Back to Journals » Cancer Management and Research » Volume 13
Concomitant Pulmonary Tuberculosis Impair Survival in Advanced Epidermal Growth Factor Receptor (EGFR) Mutant Lung Adenocarcinoma Patients Receiving EGFR-Tyrosine Kinase Inhibitor
Authors Xie Y, Su N, Zhou W, Lei A, Li X, Li W, Huang Z, Cen W, Hu J
Received 25 June 2021
Accepted for publication 11 September 2021
Published 1 October 2021 Volume 2021:13 Pages 7517—7526
DOI https://doi.org/10.2147/CMAR.S326349
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Yalin Xie,1 Ning Su,1 Wei Zhou,2 An Lei,1 Xiang Li,3 Weiwei Li,4 Zhan Huang,4 Wenchang Cen,1 Jinxing Hu5
1Department of Oncology, Guangzhou Chest Hospital, Guangzhou, People’s Republic of China; 2Department of Pathology, Guangzhou Chest Hospital, Guangzhou, People’s Republic of China; 3Department of Pharmacy, Guangzhou Chest Hospital, Guangzhou, People’s Republic of China; 4Department of Medical Business, Amoy Diagnostics Co., Ltd., Xiamen, People’s Republic of China; 5Department of Tuberculosis, Guangzhou Chest Hospital, Guangzhou, People’s Republic of China
Correspondence: Wenchang Cen; Jinxing Hu Email [email protected]; [email protected]
Objective: Limited studies have clearly demonstrated the effect of EGFR-TKI in the treatment of EGFR mutant NSCLC patients with underlying pulmonary disease, like pulmonary tuberculosis (PTB). Here, we conducted the study to evaluate the impact of PTB on survival of Chinese EGFR mutant lung adenocarcinoma (LUAD) patients that underwent EGFR-TKI treatment.
Methods: Clinicopathologic data of 1448 LUAD patients harboring EGFR mutations from the Guangzhou Chest Hospital between 2017 and 2019 were reviewed retrospectively. Patients receiving EGFR-TKI treatment were divided into PTB and non-PTB groups. The differences in response to EGFR-TKIs and survival between the two groups were assessed.
Results: After EGFR-TKIs treatment, the objective response rate (58.14% vs 47.62%) as well as disease control rate (97.67% vs 85.71%) were higher in the non-PTB group than in the PTB group, but there was no statistical difference. In the survival analysis, both the median progression-free survival (7.47 months vs 11.77 months, p = 0.038) and the overall survival (13.00 months vs 20.00 months, p = 0.001) were significantly shorter in the PTB group than in the non-PTB group. Furthermore, for patients with 19Del mutation, or metastases sites less than 3, or using first-line EGFR-TKI, EGFR-TKIs treatment significantly prolonged the median PFS and OS in patients without PTB.
Conclusion: LUAD patients with concomitant PTB have a poor response to EGFR-TKI treatment, especially in terms of survival outcome.
Keywords: advanced lung adenocarcinoma, pulmonary tuberculosis, EGFR mutation, EGFR-tyrosine kinase inhibitors, prognosis
Introduction
Lung cancer and pulmonary tuberculosis (PTB) are two major public health issues.1 Lung cancer is the leading cause of cancer mortality worldwide, with 85% of patients diagnosed with non-small-cell lung cancer (NSCLC), many presenting at an advanced stage of the disease.2 Several studies also have suggested that PTB is associated with an increased risk of lung cancer.3,4 Chronic inflammation that leads to an imbalance in DNA damage and repair mechanisms may be a possible pathophysiology of lung cancer in TB patients. Nalbandian et al proved that chronic tuberculosis infection in the lung is sufficient to cause a multi-step transformation of cell associated with PTB lesion through squamous cell dysplasia to malignant squamous cell carcinoma.5 The mortality was also much higher in the lung cancer patients with PTB than in the no-PTB patients (51.1 versus 8.2 per 10,000 person-years).4 However, the medical, surgical, and oncological treatment algorithm for this specific subgroup of patients with concomitant NSCLC and TB, especially in the Chinese population, has not been described evidently.
Targeted therapy has been developed and is widely used for the treatment of NSCLC, particularly in patients with an activating epidermal growth factor receptor (EGFR) mutation. EGFR is a 170-kDa transmembrane protein with a tyrosine kinase (TK) domain and regulates cellular proliferation, motility and apoptosis.6 Mutations in the EGFR gene are one of the most common driver oncogenes in lung adenocarcinoma (LUAD), and occur frequently in approximately 50% of Asians.7 In patients with sensitizing EGFR mutations such as exon 19 deletions (19Del) or exon 21 L858R point mutations, treatment with EGFR-TKIs leads to longer progression-free survival (PFS) compared with cytotoxic chemotherapy.8,9
Deregulation of the EGFR pathway causing aberrant EGFR signaling is associated with many respiratory diseases including chronic inflammation disease and cancer.10,11 Hwang et al revealed that the frequency of EGFR mutations was significantly higher in patients with old PTB lesions than in patients without old PTB lesions (56/100, 56% vs 127/377, 34%; p = 0.038).7 And Luo et al reported that LUAD patients with scar cancer or old PTB lesions were more likely to develop EGFR mutations, especially exon 19 deletions.12 However, various studies raised different conclusions on EGFR mutational status of LUAD patients with PTB. A retrospective data based on 405 lung cancers with PTB in China confirmed that gene mutation status was related to gender, smoking history, pathological type, and cavity formation. There was no difference in the gene mutation rate (including EGFR) of patients between the group with active or old PTB (P = 0.357).3 Furthermore, it is unclear whether the presence of PTB affects the clinical outcomes of EGFR-mutated lung cancer patients. Luo et al reported that among patients with EGFR mutations, those who had old PTB lesions survived for a shorter period than those who did not, but with or without old PTB lesions did not affect overall survival (OS) among patients with EGFR exon 19 deletions.12 In addition, Hwang et al showed that both the PFS and the OS after EGFR-TKIs were significantly shorter in patients with old PTB lesions than in patients without old PTB lesions.7
Moreover, there are scarce data on whether the concomitant PTB affect the efficacy of EGFR-TKI treatment in patients with lung cancer. Since previous in vitro studies revealed that EGFR-TKIs exposure may be affected by co-administration with drugs that inhibit or induce cytochrome P450 (CYP) enzyme (CYP3A4/5).13,14 Moreover, clinical studies show that Osimertinib exposure is significantly decreased by a strong CYP3A4 inducer (such as rifampicin) in patients with EGFRm NSCLC.15 Thus, the effectiveness and safety of EGFR-TKI for patients diagnosed simultaneously with PTB and LUAD, and the impact of PTB on clinical outcome for these patients treated with EGFR-TKIs were investigated in this study.
Materials and Methods
Patients and Data Collection
One thousand four hundred and forty-eight patients with lung cancer were diagnosed at the Guangzhou Chest Hospital between 2017 and 2019. The co-existent PTB and LUAD were found in 223 cases, among them 21 EGFR mutant patients with accessible follow-up were included and designated as PTB with EGFR mutation group [EGFR (+) NSCLC + PTB]. Of the 1225 patients with lung cancer alone, 296 had EGFR mutations, and 43 patients were selected using simple random sampling as non-PTB with EGFR mutation group [EGFR (+) NSCLC Only]. The Medical Ethics Committee of Guangzhou Chest Hospital approved this retrospective observational study. Informed consent was waived because of the retrospective nature of this study that does not expose patients to high risks. Patient data confidentiality was maintained, and the declaration of Helsinki was followed.
EGFR Mutation Testing
Formalin-fixed paraffin-embedded (FFPE) tumor tissues were genotyped for the alterations in EGFR, ALK, ROS1, KRAS, BRAF, RET, MET, HER2, NRAS, and PIK3CA genes. Genomic DNA and total RNA were extracted from FFPE samples using the AmoyDx FFPE DNA/RNA extraction kit (Amoy Diagnostics, Xiamen, China) according to the manufacturer’s instructions, and for all other type of samples using the AmoyDx Tissue DNA/RNA extraction kit (Amoy Diagnostics). EGFR/ALK/ROS1/KRAS/BRAF/RET/MET/HER2/NRAS/PIK3CA Mutations Detection Kit obtained from Amoy Diagnostics were used to detect alterations. Experimental procedure and data analysis followed the manufacturer’s instructions in detail.
Response Evaluation
Evaluation of response to EGFR-TKI treatment was classified on the basis of interval CT scans as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) using standard Response Evaluation Criteria in Solid Tumors criteria (RECIST) version 1.1.
Statistical Analyses
Statistical analyses were performed using the SPSS software for Windows (version 20.0). Associations between categorical variables were compared using the chi-square test. Overall survival was estimated using the Kaplan–Meier method, and differences in survival were examined using the Log rank test. PFS was defined as the time from the first day of targeted therapy to disease progression or death from any cause. OS was defined as the time from the start of targeted therapy to death.
Results
Patients Characteristics
Between 2017 and 2019, 1448 patients with NSCLC were available for analysis. Of these patients, 223 (15.40%) had co-existent PTB and LUAD, and 21 EGFR mutant patients with accessible follow-up data were eligible for this study and designated as PTB group [EGFR (+) NSCLC + PTB]. Forty-three LUAD patients with EGFR mutations were randomly selected and designated as non-PTB group [EGFR (+) NSCLC Only] (Figure 1), and the efficacy and safety of EGFR-TKI treatment were analyzed in these two groups.
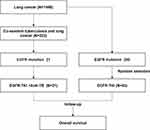 |
Figure 1 Flowchart of participant selection in this study. Data cutoff was November 7, 2020. Abbreviations: EGFR, epidermal growth factor receptor; A-TB, anti-Tuberculosis (TB) drugs. |
The clinicopathological characteristics of the patients are summarized in Table 1. The median ages of patients were 64 (PTB group) and 61 (non-PTB group) years, respectively. Histology of all the patients was adenocarcinoma. No difference was found in the baseline of the patients including age, gender, treatment method, mutation type, metastasis site and types of target drugs (Table 1). While the frequency of smoking (57.14% vs 13.95%, p = 0.001) and poor performance status (23.81% vs 6.98%, p = 0.007) were significantly higher in the PTB group compared with the non-PTB group (Table 1).
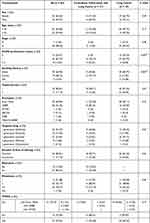 |
Table 1 Demographics and Baseline Characteristics of All Cases |
EGFR Mutant LUAD Patients with PTB Showed Inferior EGFR-TKI Efficacy
The median follow-up time in PTB and non-PTB groups was 19.0 and 32.0 months, respectively. The analysis revealed that compared with EGFR-TKI monotherapy, co-administration of anti-TB with EGFR-TKI can reduce objective response rate (ORR) (PTB vs non-PTB, 47.62 vs 58.14, P = 0.43) and disease control rate (DCR) (PTB vs non-PTB, 85.71% vs 97.67%, P = 0.06), but the difference was not significant (Table 2). As assessed by investigators, the median PFS of the non-PTB group was significantly longer than that of the PTB group (11.77 months vs 7.47 months; HR 1.89, 95% CI 1.058–4.011; P = 0.038) (Figure 2A). Compared with the PTB group, patients in the non-PTB group showed a significant survival benefit for receiving EGFR-TKI treatment (13.00 vs 20.00 months, HR 2.62, 95% CI 1.72–8.47, P = 0.0017) (Figure 2B). The PFS for patients receiving first-line EGFR-TKI therapy was not significantly different between non-PTB and PTB group (P=0.0574, Figure 2C). However, a significant difference was observed in OS (P = 0.0349, Figure 2D). To further verify the effect of rifampicin on PFS and OS in the PTB group, patients were divided into rifampin (+) subgroup and rifampin (-) subgroup. The results confirmed that rifampicin does not affect the anti-tumor efficacy of EGFR-TKI when co-administered with anti-TB therapy (Supplementary Figure 1A and B).
 |
Table 2 Efficacy of EGFR-TKI Therapy in Non-PTB Group and PTB Group |
Subgroup Analysis Indicated EGFR-TKI Response in LUAD Patients with PTB Was Dependent on Clinical and Genetic Status
Subgroup analyses of survival status were further performed according to clinicopathological parameters (exon 19Del mutation, or metastases sites less than 3, or using first-line EGFR-TKI) of all study subjects. Patients with 19Del mutation in the non-PTB group had longer PFS (5.13 vs 12.20 months, P = 0.0096, Figure 3A) and OS (11.00 vs 23.00 months, P = 0.0223, Figure 3B) than the PTB group. Compared with patients with <3 metastatic sites in the PTB group, patients with metastasis <3 in the non-PTB group had significantly longer PFS (7.47 vs 12.40 months, P = 0.0165, Figure 3C) and OS (13.00 vs 23.000 months, P = 0.0036, Figure 3D).
Adverse Events of Patients Receiving EGFR-TKIs in the Two Groups
Most treatment-related AEs were grade 1 or 2 in severity and reversible; the most common treatment-related AEs included paronychia, mucositis, diarrhea, rash, elevated transaminases, fatigue, decreased appetite and edema (Table 3). Incidence of AEs ≥ grade 3 had no significant difference between two groups (2/21 vs 1/43). The only grade 4 treatment-related adverse event (<1%) was rash in the non-PTB group (Table 3).
 |
Table 3 Adverse Events Associated with EGFR-TKI Therapy in Non-PTB and PTB Group |
Discussion
The patients with PTB were 10.9 times more likely than patients without PTB to develop lung cancer.16 Previous data demonstrated that pre-existing TB lesions were associated with more frequent EGFR mutations and poorer treatment response in patients with lung cancer.7 However, the impact of concomitant PTB on targeted therapy and clinical outcome of lung cancer patients with EGFR mutations is still unclear. The major finding in this study is that the co-existing PTB can affect the treatment efficacy of patients with EGFR-mutated LUAD, and the clinical outcome of patients with co-existent PTB and LUAD is poorer than that of patients with LUAD alone. This study also found that more than half of patients in the PTB group treated with EGFR-TKI experienced only grade 1 and grade 2 common adverse events: diarrhea and rash, which was consistent with the study in patients with lung cancer alone.17,18 These data showed that patients with co-existent PTB and LUAD were well tolerated to EGFR-TKI, and adverse events were relatively mild.
The occurrence of PTB and lung cancer as comorbidities has been widely discussed in many studies. PTB is known to be a risk factor for lung cancer, and lung cancer often develops in scars caused by PTB. In recent years, the correlation between the two diseases has attracted increasing attention in terms of the close epidemiological connection and chronic inflammation-associated carcinogenesis. Previous studies reported that 2.1% to 21% of lung cancer patients had pre-existing PTB,7,19 and 0.7% to 18.7% of lung cancer patients with active PTB.17 In the present study, 15.40% (223/1448) of all patients with available clinical information had co-existent PTB and lung cancer. These data showed that a high percentage of lung cancer cases was complicated by PTB; therefore, in the treatment of lung cancer, the possibility of concurrent PTB should be considered, while in the treatment of PTB, the possibility of concurrent lung cancer should also be kept in mind. The pathological characteristics of the patients included in this study were also shown that the vast majority of the patients (57.14%) in PTB group were former smokers, which is consistent with findings of previous studies.18,20 Although some researches have reported that the increased risk of lung cancer among PTB patients is not related to former cigarette smoking,21 it is still necessary to pay attention to the harmful effects of smoking on those infected with TB. The frequency of former smoker and poor performance status were significantly higher in the PTB group compared with the non-PTB group in this study.
For lung cancer patients with concurrent pulmonary TB, Evman et al revealed that anti-TB treatment before surgery will not increase the risk of surgical treatment for lung cancer patients, but it is necessary to rationally allocate the best time for surgery and anti-TB treatment to prevent delay in malignancy treatment or communal infectious contamination.22 And our results confirmed that rifampicin does not affect the anti-tumor efficacy of EGFR-TKI when co-administered with anti-TB therapy. While, when comparing patients with lung cancer and patients with concurrent TB and lung cancer, a study in South Korea showed that the history of PTB was related to the poor clinical response of NSCLC patients to EGFR-TKIs.7 Similar conclusion was obtained in a retrospective analysis of the data of 8265 Taiwanese patients.16 The results of this study also showed that PFS and OS of patients with co-existent PTB and LUAD were poorer than that of patients with LUAD alone. Further subgroup analysis was performed to compare the survival data of patients with EGFR-TKI as first-line treatment in the non-PTB and PTB groups. Similarly, patients in the non-PTB group had better PFS and OS than the PTB group. Therefore, combined with the above findings, it is shown that the efficacy of EGFR-TKI therapy is adversely affected in EGFR-mutant lung cancer patients with a pre-existing PTB or a co-existing PTB, however the reason for the poor response of PTB-related lung cancer patients to EGFR-TKI is still unclear. On the one hand, aggressive phenotype induced by chronic inflammation may be a possible explanation. Zhang et al proved that chronic tuberculosis infection up-regulated epiregulin, which was related to the invasiveness of EGFR-mutant lung cancer.23 On the other hand, CYP3A4 is involved in the metabolism of gefitinib; however, previous study reported that in the presence of the anti-tuberculosis drug rifampicin, a potent CYP3A4 inducer, the geometric mean maximum concentration and area under the plasma concentration-time curve of gefitinib were significantly reduced.13 A Phase I study found that in patients with advanced NSCLC, the exposure (AUC) of Osimertinib was increased by 24% when combined with itraconazole (a strong CYP3A4 inhibitor), and decreased by 78% when combined with rifampicin (a CYP3A4 inducer), and it is suggested that strong CYP3A inducers are not likely to be co-administered with Osimertinib.15 Thus, the effect of rifampicin on the prognosis of patients with co-existent PTB and LUAD was analyzed in this study. However, the result indicated that rifampicin has no significant effect on the prognosis of patients with co-existent PTB and LUAD, which may be related to the small sample size. Therefore, further large-scale investigations are required to clarify the adverse reaction mechanism of the existence of PTB to TKI treatment.
Exon 19Del and exon 21L858R are the most common EGFR mutations in NSCLC and have become common biomarkers to predict the clinical response to EGFR-TKI.24 However, inconsistent results have also been reported in some Phase III clinical trials. The results of these phase III trials showed that there was no statistically significant difference in PFS between patients with 19Del and L858R mutations treated with TKI.25 In this study, it is found that patients with 19Del mutation were more sensitive to EGFR-TKI treatment than patients with L858R mutation. Furthermore, the prognosis of patients with 19Del mutation in non-PTB group was also significantly better than that in PTB group. However, there is no significant difference in L858R subgroup analysis. This may be attributed to the small sample size of the present study. While previous studies have shown that patients with L858R mutations are prone to co-mutations and have a worse effect on EGFR-TKI than patients with 19 Del.26–28
Because most NSCLC patients have developed metastatic disease by the time they are diagnosed.20 Thus, a subgroup analysis was performed in this study based on the cancer metastasis status. In the patients included in this study, metastatic sites occurred mainly in the brain, bone, liver, and distant lymph nodes. The patients were sub-grouped according to the number of metastases ≥3, or <3. The proportion of patients with metastases ≥3 in PTB group was 42.86% (12/21), numerically higher than that in non-PTB group (27.91%,12/43). In a preclinical study, the authors found that mycobacterium tuberculosis antigens repressed the T-cell immune response and promoted tumor metastasis.29 Therefore, we postulated that patients with co-existent PTB and LUAD were more prone to develop tumor metastasis. Furthermore, for patients with metastases <3, the PFS and OS of the PTB group were significantly shorter than those of the non-PTB group. On the basis of these findings, it is suggested that metastasis is an important factor affecting the prognosis of patients with advanced NSCLC, especially in patients with co-existent PTB and LUAD.
Lung adenocarcinoma is the main pathological type of lung cancer in China. The mutation rate of EGFR in Chinese lung adenocarcinoma patients is about 40–50%, and EGFR 19Del and L858R mutations account for approximately 90%.7,12 Previous data also show that the pre-existing PTB lesions are significantly related to the increase in the EGFR mutation rate, especially the 19Del in LUAD patients.10 Similarly, 52.38% of patients in the PTB group in this study carried EGFR 19Del mutation. However, in all 1448 patients with available clinical information, the mutation rate of EGFR was only 21.89%, which was significantly lower than the level reported in the literature. The possible reason is that the early-stage patients account for the majority of these patients, and only a few of the early-stage patients have received genetic testing. Comparing the pathological characteristics of patients in the PTB group and the non-PTB group, it was found that a higher proportion of patients in the PTB group had a history of smoking, and the physical status of the patients was poor than that of the non-PTB group. These results suggested that PTB patients with a history of smoking may be more likely to develop lung cancer, further large-scale investigation is warranted to confirm the correlation between smoking history and co-existent PTB and lung cancer.
In addition to the small sample size, this study has several potential limitations: Firstly, only patients who had adenocarcinoma of the lung were included in the study, because this is a retrospective study in which selection bias is inevitable; Secondly, patients included in this study were highly heterogeneous in terms of molecular and clinical characteristics, such as patients receiving first- and second-line EGFR-TKI, patients with common and uncommon/complex mutations, and patients treated with different generation of EGFR-TKI; Thirdly, due to the limited sample size, whether PTB was a risk factor affecting the efficacy of EGFR-TKI was not analyzed in this study; Fourthly, only limited information was provided on the impact of anti-TB therapy on targeted therapy.
In conclusion, findings from the study showed that PFS and OS were numerically lower in patients with co-existent PTB and LUAD, and PTB affected the response of patients with lung cancer to EGFR-TKIs treatment. Furthermore, the number of metastases and the subtypes of EGFR mutations can help distinguish the beneficial population. However, the results of this study need to be further verified due to the small sample size. In addition, the drug resistance mechanism of EGFR-TKIs in advanced LUAD with PTB needs further exploration.
Data Sharing Statement
Author elects to not share data. Data are not shared because research is still underway.
Ethical Approval Statement
Guangzhou chest hospital Research Ethics Board granted ethics approval for this study [Chest Ethics (2021) 4].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There was no funding for this study.
Disclosure
Weiwei Li and Zhan Huang were employed by Amoy Diagnostics Co., Ltd. The authors report no other potential conflicts of interest relevant to this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Zhang L, Bai L, Liu X, et al. Factors related to rapid progression of non-small cell lung cancer in Chinese patients treated using single-agent immune checkpoint inhibitor treatment. Thorac Cancer. 2020;11(5):1170–1179. doi:10.1111/1759-7714.13370
3. Hu Y, Yang X, Nie L, Zhao D, An J, Li B. [Analysis of clinical characteristics and driver genes in 405 patients with lung cancer complicated with tuberculosis]. Zhongguo Fei Ai Za Zhi. 2020;23(5):337–342. Chinese. doi:10.3779/j.issn.1009-3419.2020.101.25
4. Yu YH, Liao CC, Hsu WH, et al. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol. 2011;6(1):32–37. doi:10.1097/JTO.0b013e3181fb4fcc
5. Nalbandian A, Yan BS, Pichugin A, Bronson RT, Kramnik I. Lung carcinogenesis induced by chronic tuberculosis infection: the experimental model and genetic control. Oncogene. 2009;28(17):1928–1938. doi:10.1038/onc.2009.32
6. Fu W, Sun H, Zhao Y, et al. BCAP31 drives TNBC development by modulating ligand-independent EGFR trafficking and spontaneous EGFR phosphorylation. Theranostics. 2019;9(22):6468–6484. doi:10.7150/thno.35383
7. Hwang IK, Paik SS, Lee SH. Impact of pulmonary tuberculosis on the EGFR mutational status and clinical outcome in patients with lung adenocarcinoma. Cancer Res Treat. 2019;51(1):158–168. doi:10.4143/crt.2018.084
8. Yu HA, Arcila ME, Hellmann MD, Kris MG, Ladanyi M, Riely GJ. Poor response to erlotinib in patients with tumors containing baseline EGFR T790M mutations found by routine clinical molecular testing. Ann Oncol. 2014;25(2):423–428. doi:10.1093/annonc/mdt573
9. Mu W, Jiang L, Zhang J, et al. Non-invasive decision support for NSCLC treatment using PET/CT radiomics. Nat Commun. 2020;11(1):5228. doi:10.1038/s41467-020-19116-x
10. Woodruff PG, Wolff M, Hohlfeld JM, et al. Safety and efficacy of an inhaled epidermal growth factor receptor inhibitor (BIBW 2948 BS) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(5):438–445. doi:10.1164/rccm.200909-1415OC
11. Vallath S, Hynds RE, Succony L, Janes SM, Giangreco A. Targeting EGFR signalling in chronic lung disease: therapeutic challenges and opportunities. Eur Respir J. 2014;44(2):513–522. doi:10.1183/09031936.00146413
12. Luo YH, Wu CH, Wu WS, et al. Association between tumor epidermal growth factor receptor mutation and pulmonary tuberculosis in patients with adenocarcinoma of the lungs. J Thorac Oncol. 2012;7(2):299–305. doi:10.1097/JTO.0b013e31823c588d
13. Swaisland HC, Ranson M, Smith RP, et al. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin Pharmacokinet. 2005;44(10):1067–1081. doi:10.2165/00003088-200544100-00005
14. Dickinson PA, Cantarini MV, Collier J, et al. Metabolic disposition of osimertinib in rats, dogs, and humans: insights into a drug designed to bind covalently to a cysteine residue of epidermal growth factor receptor. Drug Metab Dispos. 2016;44(8):1201–1212. doi:10.1124/dmd.115.069203
15. Vishwanathan K, Dickinson PA, So K, et al. The effect of itraconazole and rifampicin on the pharmacokinetics of osimertinib. Br J Clin Pharmacol. 2018;84(6):1156–1169. doi:10.1111/bcp.13534
16. Chang CH, Lee CH, Ho CC, Wang JY, Yu CJ. Gender-based impact of epidermal growth factor receptor mutation in patients with nonsmall cell lung cancer and previous tuberculosis. Medicine. 2015;94(4):e444. doi:10.1097/MD.0000000000000444
17. Christopoulos A, Saif MW, Sarris EG, Syrigos KN. Epidemiology of active tuberculosis in lung cancer patients: a systematic review. Clin Respir J. 2014;8(4):375–381. doi:10.1111/crj.12094
18. Silva DR, Valentini DF
19. Cicenas S, Vencevicius V. Lung cancer in patients with tuberculosis. World J Surg Oncol. 2007;5:22. doi:10.1186/1477-7819-5-22
20. Shiels MS, Albanes D, Virtamo J, Engels EA. Increased risk of lung cancer in men with tuberculosis in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2011;20(4):672–678. doi:10.1158/1055-9965.EPI-10-1166
21. Oh CM, Roh YH, Lim D, et al. Pulmonary tuberculosis is associated with elevated risk of lung cancer in Korea: the Nationwide Cohort Study. J Cancer. 2020;11(7):1899–1906. doi:10.7150/jca.37022
22. Evman S, Baysungur V, Alpay L, et al. Management and surgical outcomes of concurrent tuberculosis and lung cancer. Thorac Cardiovasc Surg. 2017;65(7):542–545. doi:10.1055/s-0036-1583167
23. Zhang J, Iwanaga K, Choi KC, et al. Intratumoral epiregulin is a marker of advanced disease in non-small cell lung cancer patients and confers invasive properties on EGFR-mutant cells. Cancer Prev Res. 2008;1(3):201–207. doi:10.1158/1940-6207.CAPR-08-0014
24. Choi CM, Kim MY, Lee JC, Kim HJ. Advanced lung adenocarcinoma harboring a mutation of the epidermal growth factor receptor: CT findings after tyrosine kinase inhibitor therapy. Radiology. 2014;270(2):574–582. doi:10.1148/radiol.13121824
25. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi:10.1056/NEJMoa0909530
26. Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol. 2013;24(9):2371–2376. doi:10.1093/annonc/mdt205
27. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi:10.1056/NEJMoa1913662
28. Hong S, Gao F, Fu S, et al. Concomitant genetic alterations with response to treatment and epidermal growth factor receptor tyrosine kinase inhibitors in patients with EGFR-mutant advanced non-small cell lung cancer. JAMA Oncol. 2018;4(5):739–742. doi:10.1001/jamaoncol.2018.0049
29. Cao S, Li J, Lu J, Zhong R, Zhong H. Mycobacterium tuberculosis antigens repress Th1 immune response suppression and promotes lung cancer metastasis through PD-1/PDl-1 signaling pathway. Cell Death Dis. 2019;10(2):44. doi:10.1038/s41419-018-1237-y
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


