0 was used.
Results
This study included 30 right-handed boys with a history of eye rubbing due to atopy and 27 controls. There were no differences between groups regarding the age (11.2 ± 2.1 years vs 11.3 ± 2.2, p = 0.89).
In eye rubbers, the mean CDVA was 0.006 ± 0.018 logMAR (0 to 0.091 logMAR), the mean SE was −0.44 ± 1.1 D (−2.00 to +2.00 D) and the mean IOP was 13.2 ± 2.1 mmHg (11 to 16 mmHg), with no differences between right and left eyes (p = 0.89). All eyes had an unremarkable ophthalmological evaluation.
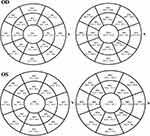
As all study participants were right-handed, performing the analysis for right and left eyes also represented a comparison of hand dominance. Mean ET and the respective differences between right and left eyes in each group are presented in Table 1. The differences between controls and eye rubbers are also presented in Table 1. There was no statistically significant difference between right and left eyes in controls. Corneal epithelial thickness was lower in eye rubbers in paracentral inferior and inferotemporal octants and in midperipheral inferior, inferotemporal and temporal octants in both right and left eyes. The epithelial thickness profiles in the eye rubbers are presented in Figure 1 and the differences between right and left eyes are presented in Table 1. There were no differences for central epithelial thickness between dominant right and non-dominant left eyes (45.8 ± 0.9 µm vs 46.1 ± 1.5 µm, p = 0.62). Epithelial thickness was lower in IT octants in right eyes in both paracentral (43.3 ± 0.9 µm vs 45.3 ± 1.5 µm, p < 0.001) and midperipheral (43.1 ± 1.2 µm vs 44.2 ± 1.8 µm, p = 0.01) areas. Unlike these areas, there were no statistically significant differences in epithelial thickness in the other octants.
Differences in mean ET between corresponding octants were found between right and left eyes. The difference between inferior and superior (I − S) was lower in right eyes (1.1 µm vs 1.9 µm; p = 0.03). The highest ET difference was registered between nasal and temporal octants and was more pronounced in right eyes (3.1 µm vs 2 µm; p < 0.001). The difference between IN and ST (1.8 µm vs 2.1 µm, p = 0.66) and between IT and SN (−0.3 µm vs 0.4 µm, p = 0.06) did not show a statistically significant difference.
Minimum ET was 40.8 ± 1.5 µm, 43.0 ± 0.9 µm and 40.0 ± 0.9 µm in right eyes and 41.5 ± 1.6 µm, 43.0 ± 0.9 µm and 40.1 ± 1.4 µm in left eyes from center to the periphery, respectively. Maximum ET was 51.1 ± 1.3 µm, 47.9 ± 1.0 µm and 48.4 ± 1.6 µm in right eyes and 51.1 ± 1.9 µm, 48.0 ± 0.8 µm and 48.9 ± 2.3 µm in left eyes from center to the periphery, respectively. The difference between the minimum and maximum (min-max) average epithelial thickness was lower in right eyes (−2.8 µm vs −3.5; p = 0.02).
The corneal full-thickness profiles in the right and left eyes are presented in Figure 1. The differences between right and left eyes are shown in Table 2. There was no statistically significant difference for central corneal thickness (533.5 ± 21.5 µm vs 532.9 ± 23.6 µm, p = 0.91). CT was lower in inferotemporal octants in right eyes in both paracentral (537.2 ± 22.5 µm vs 551.0 ± 23.4 µm, p = 0.02) and midperipheral (559.1 ± 20.4 µm vs 571.4 ± 19.9 µm, p = 0.02) areas. Unlike these areas, there were no statistically significant differences in epithelial thickness in the other octants. Finally, the current study did not find any correlations between ET and age (p = 0.89) and between ET and SE (p = 0.66).
 |
Table 2 Full Corneal Thickness Comparison (Mean ± Standard Deviation, Lower and Upper Bounds of 95% Confidence Interval; µm) Between Right and Left Eyes in Eye Rubbers |
Discussion
The present study shows that corneal epithelial thickness pattern is different in young atopic eye rubbers. The differences between eye rubbers and controls suggest that inferior and temporal areas are more affected by mechanical trauma, such as rubbing. Moreover, the inferotemporal epithelial thickness is thinner in the eye of the dominant hand’s side.
The diagnosis of KC is challenging. As the biomicroscopic signs usually appear in an advanced stage of the disease, the diagnosis strongly depends on additional diagnostic tests. Epithelial thickness mapping has been recognized as an important parameter in the diagnosis of forme fruste keratoconus in adults. Reinstein et al described how epithelial thinning at the KC apex with surrounding thickening (doughnut pattern) is an important manifestation of the earliest stages, improving sensitivity and specificity in conjunction with tomography.16,22–24
AS-OCT provides high-resolution images of the corneal epithelium with no need for contact or anesthesia. With a reproducibility of 0.93 and repeatability of 0.8, AS-OCT has been widely used to study epithelial thickness as it does not require the normal saline standoff medium employed with VHF digital ultrasound scanning.20,25–27 However, AS-OCT measurements include the tear film which can vary between 2 and 7 µm from blink to blink and diurnally.28–30 The gold standard for epithelial measurement is VHF digital ultrasound as it is performed under normal saline immersion and, therefore, epithelial measurements are made from front to back of the epithelium itself.31,32 Therefore, the epithelial thickness would be expected to be thicker in OCT. However, studies comparing the two modalities have found these to be similar. For example, Reinstein et al reported a mean central epithelial thickness of 54 µm with VHF digital ultrasound, compared to 53 µm with RTVue OCT (Optovue, Fremont, CA).33 They suggest that this may be due to an arbitrary adjustment of the refractive index used in the OCT device to calibrate the mean epithelial thickness to match that of VHF digital ultrasound. In comparison, the central epithelial thickness found in the present study using Cirrus OCT was 48 µm. This tendency for the Cirrus to measure 5–6 µm thinner than the other devices may be related to the refractive index being used for the Cirrus measurements, which may need to be recalibrated to bring the measurements into line with the other devices. The epithelial thickness results reported in the present study should therefore be interpreted in this context. Rather than considering the absolute thickness values as accurate, our findings are better related to the epithelial thickness changes due to eye rubbing. As such, these differences can be applied to epithelial measurements with other devices.
Our study included eye rubbers from 8 to 12 years with tomographically normal corneas because we believe that epithelial changes in this population could better represent the damage over the cornea due to eye rubbing and may represent the initial stages of the disease. The cut-off of 1.22 for BAD-D raises the specificity of selecting corneas with no ectasia, even fruste forms.34,35 We included patients who preferably rub the eyes with the knuckle as we believe it may better reflect the impact of eye rubbing in the cornea, according to Hafezi et al report. They studied the average amount of mechanical forces applied to the lids of KC patients during eye rubbing according to the type of eye rubbing.36 Rubbing with knuckle (proximal interphalangeal joint) was found to apply more force on the eyelids than rubbing with the fingernail or with the fingertip.36
Epithelial thickness seemed to be lower in inferior and temporal octants in both right and left eyes compared with controls.21 McMonnies has previously examined the possible causal mechanisms for rubbing-related corneal trauma and cone formation and concluded that eye rubbing leads to epithelial thinning which further increases the concentration of proinflammatory molecules in tear film of normal corneas.37 One of these molecules is interleukin-1 (IL-1) which has receptors in corneal stromal fibroblasts. Eye rubbing increases stromal sensitivity to IL-1 and accelerates keratocyte destruction, even more in KC eyes which have a four-fold greater number of IL-1 receptors. As such, we postulate that epithelial thinning could represent a very early stage of KC. On the other hand, epithelial thickness was lower in the right eyes in the inferotemporal area in paracentral and midperipheral areas. This finding also leads us to postulate that the inferotemporal site is most prone to damage by eye rubbing. Most cones are found in the inferotemporal region, which could corroborate our theory.38
Additionally, the lower epithelial thickness in the eye rubbers’ right eye illustrates that corneal damage may be directly related to the hand dominance: due to higher applied forces. Moreover, there were no differences registered between controls’ right and left eyes which lead us to postulate if hand dominance could play a role in asymmetrical nature of keratoconus. The influence of hand dominance in KC asymmetrical nature has been studied in the last decades, but there is still no consensus. Rabinowitz found a high percentage of very asymmetrical KC to occur on the dominant hand side.39 McMonnies described an association between hand dominance with the more advance eye in some cases of KC.40 On the other hand, Moran and Nakao did not find any association between KC laterality.41,42 This association is often based on retrospective reports and corneal damage depends on multiple factors, such as the frequency, technique and the force of rubbing. The frequency of rubbing is very difficult to evaluate, and asymmetric rubbing is difficult to recognize so it is very hard to clearly isolate the effect of hand dominance. As we tried to eliminate the most sources of bias as we can, we believe that under a perfect scenario of symmetric eye rubbing, the stronger dominant hand may cause more trauma because of the greater force involved.
Corneal ET pattern in healthy children was elucidated in our previous paper.21 Despite respecting the same pattern, the I-S (1.1 µm in right eyes; 1.9 µm in left eyes and 3.3 µm in healthy children) and the min-max (−2.8 µm in right eyes; −3.5 µm in left eyes and −7.2 µm in healthy children) were lower in eye rubbers as a result of inferior thinning. The difference between nasal and temporal octants was also lower in eye rubbers (3.1 µm in right eyes; 2 µm in left eyes and 0.7 µm in healthy children) due to temporal thinning.
Corneal full-thickness pattern did not show any differences between right and left eyes and between eye rubbers and healthy children. Although we know that compensatory epithelial changes to stromal irregularities were previously described by Reinstein, we clearly believe that our results come only from the mechanical damage and may represent the very early stage of KC pathogenesis.17,43–47 Another weakness was that the study did not include any left-handed children. This could be the subject of a future study to provide further evidence for the influence of hand dominance. This would also be of interest because left-handed people are often more ambidextrous, so it might be expected to less clearly illustrate the influence of hand dominance.48,49
Limitations of our study are the small sample and the fact that we only included boys. However, we prefer not to have girls due to known gender-based ET variations, which could bias our results.21,50 Moreover, another weakness of the study is not having a representative sample of confirmed left-handed subjects, so repeating the study with such a population should be the subject of further study.
Despite this, we believe our results demonstrate the effect of eye rubbing in the corneal epithelium, which could help to elucidate the pathogenesis of the disease. As such, ophthalmologists should propagate public awareness campaigns and educate their patients about the risks associated with eye rubbing. As we also believe that eye rubbing is mandatory for KC development, the differences associated with hand dominance may help to explain why KC is usually an asymmetric disease.
Conclusion
AS-OCT analyses reveal different epithelial thickness patterns between the eyes in young atopic patients, likely eye rubbers. Inferior and temporal epithelial thickness seems to be more affected by thinning in the eye of the dominant hand’s side. Further investigation is needed into the relationship between eye rubbing effect in the corneal epithelium in keratoconus cases.
Disclosure
Dr Reinstein is a consultant for Carl Zeiss Meditec (Carl Zeiss Meditec AG) and CSO Italia, reports personal fees from Carl Zeiss Meditec and CSO Italia, and has a proprietary interest in the Artemis Insight 100 technology (ArcScan, Inc) through patents administered by the Cornell Center for Technology Enterprise and Commercialization (CCTEC), Ithaca, New York (during the conduct of the study and outside the submitted work). Dr Ambrósio is a consultant for Oculus, Alcon, Zeiss, Essilor, Genom, and Mediphacos, and reports personal fees from Oculus outside the submitted work. The aforementioned authors report no other potential conflicts of interest in relation to this work. The remaining authors have no financial or proprietary interest in the materials presented herein and report no conflicts of interest in relation to this work.
References
1. Hashemi H, Heydarian S, Hooshmand E, et al. The prevalence and risk factors for keratoconus: a systematic review and meta-analysis. Cornea. 2020;39(2):263–270. doi:10.1097/ICO.0000000000002150
2. Gordon-Shaag A, Millodot M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. Biomed Res Int. 2015;2015:795738. doi:10.1155/2015/795738
3. Abu-Amero KK, Kalantan H, Al-Muammar AM. Analysis of the VSX1 gene in keratoconus patients from Saudi Arabia. Mol Vis. 2011;17:667–672.
4. Al-Raddadi HS, Al-Barry MA, Al-Harbi E, Samman MI, Albalawi AM, Basit S. Sequence analysis of the VSX1 and SOD1 genes in families with Keratoconus and a review of the literature. J Taibah Univ Med Sci. 2016;11(2):115–120. doi:10.1016/j.jtumed.2015.08.004
5. Gordon-Shaag A, Millodot M, Kaiserman I, et al. Risk factors for keratoconus in Israel: a case_control study. Ophthalmic Physiol Opt. 2015;35:673–681.
6. Galvis V, Tello A, Carreño NI, Berrospi RD, Niño CA. Risk factors for keratoconus: atopy and eye rubbing. Cornea. 2017;36(1):e1.
7. Hashemi H, Khabazkhoob M, Yazdani N, et al. The prevalence of keratoconus in a young population in Mashhad, Iran. Ophthalmic Physiol Opt. 2014;34(5):519–527. doi:10.1111/opo.12147
8. Gatinel D. Eye rubbing: a sine qua non for keratoconus? Int J Kerat Ect Cor Dis. 2016;5:6–12. doi:10.5005/jp-journals-10025-1114
9. Alio JL, Vega-Estrada A, Sanz P, et al. Corneal morphologic characteristics in patients with down syndrome. JAMA Ophthalmol. 2018;136(9):971–978. doi:10.1001/jamaophthalmol.2018.2373
10. Shajari M, Eberhardt E, Müller M, et al. Effects of atopic syndrome on keratoconus. Cornea. 2016;35(11):1416–1420.
11. Sharma N, Rao K, Maharana PK, Vajpayee RB. Ocular allergy and keratoconus. Indian J Ophthalmol. 2013;61(8):407–409. doi:10.4103/0301-4738.116063
12. Balasubramanian SA, Pye DC, Willcox MDP. Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: relevance in keratoconus. Clin Exp Optom. 2013;96(2):214–218. doi:10.1111/cxo.12038
13. Gomes JAP, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359–369.
14. Ambrósio R. Violet June: the global keratoconus awareness campaign. Ophthalmol Ther. 2020;9(3):685–688. doi:10.1007/s40123-020-00283-5
15. Kalkan Akcay E, Uysal BS, Sarac O, et al. The effect of corneal epithelium on corneal curvature in patients with keratoconus. Semin Ophthalmol. 2015. doi:10.3109/08820538.2013.874490
16. Silverman RH, Urs R, Roychoudhury A, Archer TJ, Gobbe M, Reinstein DZ. Epithelial remodeling as basis for machine-based identification of keratoconus. Investig Ophthalmol Vis Sci. 2014. doi:10.1167/iovs.13-12578
17. Li Y, Tan O, Brass R, Weiss JL, Huang D. Corneal epithelial thickness mapping by Fourier-domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology. 2012. doi:10.1016/j.ophtha.2012.06.023
18. Li Y, Meisler DM, Tang M, et al. Keratoconus diagnosis with optical coherence tomography pachymetry mapping. Ophthalmology. 2008. doi:10.1016/j.ophtha.2008.08.004
19. Li Y, Tang M, Zhang X, Salaroli CH, Ramos JL, Huang D. Pachymetric mapping with Fourier-domain optical coherence tomography. J Cataract Refract Surg. 2010. doi:10.1016/j.jcrs.2009.11.016
20. Prakash G, Agarwal A, Mazhari AI, et al. Reliability and reproducibility of assessment of corneal epithelial thickness by Fourier domain optical coherence tomography. Investig Ophthalmol Vis Sci. 2012. doi:10.1167/iovs.11-8981
21. de Oliveira Loureiro T, Rodrigues-Barros S, Lopes D, et al. Corneal epithelial thickness profile in healthy Portuguese children by high-definition optical coherence tomography. Clin Ophthalmol. 2021;15:735–743. doi:10.2147/OPTH.S293695
22. Ambrósio JR. Multimodal imaging for refractive surgery: quo vadis? Indian J Ophthalmol. 2020;68(12):2647–2649. doi:10.4103/0301-4738.301283
23. Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009. doi:10.3928/1081597X-20090610-06
24. Kanellopoulos AJ, Aslanides IM, Asimellis G. Correlation between epithelial thickness in normal corneas, untreated ectatic corneas, and ectatic corneas previously treated with CXL; is overall epithelial thickness a very early ectasia prognostic factor? Clin Ophthalmol. 2012. doi:10.2147/OPTH.S31524
25. Francoz M, Karamoko I, Baudouin C, Labbé A. Ocular surface epithelial thickness evaluation with spectral-domain optical coherence tomography. Investig Ophthalmol Vis Sci. 2011. doi:10.1167/iovs.11-7988
26. Ma XJ, Wang L, Koch DD. Repeatability of corneal epithelial thickness measurements using Fourier-domain optical coherence tomography in normal and post-LASIK eyes. Cornea. 2013. doi:10.1097/ICO.0b013e3182a7f39d
27. Ge L, Yuan Y, Shen M, Tao A, Wang J, Lu F. The role of axial resolution of optical coherence tomography on the measurement of corneal and epithelial thicknesses. Investig Ophthalmol Vis Sci. 2013. doi:10.1167/iovs.11-9308
28. Azartash K, Kwan J, Paugh JR, Nguyen AL, Jester JV, Gratton E. Pre-corneal tear film thickness in humans measured with a novel technique. Mol Vis. 2011;17:756–767.
29. Chen Q, Wang J, Tao A, Shen M, Jiao S, Lu F. Ultrahigh-resolution measurement by optical coherence tomography of dynamic tear film changes on contact lenses. Invest Ophthalmol Vis Sci. 2010;51(4):1988–1993. doi:10.1167/iovs.09-4389
30. Cui L, Wang J, Perez VL, Shen M, Yuan Y, Wang MR. Visualization of the precorneal tear film using ultrahigh resolution optical coherence tomography in dry eye. Eye Contact Lens Sci Clin Pract. 2012;38(4):240–244. doi:10.1097/icl.0b013e318257a108
31. Reinstein DZ, Silverman RH, Trokel SL, Coleman DJ. Corneal Pachymetric Topography. Ophthalmology. 1994;101(3):432–438. doi:10.1016/S0161-6420(94)31314-5
32. Reinstein DZ, Silverman RH, Rondeau MJ, Coleman DJ. Epithelial and corneal thickness measurements by high-frequency ultrasound digital signal processing. Ophthalmology. 1994;101(1):140–146. doi:10.1016/S0161-6420(94)31373-X
33. Reinstein DZ, Yap TE, Archer TJ, Gobbe M, Silverman RH. Comparison of corneal epithelial thickness measurement between Fourier-domain OCT and very high-frequency digital ultrasound. J Refract Surg. 2015. doi:10.3928/1081597X-20150623-01
34. Belin M, Khachikian S. Keratoconus/ectasia detection with the oculus pentacam: belin/ambrósio enhanced ectasia display. Highlights Ophthalmol. 2008;35(6):5–12.
35. Ambrósio R, Valbon BF, Faria-Correia F, Ramos I, Luz A. Scheimpflug imaging for laser refractive surgery. Curr Opin Ophthalmol. 2013. doi:10.1097/ICU.0b013e3283622a94
36. Hafezi F, Hafezi NL, Pajic B, et al. Assessment of the mechanical forces applied during eye rubbing. BMC Ophthalmol. 2020;20(1):301. doi:10.1186/s12886-020-01551-5
37. McMonnies CW. Mechanisms of rubbing-related corneal trauma in keratoconus. Cornea. 2009;28(6):607–615.
38. Auffarth GU, Wang L, Völcker HE. Keratoconus evaluation using the orbscan topography system. J Cataract Refract Surg. 2000;26(2):222–228.
39. Rabinowitz YS, Nesburn AB, McDonnell PJ, Ben-Meir A, Bahrl S. Videokeratography of the fellow eye in unilateral keratoconus. Cornea. 1993;12(1):181–186.
40. Mcmonnies CW, Boneham GC. Keratoconus, allergy, itch, eye‐rubbing and hand‐dominance. Clin Exp Optom. 2003;86(6):376–384. doi:10.1111/j.1444-0938.2003.tb03082.x
41. Moran S, Gomez L, Zuber K, Gatinel D, Case-Control A. Study of keratoconus risk factors. Cornea. 2020;39(6):697–701.
42. Nakao G, Koh S, Inoue R, Maeno S, Maeda N, Nishida K. The characteristics and risk factors of very asymmetric keratoconus. Eye Contact Lens. 2021;47(9):511–514.
43. Rocha KM, Perez-Straziota CE, Stulting RD, Randleman JB. SD-OCT analysis of regional epithelial thickness profiles in keratoconus, postoperative corneal ectasia, and normal eyes. J Refract Surg. 2013. doi:10.3928/1081597X-20130129-08
44. Reinstein DZ, Srivannaboon S, Gobbe M, et al. Epithelial thickness profile changes induced by myopic LASIK as measured by artemis very high-frequency digital ultrasound. J Refract Surg. 2009. doi:10.3928/1081597X-20090422-07
45. Reinstein DZ, Archer TJ, Gobbe M. Change in epithelial thickness profile 24 hours and longitudinally for 1 year after myopic LASIK: three-dimensional display with artemis very high-frequency digital ultrasound. J Refract Surg. 2012. doi:10.3928/1081597X-20120127-02
46. Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Epithelial thickness after hyperopic LASIK: three-dimensional display with artemis very high-frequency digital ultrasound. J Refract Surg. 2010. doi:10.3928/1081597X-20091105-02
47. Reinstein DZ, Archer T. Combined Artemis very high-frequency digital ultrasound-assisted transepithelial phototherapeutic keratectomy and wavefront-guided treatment following multiple corneal refractive procedures. J Cataract Refract Surg. 2006. doi:10.1016/j.jcrs.2006.07.016
48. Vuoksimaa E, Koskenvuo M, Rose RJ, Kaprio J. Origins of handedness: a nationwide study of 30 161 adults. Neuropsychologia. 2009;47(5):1294–1301. doi:10.1016/j.neuropsychologia.2009.01.007
49. de Kovel CGF, Carrión-Castillo A, Francks C. A large-scale population study of early life factors influencing left-handedness. Sci Rep. 2019;9(1):584. doi:10.1038/s41598-018-37423-8
50. Gupta PD, Johar K, Nagpal K, Vasavada AR. Sex hormone receptors in the human eye. Surv Ophthalmol. 2005. doi:10.1016/j.survophthal.2005.02.005
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.