Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 12
COPD is commonly underdiagnosed in patients with lung cancer: results from the RECOIL study (retrospective study of COPD infradiagnosis in lung cancer)
Authors Parrón Collar D, Pazos Guerra M, Rodriguez P, Gotera C, Mahíllo-Fernández I, Peces-Barba G , Seijo LM
Received 28 September 2016
Accepted for publication 21 November 2016
Published 30 March 2017 Volume 2017:12 Pages 1033—1038
DOI https://doi.org/10.2147/COPD.S123426
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Dámaso Parrón Collar,1 Mario Pazos Guerra,1 Paula Rodriguez,1,2 Carolina Gotera,1,2 Ignacio Mahíllo-Fernández,2 Germán Peces-Barba,1,2 Luis M Seijo1,2
1Pulmonary Department, Universidad Autónoma de Madrid, 2Pulmonary Department, Instituto de Investigación Sanitaria, Fundación Jiménez Díaz, CIBERES, Madrid, Spain
Introduction: Many patients with COPD are underdiagnosed, including patients with coexisting lung cancer.
Methods: We conducted a retrospective study of COPD prevalence and outcomes among all patients diagnosed with lung cancer at our institution during a 2-year period. Patients with known COPD (group A) were compared with those who received a diagnosis of COPD at the time of their oncologic workup (group B).
Results: A total of 306 patients were diagnosed with lung cancer during the study period, including 87 with COPD (28.6%). Sixty percent of patients with coexisting lung cancer and COPD were unaware of their obstructive airways disease prior to the lung cancer diagnosis. Patients in group A were older (74+9 vs 69+9 years; P=0.03), had more severe obstruction (% of predicted forced expiratory volume in one second [FEV1%] 55+17 vs 71+13; P=0.04), more emphysema (91% vs 65%; P=0.02), and worse diffusing capacity of the lungs for carbon monoxide 59+19% vs 72+22%; P=0.01) than patients in group B, but the latter had more advanced lung cancer (27.3% vs 13.8% stage IV disease; P=0.01) and consumed more outpatient resources (P=0.03). Overall mortality was similar (56% vs 58%). However, stage-adjusted mortality showed a trend toward greater mortality in group B patients (1.87 [0.91–3.85]; P=0.087).
Conclusion: COPD infradiagnosis is common in patients with coexisting lung cancer and is associated with more advanced cancer stage, greater outpatient resource consumption, and may be associated with greater stage-adjusted mortality.
Keywords: lung cancer, COPD, underdiagnosis, staging, survival
Introduction
Tobacco-related alterations in lung function (eg, COPD) and morphology (emphysema) have been linked to lung cancer incidence and prognosis in smokers. However, both COPD and emphysema are often underdiagnosed, even in symptomatic smokers. The consequences of this failure to identify patients prone to develop lung cancer cannot be overstated. Screening efforts, chemoprevention initiatives, and even lung cancer survival may be affected. Despite all these, little is known about the clinical significance of COPD underdiagnosis in lung cancer patients. We designed a retrospective hypothesis-generating study to investigate the prevalence of COPD underdiagnosis in lung cancer and its clinical implications.
Patients and methods
We conducted a retrospective, observational study of all lung cancer patients discussed at the Fundación Jimenez Diaz University Hospital’s lung tumor board meetings between May 2013 and June 2015 (Figure 1). The study protocol was approved by The Clinical Research Ethics Committee of the Fundación Jimenez Diaz University Hospital [Comité Ético de Investigación Clínica de la Fundación Jiménez Díaz] (EO62/2015_FJD). The committee did not require that written informed consent be obtained because the study was observational and retrospective. Follow-up of all patients with COPD and lung cancer was available through January 2016. Tumor board recommendations were recorded in a standardized format by a single tumor board coordinator and registered in every patient’s electronic history record. A thorough chart review was performed for each patient with coexisting lung cancer and COPD. COPD was defined as an forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) <70% on spirometry performed in pulmonary function laboratories affiliated to one of three hospitals. Seven patients were excluded from data analysis for a variety of reasons, including preexisting lung cancer diagnosis, coexisting synchronous cancer, and lack of follow-up. Key variables studied in patients with coexisting COPD and lung cancer included sex, age, body mass index (BMI), tobacco exposure, comorbidities, date of COPD diagnosis, and follow-up available prior to the lung cancer diagnosis. Global Initiative for Chronic Obstructive Lung Disease (GOLD) and cancer stage at diagnosis were also recorded, as well as COPD phenotypes (eg, frequent exacerbations, emphysema, and chronic bronchitis), dyspnea (modified Medical Research Council score), lung function study results including spirometry and carbon monoxide diffusing capacity, lung cancer histology and treatment, COPD treatment, number of outpatient visits and hospitalization before and after the lung cancer diagnosis, and survival.
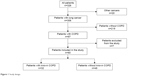  | Figure 1 Study design. |
We divided patients for the sake of statistical analysis into two groups: those with known COPD at the time of the lung cancer diagnosis (group A) and those who had COPD, but were not aware of the diagnosis until a spirometry was performed as part of their oncologic workup (group B). Patients with a known diagnosis of COPD 6 months or more prior to the lung cancer diagnosis were included in group A.
Statistical analysis
Statistical analysis was performed by a research institute–affiliated statistician with no relation to the hospital’s pulmonary department or the study investigators, using R software version 3.1.2. Qualitative variables are reported with frequencies and percentages, and quantitative variables are reported with mean values and standard deviations or medians and interquartile range. Between-group comparisons of qualitative variables were performed by chi square-analysis or Fisher’s exact test, whereas comparisons of quantitative variables were performed using Student’s t-test or Mann–Whitney U-test depending on the variable’s distribution (normal or not). Kaplan–Meier survival analysis was performed, and a P-value of less than 0.05 was considered statistically significant. Cox regression analysis was performed in order to identify variables linked to mortality, and associations are reported with hazard ratios (HRs), 95% confidence intervals, and the corresponding P-values. Lung cancer stage-adjusted mortality analysis for patients in groups A and B was also performed.
Results
A total of 306 patients with lung cancer were discussed at weekly tumor board meetings during the study period. Nearly 87 of those (28.6%) were diagnosed with COPD, including 32 patients with known COPD prior to their oncologic workup (group A) and 55 patients whose COPD diagnosis coincided with the cancer diagnosis (group B). Seven patients were excluded from data analysis for a variety of reasons. Salient patient characteristics are reported in Table 1. Group A patients were older than group B patients (73.4±8.8 vs 68.9±9.3 years; P=0.034), were predominantly male (87.5% vs 68.8%; P=0.096), had more severe obstruction (mean FEV1%: 54.6% vs 70.7% of predicted; P=0.044), had more emphysema (91% vs 65%; P=0.018), and had lower diffusing capacity of the lungs for carbon monoxide (DLCO) values (58.6% vs 72.4% of predicted; P=0.013), although GOLD stage, BMI, lung cancer histology, and comorbidities were similar (Table 2). Overall, 75% of COPD patients received combined therapy with long-acting antimuscarinic and beta agonist bronchodilators and 31% were treated with inhaled steroids. Less than 8% of patients were on home oxygen therapy at the time of their lung cancer diagnosis.
Lung adenocarcinomas (35.5% and 47.8% in groups A and B, respectively) and squamous cell cancers were the most commonly reported tumors (48.4% and 39.1% in groups A and B, respectively). Three small cell-lung cancers were diagnosed in each group, representing 8% of all tumors. The prevalence of all other histologic subtypes was anecdotal. Patients in group B had more advanced stage lung cancer (13.6% vs 48.2% of stage I; P=0.013), but similar surgical resection rates when compared to group A (P=0.146). Group B patients consumed more outpatient resources as evidenced by a greater number of mean outpatient visits following lung cancer diagnosis (30.3±22.1 vs 20.2±16.1; P=0.029), although the number of emergency room visits and hospitalizations did not differ (P=0.642). Overall 39.7% of patients with coexisting lung cancer and COPD had stage IIIb or IV non-small-cell lung cancer (Table 1). Fifty percent of patients were considered to have limited-stage small-cell carcinomas. Curative surgical resection without adjuvant therapy was the treatment of choice for 14%, whereas 26% were offered combined modality treatment including surgery. The remaining patients were treated with radiation therapy (11%), chemotherapy (11%), or a combination of chemotherapy and radiation (26%). Only 11% received no treatment. BMI (P=0.034), lung cancer stage (P=0.034), and carbon monoxide diffusing capacity (P=0.007) were associated with mortality. Despite statistically significant stage differences (Table 3), mortality was similar in both groups (58.3% vs 56.2%; P=0.891) (Figure 2). However, when adjusted for cancer stage, group B patients showed a trend toward greater mortality (HR: 1.87 [0.91–3.85]; P=0.087).
  | Figure 2 Survival of patients with coexisting COPD and lung cancer. |
Discussion
Chronic obstructive pulmonary disease is frequently underdiagnosed,1,2 with as many as 93% of patients with COPD unaware of their medical condition.2 In a Japanese study with a COPD prevalence of 11%, only 9% of patients with an abnormal spirometry had been diagnosed prior to the study.3 Furthermore, only 30%–50% of new COPD diagnoses are confirmed by spirometry suggesting that the diagnosis is often made based on symptoms alone.4,5 The prevalence of COPD in Spain is approximately 10% in subjects >40 years old and is more common in men than in women (15% vs 5.6%).6 COPD prevalence increases with age, affecting 25% of all those older than 70 years.3 Lung cancer incidence coincides with COPD prevalence peaks, since six out of ten lung cancers are diagnosed in the seventh decade of life.7 Not surprisingly, 40%–70% of patients with lung cancer also have COPD, and some studies have found that lung cancer is the most common cause of death in patients with COPD.8,9
The prevalence of COPD in our lung cancer population was approximately 30%. A study by Young et al,10 investigating the prevalence of COPD in lung cancer patients, found that COPD is six times more common in those with newly diagnosed lung cancer than in an age-matched control group of smokers.10 The prevalence of coexisting COPD and lung cancer in that study was 50%.10 Furthermore, lung cancer survival appears to be lower in patients with COPD (15% 3-year survival) than in smokers without obstructive lung disease (26% 3-year survival).11
While survival in our cohort was similar for both groups of patients with coexisting lung cancer and COPD, we found significant differences in both lung cancer and COPD stage, age, and sex, which suggest that COPD underdiagnosis is extremely prevalent in lung cancer and may have clinically relevant implications. Sixty percent of our patients with coexisting COPD and lung cancer were not aware that they had altered lung function until lung cancer was diagnosed. Patients in group B were more often women with more advanced stage disease despite being younger and having less-severe COPD. Although we can only speculate about the reasons for this finding, it is plausible that group A patients were under closer surveillance because of their COPD diagnosis, thereby benefitting from a prompt lung cancer diagnosis. At the very least, this finding implies disparities in outpatient resource consumption. More importantly, it may also condition outcomes. Stage-adjusted mortality showed a trend benefitting those with known COPD which only a larger prospective sample may confirm. Since patients in group A were older and had more severe obstruction (mean FEV1 55% vs 71%), a stage benefit may be obscured by worse prognosis associated with advanced age and poorer lung function. The differences in COPD severity between groups A and B in our study cannot be overstated since 85% of group A patients had severe obstruction compared to only 5% of those in group B. Arguably, the difference is so large that it may very well offset the survival advantage attributable to earlier stage cancer and may account for the lack of difference in curative resection rates that one might expect from that stage advantage.
Emphysema is a powerful predictor of lung cancer risk and prognosis.12–14 In our cohort, the DLCO, which can be viewed as a surrogate marker of the former,15 was low in both groups. However, patients in group A had lower DLCOs (59% vs 72%; P=0.013) suggesting that the emphysema phenotype was more prevalent and/or severe in that group. This was precisely the case since imaging findings confirmed the presence of emphysema in 91% of group A scans vs 65% of those in group B. Furthermore, DLCO in our study correlated with mortality (P=0.007). This finding may also contribute to the lack of a survival benefit described earlier, since emphysema not only increases the risk of lung cancer but also is associated with a worse prognosis.16
An intriguing finding of our study was that squamous cell carcinomas rivaled adenocarcinomas in prevalence. Both accounted for 43% of all tumors. This finding stands in stark contrast with the growing preeminence of adenocarcinoma in recent epidemiologic studies, suggesting that COPD patients have more squamous carcinomas than lung cancer patients with preserved lung function.17 It is further proof that smokers have fewer adenocarcinomas than nonsmokers and that COPD is a risk factor for squamous histology. At least one study found that squamous cell cancers are four times more common in patients with COPD than those with normal spirometry.17
The implications of our findings, if confirmed by prospective studies, for ongoing COPD screening efforts are clinically relevant. Patients with lung cancer who are unaware of their COPD have fewer symptoms, less severe obstruction, are younger, and predominantly female. However, their lung cancer is diagnosed at a more advanced stage and their survival may be even worse than older patients, predominantly male patients with more severe obstruction and more emphysema. Patients with known COPD, especially now that lung cancer screening is becoming a reality, may benefit from ongoing cancer surveillance. Furthermore, since existing evidence points to a chemopreventive effect of inhaled corticosteroids in COPD, this finding renders a timely COPD diagnosis potentially life saving by introducing therapies that not only treat COPD but also help to prevent lung cancer.18 Needless to say, smoking cessation efforts might be more effective in patients who know that they suffer from COPD since lung cancer is more common in smokers with altered lung function.19
Limitations
Our study is limited by its retrospective design. Chart review, lung function study timing, quality and reproducibility, therapeutic decisions and their impact, and follow-up are all conditioned by this design. However, the study was undertaken at a single tertiary referral center, with uniform tumor board protocols and documentation procedures for all lung cancer patients. We are now in the process of initiating a multicenter prospective study that we hope will minimize such biases and provide further evidence to support the conclusions of this hypothesis-generating study.
Conclusion
COPD is prevalent in lung cancer and commonly underdiagnosed. This finding is associated with clinically relevant and statistically significant differences in key variables including lung cancer stage, COPD severity, and the prevalence of emphysema in patients with coexisting lung cancer and COPD. This hypothesis-generating retrospective study highlights the need for further efforts leading to combined COPD and lung cancer screening and treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
Bednarek M, Maciejewski J, Wozniak M, Kuca P, Zielinski J. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax. 2008;63(5):402–407. | ||
Soriano JB, Zielinski J, Price D. Screening for and early detection of chronic obstructive pulmonary disease. Lancet. 2009;374(9691):721–732. | ||
Fukuchi Y, Nishimura M, Ichinose M, et al. COPD in Japan: the Nippon COPD Epidemiology study. Respirology. 2004;9(4):458–465. | ||
Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134(1):38–45. | ||
Bolton CE, Ionescu AA, Edwards PH, Faulkner TA, Edwards SM, Shale DJ. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005;99(4):493–500. | ||
Miravitlles M, Soriano JB, García-Río F, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64(10):863–868. | ||
Cancer Research UK. Cancer Statistics for the UK. Available from: www.cruk.org/cancerstats. Accessed July 1, 2016. | ||
Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE; Lung Health Study Research Group. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142(4):233–239. | ||
Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343(26):1902–1909. | ||
Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34(2):380–386. | ||
Kiri VA, Soriano J, Visick G, Fabbri L. Recent trends in lung cancer and its association with COPD: an analysis using the UK GP Research Database. Prim Care Respir J. 2010;19(1):57–61. | ||
Gao YH, Guan WJ, Liu Q, et al. Impact of COPD and emphysema on survival of patients with lung cancer: a meta-analysis of observational studies. Respirology. 2016;21(2):269–279. | ||
Gullón JA, Suárez I, Medina A, Rubinos G, Fernández R, González I. Role of emphysema and airway obstruction in prognosis of lung cancer. Lung Cancer. 2011;71(2):182–185. | ||
Smith BM, Pinto L, Ezer N, Sverzellati N, Muro S, Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77(1):58–63. | ||
de-Torres JP, Marín JM, Casanova C, et al. Identification of COPD patients at high risk for lung cancer mortality using the COPD-LUCSS-DLCO. Chest. 2016;149(4):936–942. | ||
Bishawi M, Moore W, Bilfinger T. Severity of emphysema predicts location of lung cancer and 5-y survival of patients with stage I non-small cell lung cancer. J Surg Res. 2013;184(1):1–5. | ||
Papi A, Casoni G, Caramori G, et al. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax. 2004;59(8):679–681. | ||
Parimon T, Chien JW, Bryson CL, McDonell MB, Udris EM, Au DH. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(7):712–719. | ||
Lee CH, Hyun MK, Jang EJ, Lee NR, Kim K, Yim JJ. Inhaled corticosteroid use and risks of lung cancer and laryngeal cancer. Respir Med. 2013;107(8):1222–1233. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



