Back to Journals » Nanotechnology, Science and Applications » Volume 13
Cordycepin Nanoencapsulated in Poly(Lactic-Co-Glycolic Acid) Exhibits Better Cytotoxicity and Lower Hemotoxicity Than Free Drug
Authors Marslin G , Khandelwal V, Franklin G
Received 20 March 2020
Accepted for publication 15 May 2020
Published 12 June 2020 Volume 2020:13 Pages 37—45
DOI https://doi.org/10.2147/NSA.S254770
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Israel (Rudi) Rubinstein
Gregory Marslin,1– 3 Vinoth Khandelwal,4 Gregory Franklin5
1School of Pharmacy, Sathyabama Institute of Science and Technology, Jeppiaar Nagar, Rajiv Gandhi Salai, Chennai 600119, India; 2Ratnam Institute of Pharmacy and Research, Nellore, 524346, India; 3College of Biological Science and Engineering, Shaanxi University of Technology, Hanzhong, Shaanxi, People’s Republic of China; 4Department of Translational Pharmacology, Santa Maria Imbaro, Italy; 5Institute of Plant Genetics of the Polish Academy of Sciencs, Strzeszyńska 34, Poznań 60-479, Wielkopolska, Poland
Correspondence: Gregory Marslin; Gregory Franklin Tel +91 4424502344
Email [email protected]; [email protected]
Purpose: Cordycepin, a natural product isolated from the fungus Cordyceps militaris, is a potential candidate for breast cancer therapy. However, due to its structural similarity with adenosine, cordycepin is rapidly metabolized into an inactive form in the body, hindering its development as a therapeutic agent. In the present study, we have prepared cordycepin as nanoparticles in poly(lactic-co-glycolic acid) (PLGA) and compared their cellular uptake, cytotoxicity and hemolytic potential with free cordycepin.
Materials and Methods: Cordycepin-loaded PLGA nanoparticles (CPNPs) were prepared by the double-emulsion solvent evaporation method. Physico-chemical characterization of the nanoparticles was done by zetasizer, transmission electron microscopy (TEM) and reverse-phase high-pressure liquid chromatography (RP-HPLC) analyses. Cellular uptake and cytotoxicity of CPNPs and free drug were tested in human breast cancer cells (MCF7). Hemolytic potential of both of these forms was evaluated in rat red blood cells (RBCs).
Results: Physico-chemical characterization revealed that CPNPs were spherical in shape, possessed a size range of 179– 246 nm, and released the encapsulated drug sustainably over a period of 10 days. CPNPs exhibited a high level of cellular uptake and cytotoxicity than the free drug in MCF-7 cells. While CPNPs were not toxic to rat RBCs even at high concentrations, free cordycepin induced hemolysis of these cells at relatively low concentration.
Conclusion: Our results reveal that delivery as CPNPs could enhance the clinical efficacy of cordycepin substantially.
Keywords: cordycepin, nanoparticles, PLGA, cellular uptake, cytotoxicity, breast cancer cells, hemolysis
Introduction
Cordycepin is an adenosine analogue (3′-deoxyadenosine) derived from the fungus Cordyceps militaris. Cordycepin possesses an array of pharmacological activities including anti-bacterial, anti-fungal, anti-inflammatory, immune modulatory, autophagy inducing, anti-atherosclerotic and anti-cancer activities.1–4 The anticancer activity is attributed mainly to the capacity of cordycepin to induce apoptosis, arrest cell cycle and reduce metastasis.5–9 As a nucleoside antagonist, cordycepin inhibits RNA synthesis and poly (ADP-ribose) polymerase pathways that repair damaged DNA and causes DNA double-strand breaks in breast cancer cells.7
Due to its structural similarity (Figure 1) with adenosine, cordycepin is rapidly metabolized by the enzyme adenosine deaminase (ADA) into an inactive metabolite: - 3ʹ deoxyhypoxanthinosine.2,10,11 ADA is produced by cells throughout the body and is associated with the activation of lymphocytes in the immune system. Although ADA is also present in normal cells, its levels are usually higher in breast cancer cells due to stimulation of cellular immunity, which would further speed up the inactivation of cordycepin.12 Deficiency of ADA activity can lead to immune deficiency and pulmonary inflammation.13 Due to these issues, strategies like co-administration with ADA inhibitors like 2ʹ-deoxycoformycin (pentostatin) to protect cordycepin have failed.2,14 Moreover, the half-life of cordycepin elimination is extremely short (ie t1/2 = 1.6 min in rats).15 As the consequences of ADA activity on cordycepin are unavoidable, a strategy that could protect the drug from this enzyme and extend its half-life in the circulation would be probably the best way to improve its clinical efficacy.
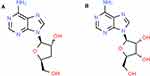 |
Figure 1 Structure of cordycepin (A) and adenosine (B) obtained from the Pubchem database showing similarity. |
Since nanoencapsulation could protect the entrapped drug molecules from harsh conditions (chemical and enzymatic degradation), encapsulation of anticancer drugs in polymeric nanoparticles has received a great deal of attention.16–18 Furthermore, these polymeric nanoparticles are biocompatible, biodegradable, and possesses the ability to maintain drug levels at therapeutic concentration for sustained periods of time.19 It has been reported that oral administration of cordycepin encapsulated in gelatin nanoparticles did not induce subacute toxicity in rats.20 Combination of cordycepin encapsulated in chitosan microspheres and photo-crosslinked hyaluronic acid methacrylate hydrogel was found to be a promising strategy for treating patients with osteoarthritis.21 Liposomal encapsulation has been shown to preserve and even enhance the in vivo anti-tumor activity of cordycepin.14 Further, conjugation of transferrin to these liposomes was found effective in the targeted delivery of cordycepin to liver cancer cells.22
PLGA is a non-toxic, biodegradable and biocompatible polymer approved by FDA for drug delivery applications that has been extensively used in the encapsulation of drug molecules to enhance their clinical efficacy. PLGA consists of monomers of polylactic acid and polyglycolic acid, which degrade through hydrolysis but are resistant to enzymatic degradation.23 PLGA nanoparticles were shown to rapidly escape from endolysosomes and enter the cytoplasm within 10 min of incubation with cells, which is important for the intracellular delivery of anticancer drugs.24,25 Additionally, PLGA nanoparticles can entrap drugs efficiently and enhance endocytosis towards cancer cells.26 Due to these reasons, nanoencapsulation in PLGA might be an excellent option to improve the clinical efficacy of drugs which have short with low half-lives and are susceptible to enzymatic degradation – such as cordycepin.
Nevertheless, cellular toxicity of cordycepin nanoformulations in general and nanoencapsulation of cordycepin in PLGA specifically have not been reported so far to the best of our knowledge. In this article, we report on the preparation of cordycepin-loaded PLGA nanoparticles, their physico-chemical characterization, cellular uptake, cytotoxicity and hemolytic activity in comparison with free cordycepin for the first time.
Materials and Methods
Materials
Cordycepin was purchased from Solarbio Life Sciences (Beijing, China). Curcumin, PLGA 50:50 (Mw 24000–38000) and polyvinyl alcohol (PVA-31000–50000 Da) were procured from Sigma-Aldrich Company (Steinheim, Germany). Tween-80 and methanol were procured from Tianjin Chemical and Reagents (Tianjin, China). All reagents used in this study were of analytical grade.
Preparation of Cordycepin-Loaded PLGA Nanoparticles
Cordycepin-loaded PLGA nanoparticles (CPNPs) were prepared by the double-emulsion solvent evaporation technique using high-speed homogenization. To optimize the formulations, various polymer concentrations (50 and 100 mg) and volumes of organic phase (5 and 7.5 mL) were tested (Table 1). Briefly, cordycepin was dissolved in 1 mL of deionized water containing 50 µL Tween 80, and PLGA was dissolved in dichloromethane (DCM). The aqueous solution containing cordycepin was emulsified in the organic phase containing PLGA and homogenized for 5 min at 15000 rpm (IKA T 25 Ultra-Turrax homogenizer) to form a water in oil (W/O) emulsion. This emulsion was added dropwise into 50 mL aqueous solution of 1% PVA and homogenized at 18000 rpm for 15 min to form a W/O/W double emulsion. Then, the DCM in the emulsion was evaporated by magnetic stirring at 800 rpm overnight to obtain CPNPs. Curcumin loaded PLGA nanoparticles were prepared in the same manner to investigate the cellular uptake of nanoparticles.27
 |
Table 1 Drug, Polymer, Solvent and Stabilizer Composition in Various Batches Tested for the Production of CPNPs |
Particle Size and Polydispersity Index
The average particle size and polydispersity index (PDI) of nanoformulations were determined by photon correlation spectroscopy in a Malvern Zetasizer (nano ZS90, Malvern Instruments). All the measurements were performed in triplicate.
Zeta Potential
The zeta potential of nanoparticles was measured at 25°C using a Malvern Zetasizer (Nano ZS90, Malvern Instruments). The samples were diluted using double distilled water and the zeta dip was used to measure the zeta potential.
Transmission Electron Microscopy (TEM)
Morphological characteristics of the nanoparticles were studied by TEM analysis. Samples were prepared by placing the nanoparticle suspension onto a copper grid and allowing it to air dry. Dried samples were negatively stained with sodium phosphotungstate solution (1%, w/v) and images were taken in a transmission electron microscope (Hitachi-HT7700, Hitachinaka, Japan).
Determination of Encapsulation Efficiency
To estimate the encapsulation efficiency, 3 mL sample from each formulation was centrifuged at 23000 rpm (44948 × g) for 30 min at 10°C. The free drug concentration was analyzed in the supernatant by reverse-phase high-pressure liquid chromatography (RP-HPLC) using an Agilent Technologies 1260 Infinity high-pressure liquid chromatograph equipped with a VL-1260 DAD detector as described previously.28 Chromatographic separation was carried out on an RP C18 (4.6×100mm) Zorbax column (Agilent Technologies, USA), using H2O: MeOH (85:15) as the mobile phases with a flow rate of 1 mL/min and 10µL injection volume. Absorbance was recorded at 260 nm and quantification was done by the external standard method. The encapsulation efficiency (EE) of nanoformulations was determined by the following equation.
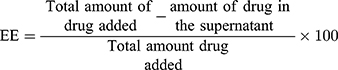
Drug-Release Profile of Nanoparticles
In vitro drug-release studies were performed using a dialysis bag (Mw 2000–14000 Da). Briefly, 3 mL of CPNPs was placed in dialysis bag, hermetically sealed and immersed into 50 mL of PBS (pH 7.4). The entire set-up was kept at 37±1°C under continuous magnetic stirring at 50 rpm. At different time points (0, 6, 12, 18, 24, 48, 72, 96, 120, 144, 168, 192, 216 and 240 h), 1 mL of PBS was withdrawn and replaced by fresh buffer solution to maintain constant volume. The concentration of cordycepin in each sample was determined by RP-HPLC as described above.
Cellular Uptake of Nanoparticles
Human breast cancer cells (MCF-7; ECACC 86012803) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen, Paisley, UK) at 37°C in a humidified incubator with 5% CO2. Cells were seeded in 8-well ibiTreat microscopy chamber (Ibidi, Martisried, Germany) and incubated for 24 h. After confirming the attachment and growth of cells, free curcumin (50 µg/mL) or curcumin loaded PLGA nanoparticles were added to the wells. After 4 h of incubation, fluorescent images were taken with a 63X objective on a Zeiss Axio Observer inverted microscope equipped with a Zeiss LSM 700 confocal module (Carl Zeiss Microimaging GmbH, Jena, Germany).
Cytotoxicity of Nanoparticles
The effect of CPNPs on the proliferation of MCF-7 cells was determined using the MTT assay. Cells were plated at a concentration of 5000 cells per well in flat bottom 96-well plates. After 24 h of incubation at 37°C, the culture medium was replaced with 200 µL of fresh medium containing different concentrations (5, 10, 20, 50, 100, 200, 400 µg/mL) of cordycepin or equivalent CPNPs and incubated as above for another 24 h. The medium was removed again and replaced with 100 µL of fresh medium containing 500 µg/mL of MTT and incubated for 1 h. Then, 100 µL SDS solution (20% w/v, water: DMF at 1:1 ratio, pH 4.7) was added to each well and further incubated for 24 h to dissolve the formazan crystals.29 The absorbance of the reaction mixture was read at 570 nm in an ELISA plate reader (Victor 1420, Perkin Elmer) to quantify formazan. The cell viability was calculated as a measure of the MTT converted into formazan.
Hemolytic Potential of Nanoparticles
The hemolytic potential of cordycepin and CPNPs was tested in rat red blood cells (RBCs). CPCSEA guidelines were followed and approval for carrying out the experiment was obtained from the Animal Ethics Committee of the Ratnam Institute of Pharmacy, Nellore, A.P, India. Fresh blood collected from the orbital sinus of animals in vials rinsed with trisodium citrate was centrifuged at 1500 rpm (211 × g) for 15 min to pellet the RBCs. The RBC pellet was washed three times with normal saline and resuspended in normal saline to obtain a 2% (v/v) suspension. Then, different concentrations of cordycepin (50 and 100 µg/mL) and equivalent CPNPs dissolved in normal saline were added to 2.5 mL of RBC suspension in glass tubes. Distilled water and normal saline were used, respectively, as positive and negative controls. After incubating the tubes for 4 h at 37ºC, the tubes were centrifuged at 587 × g for 10 min in a tabletop centrifuge. The supernatants were observed for any indication of hemolysis.
Statistical Analysis
Statistical analyses of data were performed using GraphPad Prism version 8.0.0 (GraphPad Software, San Diego, California USA). Results were expressed as the means of three replications ± standard deviation (SD). Statistical significance between different treatments was tested by two-way ANOVA followed by Bonferroni’s post hoc test.
Results and Discussion
Preparation and Characterization of Cordycepin-Loaded PLGA Nanoparticles (CPNPs)
CPNPs were successfully prepared by double-emulsion solvent evaporation method in the present study. First, we optimized the stabilizer (PVA) concentration by keeping the polymer content and solvent volume constant. Among the tested PVA concentrations (0.25%, 0.5%, 0.75% and 1%), stable nanoformulations could be obtained only at 1%. PVA concentration lower than 1% resulted in the phase separation of formulations, probably due to insufficient amount of stabilizer necessary to cover the nanoparticles. Hence, 1% PVA was selected for the preparation of different batches of CPNPs formulations. Four concentrations of PLGA (0.66%, 1%, 1.33% and 2%) in the organic phase representing two volumes (5 and 7.5 mL) were tested.
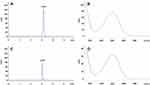
Particle size of the formulations was directly proportional to the polymer concentration and found to be in the range of 179±2.6 to 246.1±1.8 nm (Table 1). In addition to confirming the size range, TEM imaging revealed the spherical shape of CPNPs and the lack of aggregation (Figure 2A). The absence of aggregation in CPNPs formulations can be attributed to the presence of PVA, which could be seen as a bright ring covering the surface of CPNPs in the TEM images. Previous studies have also shown that PVA is a promising stabilizer for the preparation of PLGA nanoparticles as it offers stability against aggregation.30,31 Particle size distribution of the CPNPs formulation C3 is shown in Figure 2B. It is known that an increase in the polymer concentration will also increase the viscosity of the solution and will eventually reduce the rate of evaporation of the solvent from the polymer resulting in the formation of bigger particles.32 On the other hand, low concentration of polymer could provide more room for its dispersion leading to the formation of smaller particles.18 Correspondingly, when the concentration of polymer was reduced, the particle size was also decreased in the present study.
 |
Figure 2 (A) Transmission electron micrograph showing cordycepin-loaded PLGA nanoparticles (CPNPs) of C3 formulation and (B) Histogram showing the size distribution of CPNPs in the formulation. |
The zeta potential of the nanoparticles was found to be in the range of −15.2±0.8 to −18.4±0.6 mV (Table 2). The encapsulation efficiency of the nanoparticles ranged between 53.4±1.4 and 72.3±3.5% as revealed by RP-HPLC analysis (Figure 3). A direct relationship between the amount of PLGA used and the encapsulation efficiency of cordycepin was observed in our study (Table 2). With an increasing concentration of PLGA, the encapsulation efficiency also increased. Similarly, increasing the volume of organic solvent also increased the encapsulation efficiency. This may be due to the increased space availability in the particles. The C4 formulation showed the highest entrapment efficiency of 72.3±3.5%, whereas the C3 formulation was second highest (65.7±2.4).
 |
Table 2 Average Particle Size, Size Distribution, Zeta Potential and Encapsulation Efficiencies of CPNPs Obtained from Various Formulations |
Sustained Release of Cordycepin from Nanoparticles
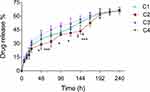
Around 30% of the drug was released from the dialysis bag into the surrounding medium within 24 h. Whereas, to reach 65% release, it took about 10 times longer ie 240 h (Figure 4). The major factors known to affect the release of drugs from PLGA nanoparticles are the solubility of drug, molecular weight of PLGA and its glycolide content. PLGA (50:50) used in the present study contains 50% of glycolic acid, which could make the resulting nanoparticles hydrophilic. Water penetration into the matrix of the CPNPs might be higher than the rate of polymer degradation leading to the bulk release of cordycepin solubilized in water initially. Once equilibrium of cordycepin concentration is achieved, the increase in cumulated drug content is very likely due to the release of encapsulated cordycepin via hydrolytic degradation of PLGA. In order to extend the release further, it is preferable to use PLGA with a lower glycolic acid content eg PLGA 75:25 or 65:35. In addition, the molecular weight and chain length of the polymer also affected the drug release. Increase in the chain length increases the lipophilicity and decreases the degradation rate of the polymer, which will affect the release kinetics of the drug.33 Thus, the drug release from the nanoparticles can be controlled also by altering the molecular weight of the polymer.
Cellular Uptake and Cytotoxic Potential of Cordycepin Nanoparticles
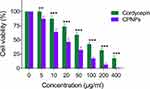
Both free and nano forms of cordycepin were evaluated for their cytotoxic potential in human breast cancer cells (MCF-7) via MTT assay (Figure 5). The IC50 value of CPNPs (16.79 µg/mL) was about 3 times lower than that of free drug (47.84 µg/mL). In order to understand the mechanism behind the robust cytotoxicity of CPNPs, we studied their uptake by MCF-7 cells. Since cordycepin does not have fluorescent properties, it is not possible to observe the uptake of cordycepin or cordycepin nanoparticles by fluorescence microscopy. Hence, we used curcumin as a fluorescent marker to evaluate the uptake efficiency in MCF-7 cells. Curcumin loaded PLGA nanoparticles were prepared in the same manner as that of cordycepin nanoparticles and incubated with the cells. Observation of cells after treatment under a confocal microscope showed high intensity green fluorescence in the curcumin nanoparticles treated cells, whereas fluorescence was weakly observed in free curcumin treated cells revealing that the nanoparticles were efficiently taken up by the cells (Figure 6), possibly via endocytosis.29,34 In a similar manner, the CPNPs might have been efficiently taken up by the MCF-7 cells. Hence, the enhanced cytotoxicity of CPNPs observed in the present study may be due to their efficient uptake by MCF-7 cells. Cellular uptake is an important factor in drug delivery because it is closely related to the therapeutic efficiency as well as the toxicity effect of drug-loaded nanoparticles.35
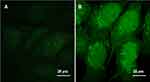 |
Figure 6 Uptake of curcumin (A) and curcumin loaded PLGA nanoparticles (B) by MCF-7 cells after 4 h of incubation as revealed by green fluorescence under the confocal microscope. |
Hemolytic Activity of Cordycepin Nanoparticles
Free cordycepin lysed rat RBCs at both the tested concentrations (50 and 100 µg/mL) in the present study (Figure 7). Similar dose-dependent induction of hemolysis has been reported for cordycepin concentrations above 150 µM (62.5 µg/mL) in mouse erythrocytes.36 CPNPs did not cause embolization or RBC lysis even at 100 µg/mL cordycepin concentration equivalent (Figure 7E). The absence of hemolysis in CPNPs treated RBCs even at this concentration, which is several times higher than that of its cytotoxicity IC50 value (16.79 µg/mL) clearly argues that nanoformulation of cordycepin could also be safely administered via intravenous route.
 |
Figure 7 Effect of free cordycepin and CPNPs on the hemolysis of in rat RBCs. (A) distilled water; (B) normal saline; (C) 50 µg/mL free cordycepin; (D) 100 µg/mL free cordycepin; (E) 100 µg/mL CPNPs. |
Conclusion
In the present study, cordycepin has been efficiently encapsulated in PVA stabilized PLGA nanoparticles, which were characterized for their physico-chemical properties for the first time. Results of the in vitro drug-release study clearly show that the half-life of cordycepin could be significantly increased by PLGA nanoencapsulation. The better cytotoxicity of CPNPs compared to free cordycepin in breast cancer cells further indicates that delivering the drug as nanoparticles could enhance its clinical efficacy. RBCs treated with CPNPs did not suffer hemolysis, emphasizing that the CPNPs may possibly be safe for administration via intravenous route as well. However, an elaborate pharmacokinetic and toxicological profiling of the CPNPs would be certainly beneficial for further development.
Acknowledgments
GM gratefully acknowledges Sathyabama Institute of Science and Technology, Ratnam Institute of Pharmacy and Shaanxi University of Technology for providing the facilities to execute the work, and Xiang Wang for the assistance in HPLC.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shi P, Huang Z, Tan X, Chen G. Proteomic detection of changes in protein expression induced by cordycepin in human hepatocellular carcinoma BEL-7402 cells. Methods Find Exp Clin Pharmacol. 2008;30:347–353. doi:10.1358/mf.2008.30.5.1186085
2. Li G, Nakagome I, Hirono S, Itoh T, Fujiwara R. Inhibition of adenosine deaminase (ADA) mediated metabolism of cordycepin by natural substances. Pharmacol Res Perspect. 2015;3:e00121. doi:10.1002/prp2.121
3. Cao T, Xu R, Xu Y, Liu Y, Qi D, Wan Q. The protective effect of Cordycepin on diabetic nephropathy through autophagy induction in vivo and in vitro. Int Urol Nephrol. 2019;51:1883–1892. doi:10.1007/s11255-019-02241-y
4. Ashraf S, Radhi M, Gowler P, et al. The polyadenylation inhibitor cordycepin reduces pain, inflammation and joint pathology in rodent models of osteoarthritis. Sci Rep. 2019;9:4696. doi:10.1038/s41598-019-41140-1
5. Cho HJ, Cho JY, Rhee MH, Kim HS, Lee HS, Park HJ. Inhibitory effects of cordycepin (3ʹ-deoxyadenosine), a component of Cordyceps militaris, on human platelet aggregation induced by thapsigargin. J Microbiol Biotechnol. 2007;17:1134–1138.
6. Tian X, Li Y, Shen Y, Li Q, Wang Q, Feng L. Apoptosis and inhibition of proliferation of cancer cells induced by cordycepin. Oncology Lett. 2015;10:595–599. doi:10.3892/ol.2015.3273
7. Lee HJ, Burger P, Vogel M, Friese K, Bruning A. The nucleoside antagonist cordycepin causes DNA double strand breaks in breast cancer cells. Invest New Drugs. 2012;30:1917–1925. doi:10.1007/s10637-012-9859-x
8. Liu C, Qi M, Li L, Yuan Y, Wu X, Fu J. Natural cordycepin induces apoptosis and suppresses metastasis in breast cancer cells by inhibiting the Hedgehog pathway. Food Funct. 2020;11:2107–2116. doi:10.1039/C9FO02879J
9. Jo E, Jang HJ, Yang KE, et al. Cordyceps militaris induces apoptosis in ovarian cancer cells through TNF-α/TNFR1-mediated inhibition of NF-κB phosphorylation. BMC Complement Med Ther. 2020;20:1. doi:10.1186/s12906-019-2780-5
10. Nakamura K, Shinozuka K, Yoshikawa N. Anticancer and antimetastatic effects of cordycepin, an active component of Cordyceps sinensis. J Pharmacol Sci. 2015;127:53–56. doi:10.1016/j.jphs.2014.09.001
11. Guan H, Qi S, Liu W, Ma C, Wang C. A rapid assay to screen adenosine deaminase inhibitors from Ligustri Lucidi Fructus against metabolism of cordycepin utilizing ultra high performance liquid chromatography–tandem mass spectrometry. Biomed Chromatogr. 2020;34:e4779. doi:10.1002/bmc.4779
12. Aghaei M, Karami-Tehrani F, Salami S, Atri M. Diagnostic value of adenosine deaminase activity in benign and malignant breast tumors. Arch Med Res. 2010;41:14–18. doi:10.1016/j.arcmed.2009.10.012
13. Blackburn MR, Kellems RE. Adenosine deaminase deficiency: metabolic basis of immune deficiency and pulmonary inflammation. Adv Immunol. 2005;86:1–41. doi:10.1016/S0065-2776(04)86001-2
14. Wu PK, Tao Z, Ouyang Z, et al. The anti-tumor effects of cordycepin-loaded liposomes on the growth of hepatoma 22 tumors in mice and human hepatoma BEL-7402 cells in culture. Drug Dev Ind Pharm. 2016;42:1424–1433. doi:10.3109/03639045.2016.1141930
15. Tsai Y-J, Lin L-C, Tsai T-H. Pharmacokinetics of adenosine and cordycepin, a bioactive constituent of Cordyceps sinensis in rat. J Agric Food Chem. 2010;58:4638–4643. doi:10.1021/jf100269g
16. Marslin G, Sheeba CJ, Kalaichelvan VK, Manavalan R, Reddy PN, Franklin G. Poly(D,L-lactic-co-glycolic acid) nanoencapsulation reduces erlotinib-induced subacute toxicity in rat. J Biomed Nanotechnol. 2009;5:464–471. doi:10.1166/jbn.2009.1075
17. Marslin G, Revina AM, Khandelwal VK, Balakumar K, Sheeba CJ, Franklin G. PEGylated ofloxacin nanoparticles render strong antibacterial activity against many clinically important human pathogens. Colloids Surf B. 2015;132:62–70. doi:10.1016/j.colsurfb.2015.04.050
18. Karanam V, Marslin G, Krishnamoorthy B, et al. Poly (e-caprolactone) nanoparticles of carboplatin: preparation, characterization and in vitro cytotoxicity evaluation in U-87 MG cell lines. Colloids Surf B. 2015;130:48–52. doi:10.1016/j.colsurfb.2015.04.005
19. Lee SS, Lee YB, Oh IJ. Cellular uptake of poly(dl-lactide-co-glycolide) nanoparticles: effects of drugs and surface characteristics of nanoparticles. J Pharm Investig. 2015;45:659–667. doi:10.1007/s40005-015-0221-0
20. Aramwit P, Porasuphatana S, Srichana T, Nakpheng T. Toxicity evaluation of cordycepin and its delivery system for sustained in vitro anti-lung cancer activity. Nanoscale Res Lett. 2015;10:015–0851. doi:10.1186/s11671-015-0851-1
21. Xia C, Chen P, Mei S, et al. Photo-crosslinked HAMA hydrogel with cordycepin encapsulated chitosan microspheres for osteoarthritis treatment. Oncotarget. 2017;8(2):2835–2849. doi:10.18632/oncotarget.13748
22. Bi YE, Zhou Y, Wang M, et al. Targeted delivery of cordycepin to liver cancer cells using transferrin-conjugated liposomes. Anticancer Res. 2017;37:5207–5214. doi:10.21873/anticanres.11944
23. Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–1397. doi:10.3390/polym3031377
24. Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–522. doi:10.1016/j.jconrel.2012.01.043
25. Vasir JK, Labhasetwar V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv Drug Deliver Rev. 2007;59:718–728. doi:10.1016/j.addr.2007.06.003
26. Rafiei P, Haddadi A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: pharmacokinetics and biodistribution profile. Int J Nanomedicine. 2017;12:935–947. doi:10.2147/IJN.S121881
27. Mayol L, Serri C, Menale C, et al. Curcumin loaded PLGA-poloxamer blend nanoparticles induce cell cycle arrest in mesothelioma cells. Eur J Pharm Biopharm. 2015;93:37–45. doi:10.1016/j.ejpb.2015.03.005
28. Zhu L, Liang Y, Lao D, Zhang T, Ito Y. Preparative separation of high-purity cordycepin from Cordyceps militaris(L.) Link by high-speed countercurrent chromatography. J Liq Chromatogr Relat Technol. 2011;37:491‐499. doi:10.1080/10826076.2011.556965
29. Ramanlal Chaudhari K, Kumar A, Megraj Khandelwal VK, et al. Bone metastasis targeting: a novel approach to reach bone using zoledronate anchored PLGA nanoparticle as carrier system loaded with Docetaxel. J Control Release. 2012;158:470–478. doi:10.1016/j.jconrel.2011.11.020
30. Rawat S, Gupta P, Kumar A, Garg P, Suri CR, Sahoo DK. Molecular mechanism of poly(vinyl alcohol) mediated prevention of aggregation and stabilization of insulin in nanoparticles. Mol Pharm. 2015;12:1018–1030. doi:10.1021/mp5003653
31. Bohrey S, Chourasiya V, Pandey A. Polymeric nanoparticles containing diazepam: preparation, optimization, characterization, in-vitro drug release and release kinetic study. Nano Converg. 2016;3:3. doi:10.1186/s40580-016-0061-2
32. Mainardes RM, Evangelista RC. PLGA nanoparticles containing praziquantel: effect of formulation variables on size distribution. Int J Pharm. 2005;290:137–144. doi:10.1016/j.ijpharm.2004.11.027
33. Mittal G, Sahana DK, Bhardwaj V, Ravi Kumar MN. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release. 2007;119:77–85. doi:10.1016/j.jconrel.2007.01.016
34. Chaudhari KR, Kumar A, Khandelwal VKM, Mishra AK, Monkkonen J, Murthy RSR. Targeting efficiency and biodistribution of zoledronate conjugated docetaxel loaded pegylated PBCA nanoparticles for bone metastasis. Adv Funct Mater. 2012;22:4101–4114. doi:10.1002/adfm.201102357
35. Xiong S, Zhao X, Heng BC, Ng KW, Loo JS. Cellular uptake of Poly-(D,L-lactide-co-glycolide) (PLGA) nanoparticles synthesized through solvent emulsion evaporation and nanoprecipitation method. Biotechnol J. 2011;6(5):501–508. doi:10.1002/biot.201000351
36. Lui JCK, Wong JWY, Suen YK, Kwok TT, Fung KP, Kong SK. Cordycepin induced eryptosis in mouse erythrocytes through a Ca2+-dependent pathway without caspase-3 activation. Arch Toxicol. 2007;81:859–865. doi:10.1007/s00204-007-0214-5
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.