Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 15
Contrast-Induced Acute Kidney Injury: Evidence in Support of Its Existence and a Review of Its Pathogenesis and Management
Authors Chaudhari H , Mahendrakar S, Baskin SE, Reddi AS
Received 21 April 2022
Accepted for publication 10 August 2022
Published 11 October 2022 Volume 2022:15 Pages 253—266
DOI https://doi.org/10.2147/IJNRD.S371700
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Pravin Singhal
Harshad Chaudhari, Smita Mahendrakar, Stuart E Baskin, Alluru S Reddi
Department of Medicine, Rutgers New Jersey Medical School, Newark, NJ, USA
Correspondence: Harshad Chaudhari, Email [email protected]
Abstract: The role of contrast-induced nephropathy (CIN) remains controversial. Many experts contend that CIN does not exist or is extremely rare. The diagnosis was previously made too frequently and inappropriately in the presence of coexisting and confounding comorbidities and risk factors making it difficult to singularly isolate the etiologic role of intravenous contrast media in acute kidney injury (AKI). It is probable that many patients were denied important diagnostic information from radiocontrast studies for fear of CIN. Recently, a new terminology for CIN was introduced, and the term CIN was replaced by two interrelated new terms: one is contrast-associated acute kidney injury (CA-AKI), and the second one is contrast-induced acute kidney injury (CI-AKI). CA-AKI occurs in association with risk factors or comorbidities, therefore, it is a correlative diagnosis. On the other hand, CI-AKI is a subtype of CA-AKI that results directly from iodinated contrast media. In this review, we present evidence from various studies that argue against CI-AKI and also those that suggest its existence but with much lower frequency. We will also provide the current status of the pathophysiology and management of CA-AKI/CI-AKI.
Keywords: contrast agents, contrast-induced acute kidney injury, contrast-associated acute kidney injury, pathophysiology, prevention, propensity score matching
Introduction
The term contrast-induced nephropathy (CIN) has been in use for decades to define the onset of acute kidney injury (AKI) following either the intravenous or intra-arterial administration of iodinated contrast media or contrast agents. The first case of contrast-induced AKI was reported by Bartels et al1 in 1954 in a 67-year-old man with multiple myeloma, who was administered 20 mL of 50% Diodone. The patient developed acute anuria following intravenous pyelography. Subsequently, a number of other cases or case series were reported with AKI following contrast studies with an incidence ranging from 0 to over 20%. In recent years, rigorous evaluation of CIN supports the concept of true CIN, but that true CIN is rare, while other conditions such as infections, nephrotoxic agents or volume depletion are often the etiology of post-contrast AKI.2 Thus, true CIN usually is AKI that occurs as a result of administration of iodinated contrast medium excluding the conditions that cause AKI. For this reason, separation of true AKI from AKI that is associated with noncontrast medium is extremely important for proper documentation of frequency rates and post-contrast complications. To this extent, a new terminology for CIN was introduced, and the term CIN was replaced by two interrelated new terms: one is contrast-associated acute kidney injury (CA-AKI), and the second one is contrast-induced acute kidney injury (CI-AKI), which is a subtype of CA-AKI. (3). The 2021 American College of Radiology (ACR) Committee on Drugs and Contrast media in combination with National Kidney Foundation recommends the definitions of CA-AKI and CI-AKI as follows3,4:
Contrast-Associated Acute Kidney Injury (CA-AKI)
CA-AKI, formerly referred to as post-contrast AKI, is currently defined as sudden decrease in kidney function within 48 h following an intravenous or intra-arterial administration of iodinated contrast medium. This decrease in kidney function is described as AKI, and occurs in those with risk factors. Thus, CA-AKI is a correlative diagnosis and does not imply a causal relationship between iodinated contrast medium and AKI.
Contrast-Induced Acute Kidney Injury (CI-AKI)
This term, formerly known as CIN, implies a sudden decrease in kidney function due to administration of iodinated contrast medium. Thus, there is a causal relationship between iodinated contrast medium and AKI. According to the 2021 ACR Committee on Drugs and Contrast media, the terms CI-AKI and CA-AKI are not synonymous and not interchangeable, but the former is a subtype of the latter.2 The incidence of CA-AKI is more frequent than that of CI-AKI. For this reason, the ACR Committee on Drugs and Contrast media states “At the current time, it is the position of ACR Committee of Drugs and Contrast Media that CI-AKI is a real, albeit rare entity”.3
This new terminology has created concerns among nephrologists and other physicians regarding the existence of CI-AKI, and the care they should take when an intravenous contrast study is ordered. The purpose of this review is to discuss briefly the evidence that questions the existence of CI-AKI and express our opinion as clinicians how to deal with this issue in practice. In order to understand the injurious effects of contrast media, it is essential to understand their properties as well as the mechanisms (pathophysiology) by which they cause AKI. Also, we will provide a logical prophylactic approach to prevent CI-AKI.
Incidence of CA-AKI and CI-AKI
Early studies used the term CIN for transient increase in serum creatinine following iodinated contrast media, and its incidence has been reported to vary from 0% to 21% in both prospective and retrospective studies.5,6 Even a higher incidence rates have been reported in some patients with several risk factors. These studies have not excluded other causes of AKI such as clinical status of the patients, different populations studied, types of procedures performed (intravenous versus intra-arterial), different definitions of AKI applied, and lack of adequate controls. Thus, every case of AKI following exposure to iodinated contrast media cannot be attributed to the contrast medium itself. Therefore, the higher incidence of CIN reported by early studies probably represents the incidence of CA-AKI rather than CI-AKI, and thus the incidence has been overstated in the literatures due to the inclusion of older, poorly conducted observational studies.3 Based on eGFR rather than serum creatinine levels, the incidence of CA-AKI and CI-AKI has been found to be much lower, as presented under risk factors below.3
Properties of Contrast Media
In addition to the patient-related risk factors, iodinated contrast media have also been identified as risk factors for CI-AKI because of their intrinsic chemical properties such as osmolality, ionic or nonionic status, viscosity, degree of polymerization, and the amount of volume injected. Table 1 shows the properties of various contrast media. Tri-iodinated benzene rings are the underlying components of all modern iodinated contrast agents. Iodine-based contrast agents can be divided according to osmolality (number of particles in solution for a given concentration of iodine in the agent, grouped as high, low, or iso- with respect to the osmolality of human plasma), ionicity (ionic meaning dissociating as a salt in solution or nonionic for agents that do not dissociate), and number of tri-iodinated benzene rings in a molecule of contrast material (monomer if one benzene ring, dimer if two). Currently, low and iso-osmolality agents are being used for intravascular iodinated contrast studies.4,7
 |
Table 1 Properties of Some Contrast Media |
Several studies have investigated the nephrotoxic potential of osmolality, the injected dose, and the route of administration of iodinated contrast media. The incidence of CA-AKI is much higher with HOCM than with LOCM.8 In a systematic review and meta-analysis, Eng et al9 found no clinically relevant difference in the risks of CA-AKI or CI-AKI between low- and iso-osmolality contrast media. Although these authors found that IOCM (Iodixanol) had a slightly lower risk for CA-AKI than LOCM, this lower risk did not exceed a criterion for clinical importance.
There is a relationship between volume of contrast agent injected and the extent of AKI.10 The general consensus is that small doses of contrast media (<70 mL) cause less kidney injury than large doses of any osmolality media. In the study by Manske et al,11 type 1 diabetic patients with a mean serum creatinine of 5.90 mg/dl who underwent coronary angiography had a dose-related CA-AKI with an odds ratio of 10.6 for contrast dose of 30 mL or higher. They calculated that for each 5-mL increment in quantity of contrast agent, the risk of CA-AKI increased by 65% (relative risk 1.44).
To minimize the incidence of CA-AKI, many equations for the accepted maximum dose of contrast have been developed. In 1989, Cigarroa et al12 predicted that the incidence of CA-AKI would be related to the dose of contrast and inversely proportional to serum creatinine. They introduced the following equation:
Accepted contrast dose = 5 mL of contrast per kg body weight/serum creatinine in mg/dl to a maximum dose of 300 mL.
When this equation was prospectively applied to 3322 patients undergoing coronary angiography, only 2% of those who remained under the limit developed CA-AKI, while 21% of those exceeding the limit developed it. Subsequently, other equations were developed: (1) contrast volume to creatinine clearance ratio (<3.7); (2) contrast volume-to-eGFR ratio (<3.7) and (3) gram of iodine-to-eGFR ratio (ideal ratio 1:1). Thus, there is a relationship between contrast volume administered and the incidence of kidney injury, particularly in patients with CKD and diabetes. The available evidence suggests that contrast doses should be limited to the g iodine-to-GFR ratio of 1, which will minimize, but not completely abolish the risk of CA-AKI.10
Route of contrast administration seems to have some impact on the incidence of CA-AKI.2,13–15 Intra-arterial route has been suggested to cause higher incidence of CA-AKI than the intravenous route, because the former route delivers more contrast to the kidneys. However, it was subsequently shown that the volume of contrast through the intra-arterial route is larger because of diagnostic and interventional procedures (coronary angiography), than the intravenous route which requires a fixed dose of contrast (CT angiography), and this may account for different incidences in CA-AKI. The meta-analysis of Eng et al9 found no difference in the risk between the intravenous and intra-arterial routes of administration.
Risk Factors for CA-AKI
The only documented risk factor is kidney dysfunction. The risk of CA-AKI increases with an increase in CKD stages or categories. The risk is 5% at an eGFR of ≥ 60 mL/min, 10% at 45–59 mL/min, 15% at 30–44 mL/min, and 30% at <30 mL/min. This risk of CA-AKI is much higher than the risk of CI-AKI because it includes many comorbidities.3,4 Although the primary risk factor is severe kidney dysfunction (eGFR <30 mL/min), studies have found an additive risk from type 1 and type 2 diabetes, hypovolemia, nephrotoxins, hypotension, albuminuria, and heart failure for CA-AKI. However, there is no convincing evidence that multiple myeloma is a risk factor for CA-AKI.
Risk Factors for CI-AKI
As in CA-AKI, the risk factor for CI-AKI is kidney dysfunction. It was reported that the risk is 0% at an eGFR of ≥45 mL/min, 0–2% at 30–44 mL/min, and 0–17% at <30 mL/min.3,4 However, previously proposed risk factors such as diabetes mellitus, diuretic use, advanced age, hypertension, hyperuricemia, and multiple iodinated contrast medium doses in a short time interval (<24 h) are not considered to cause CI-AKI for lack of confirmation by controlled intravenous iodinated contrast media studies.4
It should be noted that patients on hemo- or peritoneal dialysis with no residual kidney function may receive intravenous or intra-arterial contrast media studies anytime, as indicated. However, patients with AKI may need vigorous evaluation as to the necessity of the study based on the benefit and risk ratio. In a propensity score matched study, Yan et al16 showed no difference in the risks of further decrease in kidney function, requirement for kidney replacement therapy, and mortality in 7 and 30 days among AKI patients who received either intravenous iodinated contrast (enhanced) or no contrast (unenhanced) CT scans within 7 days after the diagnosis of AKI.
Pathogenesis of CI-AKI
The pathogenesis of CI-AKI is not completely understood. However, several possible mechanisms have been proposed: (1) kidney hemodynamic changes resulting in decreased renal blood flow (RBF); (2) direct tubular injury by iodine; (3) medullary hypoxia with impaired microcirculation; (4) intracellular signaling pathways involving cell death; and (5) inflammation, as shown in Figure 1.4,6,17–28
Intravenous iodinated contrast agents have been shown to cause renal hemodynamic changes with biphasic effect on RBF.17–21,25–28 Initially, vasodilation of afferent arterioles occurs with a transient increase in RBF, which is followed by prolonged vasoconstriction and a decrease in RBF. Vasoconstriction is due to the action of many vasoactive substances such as angiotensin II (AII), endothelin, adenosine and an increase in intracellular [Ca2+] in vascular smooth muscle cell. Also, there is also a concomitant decrease in nitric oxide and prostaglandin PGE1 and PGE2 production. Adenosine, for example, causes afferent arteriolar vasoconstriction and efferent arteriolar vasodilation, resulting in compromised GFR and AKI. This is the basis for the use of theophylline, which antagonizes the effects of adenosine.
Liu and associates21 isolated afferent and efferent arterioles from mice and demonstrated severe vasoconstriction of afferent arterioles only when perfused with iodixanol. The diameters of the afferent arterioles were significantly reduced from 9.2 to 8.3 μm, while in control arterioles diameters increased from 8.7 to 9.3 μm. This vasoconstriction seems to be mediated by decreased nitric oxide production and superoxide generation. Thus, the decrease in afferent arteriolar diameter may account for decreased RBF caused by contrast media.
Iodine is released from contrast medium by photolysis,19,22 and it has long been known that iodine has cytotoxic effect on bacteria. Iodine causes direct damage to membrane proteins, leading to the loss of cell membrane integrity. Thus, a direct injury to the kidney tubules has been proposed. Direct tubular injury by contrast media is mediated by the generation of reactive oxygen species (ROS). Apoptosis with loss of cellular membrane proteins and loss of cytochrome C from mitochondria have been observed in kidney tubular epithelial cells. Also, pathologically vacuolization in the proximal tubular cells has been observed. In animal studies, antioxidants prevented nephrotoxicity induced by contrast media. These studies formed the basis for the use of antioxidants such as N-acetylcysteine and ascorbic acid in humans to prevent CI-AKI. Also, it was shown that NaHCO3 can act as an antioxidant and prevent nephrotoxicity.
Renal medullary hypoxia is an important mechanism for CI-AKI. Normally, renal medulla functions at low O2 tension (30 mm Hg), as compared with the cortex where O2 tension is very high. Contrast media reduce outer medullary O2 tension substantially to as low as 10 mm Hg. This medullary portion (thick ascending limb of Henle’s loop) of the nephron segment requires high O2 tension because of active transport mechanisms. Also, contrast media increase the viscosity in both the tubular fluid and vasa recta with subsequent red blood cell aggregation. These changes cause low blood supply and O2 to the medulla, resulting in medullary hypoxia. ROS generation is stimulated with subsequent membrane injury and DNA damage. Mitochondrial enzyme activity is also impaired, leading to depletion of intracellular energy. The net result is cell necrosis and apoptosis.
All osmolality iodinated contrast media increase the intraluminal pressure by water reabsorption into the kidney tubular lumen and increase the hydrostatic pressure. This elevated pressure causes vasa recta constriction, resulting in medullary hypoxia and CI-AKI.
High osmolality contrast media induce osmotic diuresis via the release of atrial natriuretic peptide. Excess delivery of NaCl to the macula densa activates the tubuloglomerular feedback mechanism via adenosine, resulting in afferent arteriolar constriction and a decrease in GFR.
In both experimental animals and human subjects, contrast media induce histopathologic changes in the tubular epithelium. Following injection, iodinated contrast medium remains in the intravascular compartment without binding to the albumin, and therefore, is filtered freely at the glomerulus. Once filtered, it is not reabsorbed by the tubules, and then gets concentrated in the tubules following water and solute reabsorption. The tubular epithelial cells are then exposed to the concentrated medium, leading to cell damage. Vacuolization of proximal tubular epithelial cells is the characteristic feature of the contrast media.23
The role of inflammation on tubular injury and CI-AKI has been explored by Lau et al.24,25 According to these authors, the kidneys contain an intrinsic immune surveillance system that consists of resident macrophages and dendritic cells (resident renal phagocytes). In the mouse model, NOD-like receptor pyrin containing 3 (NLRP3) is an innate pattern recognition receptor and important immune sensor that has canonical and noncanonical roles in models of kidney disease. NLRP3 function is primarily noncanonical, regulating a platform for caspase-8 activation and apoptotic cell death. Following rapid filtration into the tubular luminal space, the contrast medium is reabsorbed and concentrated in tubular epithelial cells via the brush border enzyme dipeptidase-1 in volume depleted but not euvolemic mice. CI-AKI is a multistep process involving immune activation coordinated by resident renal phagocytes, the tubular reabsorption of contrast medium, and recruited leukocytes. Contrast activates the canonical NLRP3 inflammasome in macrophages, which contributes to CI-AKI through the regulation of inflammation. Thus, CI-AKI is a multistep process that involves immune surveillance by resident and infiltrating renal phagocytes, NLRP3-dependent inflammation, and the tubular reabsorption of contrast via dipeptidase-1.
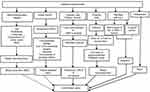 |
Figure 1 Pathogenesis of contrast-induced acute kidney injury. NLRP3, NOD-like receptor pyrin containing 3. Notes: Data from Reddi et al.17 |
Andreucci et al26 examined the survival and proliferation of HK-2 cells (human embryonic proximal tubular cells) that were exposed to iodinated contrast media, and demonstrated reduced cell survival due to decreased activation of Akt and ERK1/2 kinases. These kinases are known to be involved in cell survival/proliferation. When HK-2 cells were transfected with a constitutively active form of Akt, the inactivation was alleviated. This suggests that contrast media decreases cell survival/proliferation. In another experiment, the same investigators confirmed their observations again in HK-2 cells by exposing them to contrast drugs, and demonstrated that contrast media affect the activation/deactivation of transcription factors like FoxO3a and STAT3, which control the genes that are involved in apoptosis and cell proliferation.
Several other in vitro studies, as reviewed by Yang et al27 have confirmed decreased cell proliferation, apoptosis, mitochondrial toxicity, and direct cytotoxicity by iodinated contrast media in HK-2 cells and cultured proximal tubule cells from rat, mouse, and pig. Thus, any iodinated contrast medium is not nephroprotective. Figure 1 summarizes various mechanisms in the pathogenesis of CI-AKI.
Evidence Against CI-AKI
As early as 1978, Gelman and associates29 found no change in kidney function in the 38 patients who underwent intravenous pyelography. Similarly, Eisenberg et al30 also did not observe CI-AKI in 100 consecutive patients who had major angiographic procedures. In recent years, the concept that intravenous iodinated contrast material does not cause higher incidence of AKI in patients with either normal kidney function or chronic kidney disease was elaborated by many other investigators. Rao and Newhouse,31 Newhouse et al32 and Bruce et al33 have shown that the rates of AKI following CT scan in patients who received intravenous iodinated contrast media (or CT enhanced) were similar or identical to those patients who did not receive contrast media (or CT unenhanced). These authors identified a high incidence of AKI among control subjects who had noncontrast CT. The incidence of creatinine elevation in this group was statistically similar to that in the contrast-received group for all baseline creatinine values and all stages (categories) of CKD. Based on these results, the radiologists are left with the impression that AKI from intravenous administration of contrast material is either much less common than originally thought or does not occur at all.34,35
McDonald et al36–40 in a series of publications starting from 2013, using propensity score matching, reported no association in the incidence of AKI between patients who got CT scans with contrast and those who did not receive contrast media at any serum creatinine or eGFR levels. A matched propensity score analysis is a statistical method by which selection bias can be reduced in a retrospective study. They also used counterfactual analysis in which each patient acted as his or her own control by having both noncontrast and contrast CT within the study period, and showed no significant association between intravenous contrast media and AKI.31 Thus, this retrospective analysis suggests that intravenous iodinated contrast media may not be the causative agent for AKI after their administration for CT scans.
In a subsequent review and meta-analysis of controlled studies that involved 25,950 patients, the same authors showed no difference in the incidence of AKI, dialysis, and death between the contrast and noncontrast control group.41
In contrast, Davenport et al42 reported using propensity score analysis that intravenous low-osmolality iodinated contrast material is a nephrotoxic risk factor in patients with pre-CT serum creatinine of 1.6 mg/dl or greater, but not in patients with a stable serum creatinine level <1.5 mg/dl. The same authors also showed a graded increase in AKI rates with decrease in eGFR levels, suggesting that patients with eGFR levels <30 mL/min are at greater risk for CI-AKI than those patients with eGFR levels >45 mL/min.43
In a retrospective analysis of Emergency Department patients using propensity score matching, Hinson et al44 found no causal association between contrast administration and AKI. Also, they observed that contrast administration was not associated with increased incidence of either CKD, dialysis or kidney transplant at 6 months. Similarly, a meta-analysis by Aycock et al45 that included 28 studies with 107,335 patients who came to the emergency department also found no significant differences between patients receiving CT with contrast material and those with no contrast material in the incidence of either AKI, need for kidney replacement therapy, or all-cause mortality.
Similar to McDonald et al40 observations in ICU patients, Ehrmann et al46 in a prospective observational propensity-matched cohort study found no difference in the incidence of AKI between contrast and noncontrast groups.
Table 2 shows representative rates of AKI stratified according to eGFR levels by the studies discussed above. As evident from the table, only the studies of Davenport et al showed that AKI rates are significantly higher with contrast exposure in patients with an eGFR <30 mL/min. Also, the trend is similar when AKI rates were stratified with serum creatinine levels.
 |
Table 2 Representative Propensity Score Matching Rates of Acute Kidney Injury (AKI) Stratified According to eGFR Levels in Patients with and without Contrast Study |
A non-propensity score analysis by Wilhelm-Leen et al47 also showed no difference in the incidence of AKI between contrast and noncontrast exposure groups. Using the entire Nationwide Inpatient Sample dataset for 2009, these authors analyzed 5,931,523 hospitalizations for CI-AKI. Overall, the AKI rates were 5.5% for contrast-received patients and 5.6% for noncontrast patients, respectively. However, disease specific-risks were rather different and interesting to note. For example, radiocontrast administration was associated with a higher risk of AKI (contrast versus noncontrast): sepsis (35.8% versus 32.9%), pneumonia (16.3% versus 12.7%), urinary tract infection/pyelonephritis (17.4% versus 15.7%), peritonitis (31.4% versus 28.9%), gastrointestinal bleeding (16.8% versus 13.8%), chronic obstructive pulmonary disease exacerbation (16.3% versus 15.1%), and acute pancreatitis (16.4% versus 8.2%).On the other hand, radiocontrast administration had a lower rate of risk in patients with exacerbation of heart failure (16.6% versus 19%) and acute coronary syndrome (6.4% versus 17.4%). Despite the above interesting findings, the authors believe that the relationship “between radiocontrast administration and AKI is highly confounded, unpredictable, and sometimes bidirectional.” Interestingly, the authors also admit that the risk of CI-AKI is “likely low but not likely zero.”
In patients with acute ischemic stroke, Lima et al48 showed no increase in AKI after receiving either CT angiography (CTA) or CT perfusion (CTP) or both. Some of the patients also had conventional angiography after CTA/CTP. The incidence of AKI was 5% in the contrast and 10% in the noncontrast group. Patients who underwent conventional angiography after contrast CT were at no greater risk of AKI than patients who underwent CTA/CTP alone. Similar conclusions were reached by Brinjikji and associates49 in a meta-analysis involving 5727 patients with acute stroke. This meta-analysis included six case–control and eight single-arm studies. In these studies, 5727 patients received CTA/CTP and 981 received noncontrast computed tomography. In case–control studies, AKI was significantly lower among CTA/CTP patients compared with noncontrast patients. Adjusting for baseline creatinine, there was no difference in AKI rates between groups. The overall rate of AKI in CTA/CTP patients was 3%. The overall rate of hemodialysis in the CTA/CTP group was 0.07% (3 of 4373). Among patients with CKD, the overall rate of AKI following CTA/CTP was 2.3% (14 of 609) compared with 3.7% for the non-CKD group (65 of 1780). In unadjusted meta-analysis, patients with CKD had similar odds of developing AKI than patients without CKD. This study emphasizes that concerns about AKI should not deter physicians from pursuing their imaging studies for management of patients with acute ischemic stroke.
Another recent Swedish study50 attests to the existence of CA-AKI. This prospective study of 1009 participants with an eGFR ≥50 mL/min who underwent coronary CT angiography with iohexol showed a median increase in serum creatinine of 0–2 μmol/L 2–4 days post procedure. Post-contrast AKI was observed in 12/1009 individuals (1.2%) when AKI was defined as >25% or >44 μmol/L increase in serum creatinine and 2 individuals (0.2%) when defined as ≥50% or ≥27 μmol/L increase in serum creatinine. Possible risk factors (eg, diabetes, age, eGFR, NSAID use) did not show increased risk of developing post contrast-AKI. This study suggests that iohexol administration to a randomly selected cohort with mildly reduced eGFR is safe, and post-contrast-AKI is very rare.
Some Concerns of the Above Studies
- The studies of McDonald et al and Davenport et al were retrospective and observational, and they used propensity score matching in analyzing their data. This analysis does not avoid selection bias as to who undergoes contrast procedure and who does not. In this context, Nyman et al51 raised the following analytical issues: (1) comparison of noncontrast group with contrast group; (2) use of relative rather than absolute eGFRs for kidney function risk stratification; (3) failure to use the dose of contrast material in relation to kidney function risk evaluation; and (4) insufficient attention in the evaluation of nonrenal risk factors. Others also raised some concerns for not using appropriate controls as in randomized controlled studies, while realizing at the same time that the use of such placebo controls in contrast studies is rather difficult because of ethical considerations.52
- In propensity score matching, patients with eGFRs <20 mL/min were not included. Although both McDonald et al and Davenport et al used the same propensity score matching, the AKI risk was different. McDonald et al found no difference in AKI risk at any eGFR levels, but Davenport et al found graded risk at different eGFR levels.
- Dataset represents the data that have been entered into the records, but it may not have included some other comorbidities that precluded contrast studies.47
- Patients who underwent contrast studies may have received adequate preventive measures compared to those who did not receive contrast, and this may have obscured the nephrotoxic potential of the contrast media.
- Daily variability of serum creatinine levels is well known. This is more profound in critically ill patients because of several reasons. Among these reasons, fasting and muscle mass are extremely important. Some patients may have attained the highest level of serum creatinine level prior to contrast study, and post-contrast increase in serum creatinine may be minimal and may not qualify for stage 1 AKI. Also, overzealous post-contrast hydration may obscure the elevation in serum creatinine level.
Does CI-AKI Really Exist?
Based on the above studies, CI-AKI is real and does exist, but the incidence is low, as suggested by the ACR Committee on Drugs and Contrast Media.4 The studies of Davenport et al42,43 reveal the existence of this clinical entity. Also, the dataset analysis by Wilhelm-Leen et al47 points out that the risk of CI-AKI is “likely low but not likely zero.” In addition, the following studies have suggested the existence of CI-AKI.
A meta-analysis compared the incidence of AKI with iodinated contrast media (ICM) versus carbon dioxide (CO2) study. In total, 677 patients underwent 754 peripheral angiographic procedures. ICM caused AKI in 11.1% and CO2 in 4.3%, suggesting that ICM is nephrotoxic.53
Also, another propensity-matched study evaluated the ates of AKI in patients with stage G3 or G4 CKD who underwent coronary angiography, contrast-enhanced CT, or nonenhanced CT. Post-contrast AKI occurred in 27%, 24%, and 24% of the patients, respectively. It was found that the incidence of CI-AKI was higher in the coronary angiography group (16.5%) than contrast (12.5%) group.54
Similar propensity score matching studies of Medalion et al.55 Sigterman et al56 and shah et al57 also showed nephrotoxicity following intra-arterial administration of contrast material during coronary angiography.
Based on the pathophysiologic mechanisms and some of the above mentioned studies that reported lower rates of AKI, it is our position that CI-AKI is real and does exist in patients even with prophylactic measures, particularly after coronary angiography than CT enhanced scans. We suggest that clinicians evaluate the risk and benefit ratio when ordering a contrast study in patients with an eGFR <30 mL/min and AKI. Although discussion between the ordering physician and radiologist or cardiologist is valuable, it does not happen in real world, and the clinician should not hesitate requesting a contrast study for diagnostic and therapeutic measures when indicated.
Preventive Strategies of CA-AKI/CI-AKI
The most important strategies to prevent the incidence and severity of CA-AKI/CI-AKI are as follows: (1) identification of high-risk patients; (2) hydration; (3) withhold nephrotoxic medications; (4) use of low or iso-osmolar iodinated contrast media; (4) dose reduction that is sufficient enough for diagnostic images; and (5) communication with a radiologist prior to the study. Except for hydration and nephrotoxic agents, we have already presented other aspects of prevention strategies. Most studies have shown that volume expansion (hydration) with normal saline is the most important measure to prevent CA-AKI/CI-AKI (see Table 3). Hydration, besides low cost, it increases urine flow rates, reduces the concentration of contrast media in the tubule, and promotes excretion of contrast media, thus reducing the length of exposure time of tubular cells to the toxic effects of contrast media. In addition, hydration suppresses of renin-angiotensin-aldosterone system, thus preventing renal vasoconstriction caused by the contrast media. It was also shown that high urine flow rates may lead to vasodilatation in some regions of the renal medulla, possibly through increasing the production of prostacycline.58 However, it is interesting to note that one study did not find any effect of volume expansion on AKI rates.59 In this Maastricht Contrast-Induced Nephropathy Guideline (AMACING) trial, which is a prospective randomized controlled trial in high-risk patients with an eGFR of 30–59 mL/min, compared prophylactic effect of hydration versus no hydration on the incidence of CA-AKI/CI-AKI. The data showed that no prophylactic treatment was noninferior to prophylactic IV hydration in the prevention of CA-AKI/CI-AKI. This issue was addressed by Lee et al60 in a meta-analysis. They concluded that prophylactic hydration is not necessary if the contrast volume is <100 mL regardless of eGFR levels, but prophylactic hydration is recommended to those who receive large volume (>100 mL) of contrast medium to prevent CA-AKI/CI-AKI.
 |
Table 3 Prevention of CA-AKI* |
The aim of the fluid therapy is to maintain euvolemia and avoidance of overhydration. This can be achieved by careful peripheral IV administration of fluids. Besides peripheral IV, other potentially useful interventions such as left ventricular end-diastolic pressure (LVEDP)-guided sliding-scale hydration with normal saline and forced diuresis automated system (eg, RenalGuard system) were applied to patients undergoing cardiac catheterization.
In the Prevention of Contrast Renal Injury with Different Hydration Strategies (POSEIDON) trial, Brar et al61 investigated different rates of fluid administration guided by the LVEDP in patients undergoing cardiac catheterization. LVEDP is routinely used as a measure of intravascular volume status. The authors administered 5 mL/kg/h for LVEDP <13 mm Hg, 3 mL/kg/h for 13–18 mm Hg, and 1.5 mL/kg/h for >18 mm Hg of normal saline to patients with ≤60 mL/min and at least 1 risk factor. The control group received 1.5 mL/kg/h of normal saline. The fluid rate was started before contrast exposure, continued during and for 4 h after procedure in both groups. The results showed that CA-AKI was less (6.7%) in LVEDP group than in the control group (16.3%). Thus, LVEDP-guided hydration with normal saline during cardiac catheterization seems to lower the rate of CA-AKI than peripheral IV hydration. In both groups, an equal number of patients had to stop the procedure because of shortness of breath. However, a study by Marashizadeh et al62 did not find any difference in the rate of CA-AKI between LVEDP-guided and peripheral IV hydration with normal saline in patients with eGFR ≤60 mL/min.
A high urinary flow rate of ≥150 mL/h has been shown to be protective in CA-AKI/CI-AKI.
Increased urine output dilutes the concentration of contrast, like volume expansion, within the kidney tubule lumen and decreases its contact time with kidney tubule cells. Thus, volume expansion and diuresis are associated with enhanced resistance to oxidative injury.63 At times, it is difficult to maintain euvolemia and adequate urine output. To maintain adequate intravascular volume and urine output, RenalGuard system was developed. This RenalGuard system measures urine flow rate induced by furosemide, and thus urine volume is replaced by an equivalent amount of normal saline so that euvolemia is maintained. During the process of this system, the contrast is diluted to minimize the viscosity, and also limit the exposure time of kidney tubules to contrast medium while maintaining renal blood flow. In the Renal Insufficiency After Contrast Media Administration Trial II (REMEDIAL II) trial, Briguori et al64 showed that RenalGuard therapy reduced the incidence of CA-AKI/CI-AKI in patients with low eGFR (half had an eGFR ≤30 mL/min) who underwent coronary and/or peripheral angiography/angioplasty. In this trial, the control group received NaHCO3 plus N-acetylcysteine (NAC) and the RenalGuard group received hydration with normal saline plus NAC controlled by the RenalGuard system. The incidence of CA-AKI/CI-AKI in the control group was 20.5% compared to 11% in the RenalGuard group. Thus, maintenance of hydration based on forced urine flow rate ≥150 mL/h can reduce the incidence of CA-AKI/CI-AKI in high-risk patients. Additional data from this and two other trials including 586 CKD patients showed decreased risk of CA-AKI/CI-AKI by 60% major adverse clinical events rate by 59%, and the need for kidney replacement therapy by 78%, compared with the standard of care.65
The disadvantage of both LVEDP and RenalGuard system is that their use is limited to patients undergoing intra-arterial procedures and probably coronary interventions in elective and acute settings. Also, one can question their use in patients with stable eGFR 30–44 mL/min. In LVEDP-guided sliding-scale hydration, those patients who received 3 or 5 mL/kg of normal saline may have diluted their serum creatinine, thus obscuring the real creatinine level. This could have been corrected by a factor that can be obtained by the difference between pre- and post-procedure weights to observe real creatinine level. Additional studies are needed before their routine use in clinical practice.
We recommend the following flow chart (Figure 2) for patients who desire intravenous or intra-arterial studies with or without contrast and with different eGFR levels to minimize CA-AKI/CI-AKI.
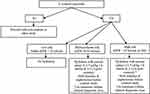 |
Figure 2 Flow chart for patients with different eGFR levels to minimize Ca-AKI/CI-AKI, *Hold hydration in hypervolemic (dialysis or heart failure with EF <30%) patients. |
Clinical Course of CA-AKI and CI-AKI
The clinical course of both CA-AKI and CI-AKI depends on several factors, including baseline kidney function, coexisting comorbidities, and degree of hydration. Typically, serum creatinine level starts rising within 24 h, peaks in 4 days, and gradually returns to baseline in 7–10 days following intravascular iodinated contrast medium administration.
Several studies have shown that patients with CA-AKI and CI-AKI experience, besides longer hospital stays, higher incidences of cardiovascular, kidney, and neurologic events, and increased mortality. Increased mortality is attributed to cardiovascular disease.66–68 However, the 2021 ACR Committee on Drugs and Contrast Media states that many studies reporting CI-AKI and its short- and long-term consequences have failed to include a control group; therefore, the contrast associated CI-AKI may be related to noncontrast causes of CA-AKI.4 Also, the committee states that “It is unusual for patients to develop permanent renal dysfunction”. In order to resolve this issue, larger studies with proper control groups are needed.
Conclusion
CI-AKI is a really entity, and its incidence is much lower than previously thought. Although CKD patients with an eGFR <30 mL/min and AKI patients are at risk for CI-AKI, controversy still exists regarding the causal relationship between intravascular iodinated contrast media and the development of AKI because prospective randomized controlled trials with adequate number of patients are lacking. Such studies are difficult to conduct because each patient is exposed twice to unnecessary radiation (unenhanced and enhanced CT scans). Also, ethical and cost considerations may prohibit from conducting such control studies. Meanwhile, analysis of data by propensity score matching methods in observational studies may provide useful information whether AKI after contrast medium is either correlative (CA-AKI) or causal (CI-AKI).
Also, the clinician should evaluate individually the indication for intravenous contrast study based on risk/benefit considerations. Comorbidities for CA-AKI should be carefully evaluated. An early study reported that diabetes alone is not a risk factor, but diabetes with CKD (eGFR 30–44 mL/min) is a risk factor for CA-AKI.69 When there is a clear indication, prophylaxis is advised for high-risk patients, and the clinician should not hesitate to request a contrast study. It always benefits the patient, if the ordering physician communicates with the radiologist to discuss the risks and benefits of the contrast administration. If iodinated contrast medium administration deems necessary for life-threatening diagnosis, it should be done irrespective of kidney function. Such a patient should receive prophylactic saline administration, if no contraindications.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bartels ED, Brun GC, Gammeltoft A, et al. Acute anuria following intravenous pyelography in a patient with myelomatosis. Acta Med Scand. 1954;40:297–302.
2. Davenport MS, Cohan RH, Khalatbari S, Ellis JH. The challenges in assessing contrast-induced nephropathy: where are we now? Am J Roentgenol. 2014;202:784–789. doi:10.2214/AJR.13.11369
3. Davenport MS, Perazella MA, Yee J, et al. Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2020;294:660–668. doi:10.1148/radiol.2019192094
4. ACR Committee on Drugs and Contrast Media. ACR manual on contrast media. In: Contrast-Associated Acute Kidney Injury and Contrast-Induced Acute Kidney Injury in Adults. ACR Committee on Drugs and Contrast Media; 2021:33–43.
5. Shah R, Le FK, Labroo A, et al. Contrast-associated acute kidney injury. Quant Imaging Med Surg. 2020;10:891–894. doi:10.21037/qims.2020.03.20
6. Azzalini L, Spagnoli V, Ly HQ. Contrast-induced nephropathy: from pathophysiology to preventive strategies. Can J Cardiol. 2016;32:247–255. doi:10.1016/j.cjca.2015.05.013
7. Grainger RG, Thomsen HS, Marcos SK, et al. Intravasular contrast media for radiology, CT and MRI. In: Adam A, Dixon AK, editors. Grainger & Allison’s Diagnostic Radiology. A Textbook of Medical Imaging,
8. Barrett BJ, Carlisle EJ. Meta-analysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology. 1993;188:171–178. doi:10.1148/radiology.188.1.8511292
9. Eng J, Wilson RF, Subramaniam RM, et al. Comparative effect of contrast media type on the incidence of contrast-induced nephropathy: a systematic review and meta-analysis. Ann Intern Med. 2016;164:417–424. doi:10.7326/M15-1402
10. Keaney JJ, Hannon CM, Murray PT. Contrast-induced acute kidney injury: how much contrast is safe? Nephrol Dial Transplant. 2013;28:1376–1383. doi:10.1093/ndt/gfs602
11. Manske CL, Sprafka JM, Strony JT, et al. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med. 1990;89:615–620. doi:10.1016/0002-9343(90)90180-L
12. Cigarroa RG, Lange RA, Williams RH, et al. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am J Med. 1989;86:649–652. doi:10.1016/0002-9343(89)90437-3
13. Karlsberg RP, Dohad SY, Sheng R. Contrast medium-induced acute kidney injury: comparison of intravenous and intraarterial administration of iodinated contrast medium. J Vasc Intervent Radiol. 2011;22:1159–1165. doi:10.1016/j.jvir.2011.03.020
14. Nyman U, Almén T, Jacobsson B, et al. Are intravenous injections of contrast media really less nephrotoxic than intraarterial injections? Eur Radiology. 2012;22:1366–1371. doi:10.1007/s00330-011-2371-4
15. Dekkers IA, van der Molen AJ. Propensity score matching as a substitute for randomized controlled trials on acute kidney injury after contrast media administration: a systematic review. AJR. 2018;211:822–826. doi:10.2214/AJR.17.19499
16. Yan P, Zhang N-Y, Luo X-Q, et al. Is intravenous iodinated contrast medium administration really harmful in hospitalized acute kidney injury patients: a propensity score–matched study. Eur Radiology. 2022;32:1163–1172. doi:10.1007/s00330-021-08192-2
17. Reddi TWG, Kothari N. Periprocedural planning for neuroendovascular procedures. In: Khatri E, editor. Complications of Neuroendovascular Procedures and Bailout Techniques. Cambridge: Cambridge University Press; 2016:163–179.
18. Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005;68:14–22. doi:10.1111/j.1523-1755.2005.00377.x
19. Seeliger E, Sendeski M, Riha CS, et al. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012;33:2007–2015. doi:10.1093/eurheartj/ehr494
20. Wong PC, Li Z, Guo J, Zhang A. Pathophysiology of contrast-induced nephropathy. Int J Cardiol. 2012;158:186–192. doi:10.1016/j.ijcard.2011.06.115
21. Liu ZZ, Viegas VU, Perlewitz A, et al. Iodinated contrast media differentially affect afferent and efferent arteriolar tone and reactivity in mice: a possible explanation for reduced glomerular filtration rate. Radiology. 2012;265(3):762–771. doi:10.1148/radiol.12120044
22. Eloy R, Corot C, Belleville J. Contrast media for angiography: physicochemical properties, pharmacokinetics and biocompatibility. Clin Mater. 1991;7:89–197. doi:10.1016/0267-6605(91)90045-h
23. Kiss N, Hamar P. Histopathological evaluation of contrast-induced acute kidney injury rodent models. BioMed Res Int. 2016;2016:15.
24. Lau A, Chung H, Komada T, et al. Renal immune surveillance and dipeptidase1 contribute to contrast-induced acute kidney injury. J Clin Invest. 2018;128:2894–2913. doi:10.1172/JCI96640
25. Hirematha S, Velez JCQ. Preventing a nonexistent entity: the curious case of contrast and acute kidney injury. Curr Opin Nephrol Hypertens. 2020;29:152–160. doi:10.1097/MNH.0000000000000562
26. Andreucci M, Faga T, Serra R, et al. Update on the renal toxicity of iodinated contrast drugs used in clinical medicine. Drug Healthc Patient Saf. 2017;9:25–37. doi:10.2147/DHPS.S122207
27. Yang J-S, Peng Y-R, Tsai S-C, et al. The molecular mechanism of contrast-induced nephropathy (CIN) and its link to in vitro studies on iodinated contrast media (CM). BioMedicine. 2018;8(1):1–11. doi:10.1051/bmdcn/2018080101
28. Faucona A-L, Bobriea G, Clément O. Nephrotoxicity of iodinated contrast media: from pathophysiology to prevention strategies. Eur J Radiol. 2019;116:231–241. doi:10.1016/j.ejrad.2019.03.008
29. Gelman LM, Rowe JW, Coggins CH. Effects of an angiographic contrast agent on renal function. Cardiovasc Med. 1978;4:313.
30. Eisenberg RL, Bank WD, Hedglock MW. Renal function after major angiography. Am J Med. 1980;68:43–46. doi:10.1016/0002-9343(80)90163-1
31. Rao QA, Newhouse JH. Risk of nephropathy after intravenous administration of contrast material: a critical literature analysis. Radiology. 2006;239(2):392–397. doi:10.1148/radiol.2392050413
32. Newhouse JH, Kho D, Rao QA, Starren J. Frequency of serum creatinine changes in the absence of iodinated contrast material: implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376–382. doi:10.2214/AJR.07.3280
33. Bruce RJ, Djamali A, Shinki K, Michel SJ, Fine JP, Pozniak MA. Background fluctuation of kidney function versus contrast-induced nephrotoxicity. AJR Am J Roentgenol. 2009;192(3):711–718. doi:10.2214/AJR.08.1413
34. Baumgarten DA, Ellis JH. Contrast induced nephropathy: contrast material not required? AJR Am J Roentgenol. 2008;191(2):383–386. doi:10.2214/AJR.08.1310
35. Katzberg RW, Newhouse JH. Intravenous contrast medium-induced nephrotoxicity: is the medical risk really as great as we have come to believe? Radiology. 2010;256:21–28. doi:10.1148/radiol.10092000
36. McDonald RJ, McDonald JS, Bida JP, et al. Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology. 2013;267:106–118. doi:10.1148/radiol.12121823
37. McDonald JS, McDonald RJ, Carter RE, Katzberg RW, Kallmes DF, Williamson EE. Risk of intravenous contrast material-mediated acute kidney injury: a propensity score matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65–73. doi:10.1148/radiol.13130775
38. McDonald JS, McDonald RJ, Lieske JC, et al. Risk of acute kidney injury, dialysis, and mortality in patients with chronic kidney disease after intravenous contrast material exposure. Mayo Clin Proc. 2015;90(8):1046–1053. doi:10.1016/j.mayocp.2015.05.016
39. McDonald JS, McDonald RJ, Williamson EE, Kallmes DF. Is intravenous administration of iodixanol associated with increased risk of acute kidney injury, dialysis, or mortality? A propensity score-adjusted study. Radiology. 2017;285(2):414–424. doi:10.1148/radiol.2017161573
40. McDonald JS, McDonald RJ, Williamson EE, Kallmes DF, Kashani K. Post-contrast acute kidney injury in intensive care unit patients: a propensity score-adjusted study. Intensive Care Med. 2017;43(6):774–784. doi:10.1007/s00134-017-4699-y
41. McDonald JS, McDonald RJ, Comin J, et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267:119–128. doi:10.1148/radiol.12121460
42. Davenport MS, Khalatbari S, Dillman JR, et al. Contrast material-induced nephrotoxicity and intravenous low osmolality iodinated contrast material. Radiology. 2013;267:94–105. doi:10.1148/radiol.12121394
43. Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719–728. doi:10.1148/radiol.13122276
44. Hinson JS, Ehmann MR, Fine DM, et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577–586. doi:10.1016/j.annemergmed.2016.11.021
45. Aycock RD, Westafer LM, Boxen JL, et al. Acute kidney injury after computed tomography: a meta-analysis. Ann Emerg Med. 2018;71:44–53. doi:10.1016/j.annemergmed.2017.06.041
46. Ehrmann S, Badin J, Savath L, et al. Acute kidney injury in the critically ill: is iodinated contrast medium really harmful? Crit Care Med. 2013;41:1017–1026. doi:10.1097/CCM.0b013e318275871a
47. Wilhelm-Leen E, Montez-Rath ME, Chertow G. Estimating the risk of radiocontrast-associated nephropathy. J Am Soc Nephrol. 2017;28:653–659. doi:10.1681/ASN.2016010021
48. Lima FO, Lev MH, Levy RA. Functional contrast-enhanced CT for evaluation of acute ischemic stroke does not increase the risk of contrast-induced nephropathy. Am J Neuroradiol. 2010;31:817–821. doi:10.3174/ajnr.A1927
49. Brinjikji W, Demchuk AM, Murad MH, et al. Neurons over nephrons: systematic review and meta-analysis of contrast-induced nephropathy in patients with acute stroke. Stroke. 2017;48:1862–1868. doi:10.1161/STROKEAHA.117.016771
50. Carlqvist J, Nyman U, Sterner G, et al. Minimal risk of contrast-induced kidney injury in a randomly selected cohort with mildly reduced GFR. Eur Radiol. 2021;31:3248–3257. doi:10.1007/s00330-020-07429-w
51. Nyman U, Aspelin P, Jakobsen J, et al. Controversies in contrast material–induced acute kidney injury: propensity score matching of patients with different dose/absolute glomerular filtration rate ratios. Radiology. 2015;277(3):633–637. doi:10.1148/radiol.2015151341
52. Thomsen HS, Stacul F. CIN: can we forget it? Acta Radiologica. 2014;55(9):1027–1030. doi:10.1177/0284185114545153
53. Ghumman SS, Weinerman J, Khan A, et al. Contrast induced-acute kidney injury following peripheral angiography with carbon dioxide versus iodinated contrast media: a meta-analysis and systematic review of current literature. Catheter Cardiovasc Interv. 2017;90(3):437–448. doi:10.1002/ccd.27051
54. Chaudhury P, Armanyous S, Harb SC, et al. Intra-arterial versus intravenous contrast and renal injury in chronic kidney disease: a propensity-matched analysis. Nephron. 2019;141(1):31–40. doi:10.1159/000494047
55. Medalion B, Cohen H, Assali A, et al. The effect of cardiac angiography timing, contrast media dose, and preoperative renal function on acute renal failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2010;139:1539–1544. doi:10.1016/j.jtcvs.2009.08.042
56. Sigterman TA, Bolt LJ, Krasznai AG, et al. Loss of kidney function after endovascular treatment of peripheral arterial disease. Ann Vasc Surg. 2017;40:231–238. doi:10.1016/j.avsg.2016.07.100
57. Shah M, Gajanana D, Wheeler DS, et al. Effects of staged versus ad hoc percutaneous coronary interventions on renal function: is there a benefit to staging? Cardiovasc Revasc Med. 2017;18:344–348. doi:10.1016/j.carrev.2017.02.017
58. Ellis JH, Cohan RH. Prevention of contrast induced nephropathy: an overview. Radiol Clin N Am. 2009;47:801–811. doi:10.1016/j.rcl.2009.06.003
59. Nijssen EC, Rennenberg RJ, Nelemans PJ, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, Phase 3, controlled, open-label, non-inferiority trial. Lancet. 2017;389:1312–1322. doi:10.1016/S0140-6736(17)30057-0
60. Lee H-C, Chuang K-I, Lu C-F, et al. use of contrast medium volume to guide prophylactic hydration to prevent acute kidney injury after contrast administration: a meta-analysis. Am J Radiol. 2020;215:15–24.
61. Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383:1814–1823. doi:10.1016/S0140-6736(14)60689-9
62. Marashizadeh A, Sanati HR, Sadeghipour P, et al. Left ventricular end-diastolic pressure-guided hydration for the prevention of contrast-induced acute kidney injury in patients with stable ischemic heart disease: the LAKESIDE trial. Int Urol Nephrol. 2019;51(10):1815–1822. doi:10.1007/s11255-019-02235-w
63. Solomon R. Forced diuresis with the RenalGuard system: impact on contrast induced acute kidney injury. J Cardiol. 2014;63:9–13. doi:10.1016/j.jjcc.2013.10.001
64. Briguori C, Visconti G, Focaccio A, et al. Renal insufficiency after contrast media administration trial II (REMEDIAL II): renalGuard system in high-risk patients for contrast-induced acute kidney injury. Circulation. 2011;124(11):1260–1269. doi:10.1161/CIRCULATIONAHA.111.030759
65. Shah R, Wood SJ, Khan SA, et al. High-volume forced diuresis with matched hydration using the RenalGuard System to prevent contrast-induced nephropathy: a meta-analysis of randomized trials. Clin Cardiol. 2017;40:1242–1246. doi:10.1002/clc.22817
66. Brown J, Malenka DJ, DeVries JT, et al. Transient and persistent renal dysfunction are predictors of survival after percutaneous coronary intervention: insights from the Dartmouth Dynamic Registry. Catheter Cardiovasc Interv. 2008;72:347–354. doi:10.1002/ccd.21619
67. Solomon RJ, Mehran R, Natarajan MK, et al. Contrast-induced nephropathy and long-term adverse events: cause and effect? Clin J Am Soc Nephrol. 2009;4:1162–1169. doi:10.2215/CJN.00550109
68. Cashion W, Weisbord SD. Radiographic contrast media and the kidney. Clin J Am Soc Nephrol. 2022;17:1234–1242. doi:10.2215/CJN.16311221
69. Rudnick MR, Goldfarb S, Wexler L, et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. The Iohexol Cooperative Study. Kidney Int. 1995;47:254–261. doi:10.1038/ki.1995.32
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
