Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Consistency Evaluation of Two Loading Devices in Measuring the Perception of Dyspnea
Authors Song J , Yin D , Liu X, Li X, Huang K
Received 20 March 2022
Accepted for publication 9 August 2022
Published 26 August 2022 Volume 2022:17 Pages 1963—1973
DOI https://doi.org/10.2147/COPD.S367213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Jie Song,1 Danfeng Yin,2 Xiaohui Liu,3 Xiaohui Li,1 Kewu Huang2
1Department of Respiratory and Critical Care Medicine, Beijing Luhe Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine and Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Department of Evidence-Based Medicine, Beijing Luhe Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Kewu Huang, Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine and Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China, Tel +861085231167, Email [email protected]
Purpose: This study aimed to assess the consistency of hand-held electronic incremental threshold loading device (I-TLD) and traditional constant threshold loading device (C-TLD) in measuring the perception of dyspnea (POD) in humans.
Patients and methods: Thirty-eight patients with stable chronic obstructive pulmonary disease (COPD) and 41 non-COPD subjects were recruited for the study, all of whom were subjected to an external loading breathing test by gradually increasing the inspiratory load starting from 0 to 5, 10, 20, and 30 cmH2O oral pressure using I-TLD and C-TLD. The Borg score measurement was performed immediately after the loading breath of each level. The linear regression slope a of Borg scores vs percentage of oral pressure from the patients’ maximum represented patients’ POD. The consistency of POD measured by the two devices was analyzed by two Related Samples Wilcoxon test, Spearman correlation analysis, and Bland-Altman analysis.
Results: There was no significant difference in slope a measured by the two devices in all subjects. The Spearman correlation analysis revealed that the slope a measured by the two devices in the inspiratory loading breath test had a significant correlation: in COPD patients, r = 0.678, (p < 0.001) and in non-COPD subjects, r = 0.603, (p < 0.001). For the results of the Bland-Altman analysis of the whole subjects, 3.8% (3/79) points were outside of the 95% LoA confidence interval (CI) (− 10.380, 9.457), and the LoA CI was acceptable, which depicted that the two devices were consistent in their estimation.
Conclusion: I-TLD was consistent with C-TLD in measuring POD in COPD patients and non-COPD subjects. I-TLD may be used as an alternative method to replace C-TLD to measure POD in COPD patients and non-COPD subjects.
Keywords: consistency evaluation, chronic obstructive pulmonary disease, external load breathing test, perception of dyspnea
Introduction
Dyspnea is a common distressing symptom of cardiopulmonary and neuromuscular diseases. In COPD patients, dyspnea, particularly during exertion, is a cardinal symptom.1,2 The degree of dyspnea is usually determined by utilizing patients’ experience1,3 and is often independent of forced expiratory volume in one second (FEV1) of patients.4,5 Some patients with COPD perceive the severity of their disease rather poorly, and those patients may not receive optimal therapy because of the underpresentation of their respiratory symptoms. Conversely, other patients with overperception are at risk of increased and inappropriate use of reliever medications and antibiotics since they frequently seek medical assistance.2,6 Thus, objective evaluation of the perception of dyspnea (POD) has an important clinical significance in identifying such patients.2 For this purpose, some laboratory methods are often used to simulate the dyspnea sensation experienced by patients. The external respiratory loading breath test is a commonly used method for measuring POD. This method made the subjects experience laborious breathing by artificially setting respiratory resistance. POD is usually measured by evaluating the sensation of dyspnea under different resistance.7
Previous studies have reported using a traditional constant threshold loading device (C-TLD) to measure POD, which also finds its application in respiratory muscle strength training. The basic principle of the device is based on setting the respiratory resistance at a constant level, and the subject must continue to overcome the resistance for breathing within the set time. The calculation of POD is then done based on the scale to score dyspnea sensation.
Recently, with the continuous update of respiratory muscle strength training instruments, a hand-held electronic incremental threshold loading device (I-TLD) has been applied for respiratory muscle strength training. In contrast to C-TLD, following setting up the resistance, the respiratory resistance applied by the device achieves the pre-set resistance value by gradually increasing the resistance. I-TLD has enabled the subject to gradually adapt before reaching the pre-set resistance value, which potentially reduces the patient’s resentment to loading breath. Similarly, applying I-TLD has been found to be more convenient as it relies on the software system for recording patients’ respiratory data.
In view of the significance of POD measurement in the management of COPD patients, it is necessary to choose easier and more patient-acceptable instruments to assess POD in COPD patients. However, whether I-TLD can be applied for estimating POD with good consistency with C-TLD has not yet been reported in the previous literature. In the present study, we investigated whether the assessment of POD by an I-TLD test produces results comparable with those of a C-TLD test for COPD and non-COPD participants.
Participants and Methods
Participants
This study recruited the subjects who underwent physical examination in a community in Tongzhou District Beijing between June 2021 and October 2021.
The recruitment criteria for COPD were in accordance with the Global Initiative for Chronic Obstructive Lung Disease (GOLD),2 and subjects were clinically stable for at least three months before testing. The exclusion criteria of COPD subjects included those having experienced a respiratory infection or acute exacerbation of COPD in the past three months, those suffering from a large number of lung tissue destructive diseases, such as severe bronchiectasis or pulmonary tuberculosis, those who underwent chest surgery or abdominal surgery in the past three months, those whose heart rate was >120 beats/min, those who were undergoing anti-tuberculosis treatment, those who were having other serious uncontrolled systemic diseases, pregnant and lactating mothers, those who have participated in other clinical trials, and those with heart failure, respiratory failure, severe neuromuscular diseases, or current oral sedatives.
The recruitment criteria for non-COPD subjects were defined as those having an FEV1/ FVC of >75% before bronchodilator inhalation, while those non-COPD subjects who had a history of chronic respiratory diseases or present acute respiratory diseases, those who underwent chest surgery or abdominal surgery in the past three months, those who had a heart rate of >120 beats/min, those who had anti-tuberculosis treatment, those who had other combined uncontrolled systemic diseases, pregnant or lactating mothers, those who have participated in other clinical trials, and those with heart failure, respiratory failure, severe neuromuscular diseases, severe mental diseases, or current oral sedatives were excluded from the study.
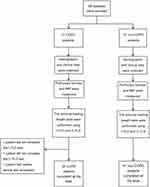
The demographic and clinical data were collected from all recruited subjects, including gender, age, smoking history, respiratory symptoms, lung function, etc., followed by measuring the maximum inspiratory pressure. Following this, the POD was measured with two devices. In 41 COPD patients, one patient did not complete the I-TLD test, one patient did not complete the C-TLD test, and one patient had neither device test completed. In 41 non-COPD subjects, both device tests were completed. The subject’s recruitment and test processes are summarized in Figure 1.
Methods
Spirometry
Trained and certified technicians performed pulmonary function tests before or after bronchodilator inhalation (salbutamol 400 µg) using a MasterScreen Pneumo PC spirometer (Masterscreen Pneumo, Carefusion, Hochberg, Germany) according to a standard protocol.8 FVC and FEV1 were measured in triplicates, and the best value was selected. The subjects were examined for pulmonary function from 8:00 to 12:00.
Maximum Inspiratory Pressure (MIP)
MIP was measured following a pulmonary function measurement. All subjects were seated comfortably and briefed about the process by an experienced operator. The subjects were made to perform the maximum inspiratory action at or near the residual volume (RV) (Mueller method), and then the MIP was measured via respiratory muscle strength measurement program in the I-TLD (Saike [Xiamen] Medical Devices Co. Ltd., China). The I-TLD had the flow channel design structure comprising a valve head and a sensor. The latter detects the pressure difference signal between exhalation and inhalation. The microcontrol unit (MCU) processor calculates various ventilation function parameters, such as volume through flow rate integration. The MIP test was repeated three times to select the best value.8
External Loading Breath Test
The subjects also performed an external loading breath test using C-TLD and I-TLD. The order of device use was random where, following each level of loading breath, Borg scale was used to score dyspnea sensation. The subjects received complete rest for 30 min between the two device tests.
1. C-TLD test: The external loading test was conducted using breathing trainer-C2 (Hangzhou Julu Medical Instrument Co. Ltd., China), as proposed by Larson.9 The opening of the inspiratory valve is guarded by a compressed spring with the ability of inspiratory load adjustment between –6 cmH2O and –41 cmH2O. The inspiratory load rising mode of the device is depicted in Figure 2A. During the procedure, the subject must overcome the pre-set inspiratory load to switch on the device's inspiratory valve to allow the airflow to pass through it. The study was performed adopting a previously reported protocol.10,11 Briefly, the subjects were allowed to breathe against a progressive load at 1-min intervals to achieve oral pressure of 0 cmH2O (no load), followed by a gradually increasing pressure to 5, 10, 20, and 30 cmH2O. Following breathing for 1 min at each load level, the subjects were allowed to rate the sensation of dyspnea using Borg scale.12 In instances where the subjects could not tolerate the respiratory resistance, the test was immediately halted, and the subjects were allowed to score the sensation of the last loading breath.
2. I-TLD test: The external loading test was performed using a hand-held electronic incremental loading device (Saike [Xiamen] Medical Devices Co. Ltd., China), where the MCU processor of the device controls the drive gear through an automatic command system to increase or decrease the valve area as per the corresponding impedance position during operation. The subjects were supposed to overcome the resistance to applying the inspiratory load during the inspiratory phase. The rising mode of the inspiratory load of the device is depicted in Figure 2B. In this experiment, a manual selection mode was applied to increase the respiratory load where the levels of 0 (no load), 5, 10, 20, and 30 cmH2O were selected in a defined sequence. The first two breaths at each level were resistance-free detection, followed by gradually increasing the load to 25%, 50%, 75%, and 100% in the subsequent four breaths, and six effective breaths were recorded at each level of the inspiratory load. All subjects were allowed to rest for 1 min between the two consecutive levels of resistance, during which dyspnea sensation was scored using Borg score. In instances where the subjects could not tolerate the respiratory resistance, the test was immediately halted, and the subjects were allowed to score the sensation of the last loading breath.
3. Measurement of dyspnea sensation: Following each level of loading breath, the sensation of dyspnea was scored using Borg scale, which is a numerical linear scale ranking the level of dyspnea from 0 (none) to 10 (maximum).
Statistical Analysis
All experimental data obtained were statistically analyzed. The quantitative data did not conform to the normal distribution, which was thus described by the median (5–95 percentile), and the classified data was described by the sample size (percentage). To quantify the perception of dyspnea, the linear regression slope of Borg dyspnea score vs percentage of oral pressure from the patient’s maximum was calculated by using the following equation: Borg = y + a %MIP.13 The “slope a” represented the sensitivity to dyspnea caused by changes in the external respiratory resistance, ie, POD. Borg scores of the two devices at each resistance level were compared by two related samples Wilcoxon test, while the consistency of POD measured by the two devices was analyzed by two related samples Wilcoxon test, Spearman correlation analysis, and Bland-Altman analysis.
Results
Subjects
The general characteristics of the subjects are shown in Table 1. The subjects with COPD were 36–80 years old (median age of 57.5 years). Simultaneously, non-COPD subjects were 35–75 years old (median age of 54.0 years). The total proportion of male COPD subjects was 76.3% of the sample size, with a higher smoking rate (60.5%) than in non-COPD subjects (41.5%). The pulmonary function grades of COPD patients were GOLD I (34.2%), GOLD II (44.7%), GOLD III (15.8%), and GOLD IV (5.3%), respectively.
 |
Table 1 General Characteristics of Enrolled COPD and Non-COPD Subjects |
Measurement results of Threshold Loading Breath Test of the Two Devices
Borg score obtained using two devices at the resistance level of 5 (p = 0.002) and 10 cmH2O (p = 0.039) was found to be statistically significant for COPD subjects (as shown in Table 2) with a high Borg score, while no significant difference was observed at resistance levels of 0, 20, and 30 cmH2O. Similarly, no significant difference in Borg score was observed with both devices at each resistance level in non-COPD subjects. In all subjects, the difference in Borg scores at the 5-cm H2O resistance level was statistically significant (p = 0.001), while the Borg scores of other resistance levels were not significant. There was no significant difference in slope a measured by the two devices in either of the subjects.
 |
Table 2 Borg Scores and Slope a Measured by the Two Devices in COPD and Non-COPD Subjects |
The distributions of slope a in COPD and non-COPD subjects are illustrated in Figure 3A and B. The slope a measured demonstrated a non-normal distribution using two different devices where the median slope a of patients with COPD was 8.1 (5–95%, percentile: 1.3–18.3) for the I-TLD test and 8.1 (5–95%, percentile: 1.6–30.7) for the C-TLD test. Meanwhile, in non-COPD subjects, the median slope a of the I-TLD test was 8.3 (5–95%, percentile: 4.1–20.9), and 8.1 (5–95%, percentile: 1.2–23.0) for C-TLD test. Compared to C-TLD, the slope a value distribution of I-TLD was more concentrated.
A significant correlation between the slope a was found for both devices in the inspiratory loading breath test for COPD subjects (r = 0.678, p < 0.001, Figure 4A), while r = 0.603, p < 0.001 (Figure 4B) was observed in non-COPD subjects. The Bland-Altman method was also employed to analyze the slope a measured using both devices (Figure 5), where 3.8% (3/79) points were outside of the 95% LoA CI (−10.380, 9.457) in all subjects, and the LoA CI was acceptable, which depicted that the two devices were consistent in their estimation.
 |
Figure 5 Consistency evaluation of slope a in all subjects for the two devices. Abbreviation: slope a, Borg scores vs percentage of oral pressure from the patient’s maximum. |
Discussion
This study aimed to measure POD using I-TLD and C-TLD during a threshold loading breath test in COPD and non-COPD subjects and evaluate the consistency of the two devices. Our results showed that I-TLD was consistent with C-TLD in measuring POD in COPD patients and non-COPD subjects. Meanwhile, we observed that Borg scores for I-TLD were lower at the beginning of the test in subjects with COPD, implying that the I-TLD maneuver is more likely to be acceptable by the subjects.
The external loading breathing test has been widely used to study the mechanism and perception of dyspnea in healthy subjects14–17 and COPD patients7,18–22 to evaluate the impact of dyspnea perception on diseases and explore new strategies to reduce dyspnea. During loading breath, inspiratory muscle effort evokes large rib cage and neck muscle activation. Meanwhile, there is a decrease in the rib cage and neck muscle tissue oxygen saturation, which may indicate a mismatch between inspiratory muscle oxygen delivery and utilization.23 The external load breathing test mainly creates a sense of breathing effort in a patient.3 Since breathing effort is the main manifestation experienced by COPD patients,24,25 and the airway resistance in COPD patients in acute exacerbation is high in comparison to remission,26 the dyspnea simulated by external loading breath test might help get a close to the real sensation in COPD patients. This method has the inherent merits of being easy to standardize and observe a wider range of dyspnea sensations within the ethical range and measuring POD in healthy subjects16 and COPD patients7 in a repetitive manner.
In the present study, we measured the POD by calculating the sensitivity to dyspnea caused by changes in the external respiratory resistance. The threshold loading breath test instrument27 works on the principle that the subject’s oral pressure must exceed a certain threshold limit to produce a significant inspiratory flow while breathing under a constant inspiratory load. Following improvements made by Larson,7 a discontinuous incremental loading breath test can be used as an alternative to increasing the rest time of the patients during the test, which may translate into a significant reduction of potentially unpleasant sensations of subjects to the test. Additionally, its reliability of repeated measurements of POD in patients with COPD has also been confirmed. Following that, Larson et al9 have improved the threshold loading breath device and developed a compressed spring system to limit the opening of the inlet flap, making the device more convenient to use. However, the major demerit of the device is that the patient must breathe under constant resistance once the device is set at a certain load value until and unless the load value is changed artificially.
With the advent of modern and updated respiratory training instruments, I-TLD has been employed in respiratory muscle strength training. This device has been more convenient and has had an edge in recording patients’ respiratory data by relying on the software system, yet it has not been applied for measuring POD. Compared to C-TLD, the initial loading at each loading level is low due to the gradual pressure increment, but the subjects still need to reach the pre-set resistance value at the end of each level. This gradual increase in the load gave the subjects an adaptation relay, but it must finally reach the pre-set respiratory loading level.
In the present study, we evaluated the consistency between the domestic I-TLD and C-TLD in measuring the POD of patients with COPD by using the linear regression slope a between Borg score and the percentage of patients’ oral pressure in MIP to express the sensitivity of subjects to changes in external respiratory load. Although the two devices have different ways of increasing load, we observed that there was no significant difference in slope a measured by the two devices for either all subjects and the slope a was correlated in both COPD and non-COPD subjects, where the slope a of the total participants was consistent between the two devices through the Bland-Altman analysis.
In our study, the differences in Brog scores and slope a were insignificant for both devices in non-COPD subjects, while a significant difference was observed in COPD patients at the first two loading levels where Borg scores for C-TLD were higher. Furthermore, with an increased loading level, the difference became statistically insignificant. The possible reason could be that COPD subjects experienced more unwanted sensations during C-TLD in the early stages of the test, ie, 5- and 10-cmH2O loading levels, and despite successfully overcoming the respiratory resistance, the dyspnea score was higher. However, interestingly, in the later stages of loading levels, ie, 20 and 30 cmH2O, the influence of unwanted sensation weakened, which might be because of subjects’ adaptation to the test resulting in an insignificant difference in Borg scores between both devices. Many previous studies have demonstrated that dyspnea among COPD patients could also be attributed to emotional and psychological aspects of the patients,4,21,28 where the patients were more conscious of dyspnea attention and aversion. Similarly, Esser and his colleagues have found significant involvement of emotional brain regions (hippocampus and amygdala) in the COPD patients before the loading breath test,21 where they observed no significant difference between the test and rest states of the patients. Since the increase in loading level for I-TLD was gradual, it could be of pivotal importance in reducing the impact of patients’ psychological impact on the test.
Additionally, I-TLD had the advantage of time-saving, being an efficient method primarily based on the number of breaths with each loading level of 6 breaths (about 30 s), which is less time-consuming than C-TLD, which involves breathing for 1 min at each loading level.
Finally, the subjects’ tolerance to both devices was similar, and the difference in the number of subjects who failed to complete the test was not statistically significant, while I-TLD did not confirm that more subjects completed the test. The possible reason could be that the requirements of the two devices on the patients’ respiratory muscle strength were similar. At the same level of a loading breath test, the subjects must overcome the same set load to complete the test.
Currently, most reported studies on POD in COPD patients have had small sample sizes, and a comprehensive understanding of POD characteristics in large COPD populations is still lacking. One of the reasons is that loading breathing test is not easy to implement. However, our present study showed the consistency of I-TLD and C-TLD in measuring POD, and I-TLD also had potential advantages in terms of time-saving and patient compliance.
In the present study, we also preliminarily compared the POD between the COPD and non-COPD subjects, and the result showed no significant difference between COPD and non-COPD subjects, which was consistent with a previous report,29 and suggested that COPD might not interfere and damage the central nervous system and conduction pathways to affect the dyspnea perception, although it still requires further studies to confirm.
In the present study, we selected fixed incremental loads as the protocol, which has been used in some previous studies.10,11 In the present study, we chose this protocol for the following reasons. First, the loading levels chosen for each patient in both device tests were the same for each individual, and the fixed resistance represented the same percentage of the individual’s MIP; hence, this protocol did not affect the consistency comparison of the two devices in measuring POD. Second, the present protocol is more convenient, eliminating the step of calculating resistance as a percentage of MIP during the test. Certainly, we also agree that a progressive load based on values relative to MIP for each individual (ie, 10%, 20%, 30%, and 40% of MIP) may be an alternative in future clinical practice.
There are some limitations to our study. First, similar to other COPD studies, the majority of COPD patients enrolled were males. Second, because the patients with COPD enrolled in the present study came from the screening for COPD conducted in a general population, they had fewer symptoms, and the majority of them were at GOLD I and II stages, which does not represent all COPD subjects. Furthermore, smokers were not excluded from the healthy subjects, and it was also unclear whether smoking may affect the POD results of the subjects or not. Additionally, the reproducibility of POD measurement using I-TLD was not evaluated, although the reproducibility has been confirmed for C-TLD.7
Conclusion
I-TLD showed good consistency with C-TLD for measuring POD in COPD and non-COPD subjects, which can be used as a novel alternative to C-TLD for measuring POD in both COPD and non-COPD subjects. Furthermore, compared to C-TLD, I-TLD proved the merit of time-saving, easy data recording, and helping reduce the emotional interference with the test. Therefore, our findings contribute to the wide application of I-TLD for measuring POD in patients with COPD, which will help identify differential characteristics of patients with COPD and define a new phenotype that, if identified early, would allow performing interventions to prevent a negative impact on the morbidity/mortality of these patients.
Abbreviations
I-TLD, hand-held electronic incremental threshold loading device; C-TLD, traditional threshold loading device; POD, perception of dyspnea; COPD, chronic obstructive pulmonary disease; GOLD, global Initiative for chronic obstructive lung disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FEV1/FVC, ratio of forced expiratory volume in one second to forced vital capacity; MIP, maximum inspiratory pressure; RV, residual volume; MCU, microcontrol unit; slope a, Borg scores vs percentage of oral pressure from the patient’s maximum.
Data Sharing Statement
Researchers may request datasets that were used in this study from the corresponding author with a reasonable request.
Ethics Approval and Informed Consent
All subjects voluntarily entered the study and signed the written informed consent. The study was conducted in accordance with the Declaration of Helsinki. The study was approved by the institutional ethical committee of Beijing Chao-Yang Hospital, Capital Medical University (ethics batch No.: 2021-ke-419).
Consent for Publication
All authors agreed with the publication.
Acknowledgments
We would like to acknowledge the Liyuan Community Health Service Center, Tongzhou District, Beijing, for assistance in screening the subjects.
Funding
This study was supported by the National Natural Science Foundation of China (81870032) and the “Summit” Talent Training Program, Beijing Hospital Authority (DFL20190301), but these had no role in the writing or submission of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. O’Donnell DE, Milne KM, James MD, de Torres JP, Neder JA. Dyspnea in COPD: new mechanistic insights and management implications. Adv Ther. 2020;37(1):41–60. doi:10.1007/s12325-019-01128-9
2. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease report; 2022. Available from: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf.
3. Parshall MB, Schwartzstein RM, Adams L, et al. An Official American Thoracic Society Statement: update on the mechanisms, assessment, and management of dyspnea. Am J Resp Crit Care. 2012;185(4):435–452. doi:10.1164/rccm.201111-2042ST
4. Herigstad M, Hayen A, Evans E, et al. Dyspnea-related cues engage the prefrontal cortex: evidence from functional brain imaging in COPD. Chest. 2015;148(4):953–961. doi:10.1378/chest.15-0416
5. Ottanelli R, Rosi E, Ronchi MC, et al. Perception of bronchoconstriction in smokers with airflow limitation. Clin Sci. 2001;101(5):515. doi:10.1042/CS20000339
6. Scioscia G, Blanco I, Arismendi E, et al. Different dyspnoea perception in COPD patients with frequent and infrequent exacerbations. Thorax. 2017;72(2):117–121. doi:10.1136/thoraxjnl-2016-208332
7. Larson JL, Covey MK, Berry J, Wirtz S, Alex CG, Matsuo M. Discontinuous incremental threshold loading test: measure of respiratory muscle endurance in patients with COPD. Chest. 1999;115(1):60–67. doi:10.1378/chest.115.1.60
8. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
9. Larson JL, Kim MJ, Sharp JT, Larson DA. Inspiratory muscle training with a pressure threshold breathing device in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1988;138(3):689–696. doi:10.1164/ajrccm/138.3.689
10. Magadle R, Berar-Yanay N, Weiner P. The risk of hospitalization and near-fatal and fatal asthma in relation to the perception of dyspnea. Chest. 2002;121(2):329–333. doi:10.1378/chest.121.2.329
11. Weiner P, Magadle R, Beckerman M, Weiner M, Berar-Yanay N. Maintenance of inspiratory muscle training in COPD patients: one year follow-up. Eur Respir J. 2004;23(1):61–65. doi:10.1183/09031936.03.00059503
12. El-Manshawi A, Killian KJ, Summers E, Jones NL. Breathlessness during exercise with and without resistive loading. J Appl Physiol. 1986;61(3):896–905. doi:10.1152/jappl.1986.61.3.896
13. Bijl-Hofland ID, Cloosterman SG, van Schayck CP, V. D. Elshout FJJ, Akkermans RP, Folgering HTM. Perception of respiratory sensation assessed by means of histamine challenge and threshold loading tests. Chest. 2000;117(4):954–959. doi:10.1378/chest.117.4.954
14. Ziegler B, Fernandes AK, Sanches PRS, Konzen GL, Dalcin PDTR. Variability of the perception of dyspnea in healthy subjects assessed through inspiratory resistive loading. J Bras Pneumol. 2015;41(2):143–150. doi:10.1590/S1806-37132015000004409
15. Boswell-Ruys CL, Lewis C, McBain RA, Gandevia SC, Butler JE. The reliability of inspiratory resistive load magnitude and detection testing. Respir Physiol Neurobiol. 2020;281:103490. doi:10.1016/j.resp.2020.103490
16. Fernandes AK, Ziegler B, Konzen GL, et al. Repeatability of the evaluation of perception of dyspnea in normal subjects assessed through inspiratory resistive loads. Open Respir Med J. 2014;8:41–47. doi:10.2174/1874306401408010041
17. Herzog M, Sucec J, Van Diest I, et al. Reduced neural gating of respiratory sensations is associated with increased dyspnoea perception. Eur Respir J. 2018;52(1):1800559. doi:10.1183/13993003.00559-2018
18. Patessio A, Rampulla C, Fracchia C, et al. Relationship between the perception of breathlessness and inspiratory resistive loading: report on a clinical trial. Eur Respir J Suppl. 1989;7:587s–591s.
19. Livermore N, Butler JE, Sharpe L, McBain RA, Gandevia SC, McKenzie DK. Panic attacks and perception of inspiratory resistive loads in chronic obstructive pulmonary disease. Am J Resp Crit Care. 2008;178(1):7–12. doi:10.1164/rccm.200711-1700OC
20. Livermore N, Dimitri A, Sharpe L, McKenzie DK, Gandevia SC, Butler JE. Cognitive behaviour therapy reduces dyspnoea ratings in patients with chronic obstructive pulmonary disease (COPD). Respir Physiol Neurobiol. 2015;216:35–42. doi:10.1016/j.resp.2015.05.013
21. Esser RW, Stoeckel MC, Kirsten A, et al. Brain activation during perception and anticipation of dyspnea in chronic obstructive pulmonary disease. Front Physiol. 2017;8. doi:10.3389/fphys.2017.00617
22. Mahler DA, Gifford AH, Waterman LA, et al. Effect of increased blood levels of β-endorphin on perception of breathlessness. Chest. 2013;143(5):1378–1385. doi:10.1378/chest.12-1541
23. Rodrigues A, Louvaris Z, Dacha S, et al. Differences in respiratory muscle responses to hyperpnea or loaded breathing in COPD. Med Sci Sports Exerc. 2020;52(5):1126–1134. doi:10.1249/MSS.0000000000002222
24. O’Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med. 1997;155(1):109–115. doi:10.1164/ajrccm.155.1.9001298
25. Mahler DA, Harver A, Lentine T, Scott JA, Beck K, Schwartzstein RM. Descriptors of breathlessness in cardiorespiratory diseases. Am J Respir Crit Care Med. 1996;154(5):1357–1363. doi:10.1164/ajrccm.154.5.8912748
26. Jalusic-Gluncic T. What happens with airway resistance (RAW) in asthma and COPD exacerbation. Med Arh. 2011;65(5):270–273.
27. Nickerson BG, Keens TG. Measuring ventilatory muscle endurance in humans as sustainable inspiratory pressure. J Appl Physiol Respir Environ Exerc Physiol. 1982;52(3):768–772. doi:10.1152/jappl.1982.52.3.768
28. Reijnders T, Troosters T, Janssens W, et al. Brain activations to dyspnea in patients with COPD. Front Physiol. 2020;11:7. doi:10.3389/fphys.2020.00007
29. Mahler DA, Rosiello RA, Harver A, Lentine T, McGovern JF, Daubenspeck JA. Comparison of clinical dyspnea ratings and psychophysical measurements of respiratory sensation in obstructive airway disease. Am Rev Respir Dis. 1987;135(6):1229–1233. doi:10.1164/arrd.1987.135.6.1229
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.