Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
COMT Val158Met Polymorphism Influences the Cerebral Blood Flow Changes Related to Psychomotor Retardation in Major Depressive Disorder
Authors Yin Y , Xie C, Zhang H, Zhang H, Zhang Z, Yuan Y
Received 29 June 2022
Accepted for publication 14 September 2022
Published 25 September 2022 Volume 2022:18 Pages 2159—2169
DOI https://doi.org/10.2147/NDT.S379146
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yingying Yin,1,2 Chunming Xie,3 Haisan Zhang,4 Hongxing Zhang,5 Zhijun Zhang,3 Yonggui Yuan1,2
1Department of Psychosomatics and Psychiatry, ZhongDa Hospital, School of Medicine, Southeast University, Nanjing, 210009, People’s Republic of China; 2Institute of Psychosomatics, Medical School of Southeast University, Nanjing, 210009, People’s Republic of China; 3Department of Neurology, ZhongDa Hospital, School of Medicine, Southeast University, Nanjing, Jiangsu, 210009, People’s Republic of China; 4Departments of Clinical Magnetic Resonance Imaging, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, Henan, 453002, People’s Republic of China; 5Departments of Psychiatry, the Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, Henan, 453002, People’s Republic of China
Correspondence: Yonggui Yuan; Yingying Yin, Department of Psychosomatics and Psychiatry, ZhongDa Hospital, School of Medicine, Southeast University, No. 87 Dingjiaqiao Road, Nanjing, 210009, People’s Republic of China, Email [email protected]; [email protected]
Background: Previous studies revealed different cerebral blood flow (CBF) changes of major depressive disorder (MDD) patients with psychomotor retardation (PMR). These different changes might result from the modulation of other factors, such as genes. This study aimed to investigate the influence of COMT Val158Met polymorphism on the CBF alterations in MDD patients with PMR.
Methods: COMT Val158Met genotypes and arterial spin labeling-magnetic resonance imaging (ASL-MRI) data of 103 Chinese Han participants (63 MDD, 40 NCs) were collected in this study. MDD patients were divided into PMR group (N = 23) and NPMR group (N = 40) according to the Salpetriere Retardation Rating Scale score. PMR, NPMR and NCs groups were further divided into two subgroups, respectively, based on the COMT Val158Met genotype. CBF throughout the whole brain was calculated based on the ASL-MRI data. A two-way factorial analysis of covariance was used to investigate the main effects of PMR, COMT Met allele, as well as the interactions between COMT genotype and PMR on the CBF in a voxel-wise manner. Partial correlation analyses were also applied to evaluate the association between the CBF of significant brain regions and the PMR severity.
Results: Main effect of PMR mainly influenced the CBF of the prefrontal cortex (PFC). Main effect of COMT Met allele mainly influenced the CBF of the thalamus. The interaction between PMR and COMT Met allele primarily influenced the CBF of left precuneus and right caudate. The CBF of PFC was positively correlated with the PMR severity.
Conclusion: Our findings indicate that the COMT Met allele could modulate the CBF changes of the left precuneus and right caudate in MDD patients with PMR, providing additional layer of information regarding earlier reports for different CBF changes of MDD patients with psychomotor retardation in the literature, which were assessed irrespective of polymorphisms among patients.
Keywords: major depressive disorder, psychomotor retardation, ASL, cerebral blood flow, COMT Val158Met polymorphism
Introduction
Previous studies revealed cerebral blood flow (CBF) changes of major depressive disorder (MDD) patients with psychomotor retardation (PMR). The decreased CBF of prefrontal cortex (PFC) was consistently reported associated with PMR,1–5 while other findings, such as decreased CBF in basal ganglia or precuneus, were not well replicated.6,7 Recent studies indicated that the psychomotor mechanism is primarily related to the dopaminergic-based subcortical–cortical motor circuit.8 There are complex interactions between serotonin and dopamine and their effects on intrinsic brain activity.9 Therefore, the different CBF changes might result from the complex modulation of other factors. The dopamine dysfunction in MDD patients with PMR has been widely reported. Evidence from single-photon emission computed tomography (SPECT) and positron emission tomography (PET) studies10,11 both found that decreased dopamine function in the striatum is associated with PMR. A PET study also observed an elevation of D2 binding in bilateral putamen in MDD patients with PMR.12 Here, we deduced that dopaminergic polymorphisms might modulate the CBF changes related to PMR.
Catechol-O-methyltransferase (COMT) catalyzes the degradation of synaptic dopamine in the brain. The COMT gene is located at chromosome 22q11 and contains a G-to-A transition in codon 158, which converts a valine (val) high-activity allele to a methionine (met) low-activity allele (Val158Met).13 COMT Val158Met polymorphism significantly influences the expression level of COMT, the crucial enzyme modulating the degradation of synaptic dopamine. The enzymatic activity of Met-allele carriers decreases fourfold than Val&Val genotype carriers.14 Previous studies have found an association between the high activity Val allele and MDD,15 and COMT Val158Met polymorphism could influence healthy subjects’ brain structure and function.16–22 In addition, a few studies also reported the influences of the polymorphism on brain function,23 gray matter volume,24 and white matter connectivity.25
As a dopamine-driven symptom of MDD, PMR is likely to be associated with COMT Val158Met polymorphism. However, no study has reported the effect of the polymorphism on brain dysfunction of PMR in MDD. This study aimed to investigate the influence of COMT Val158Met polymorphism on the cerebral blood flow (CBF) changes in MDD patients with PMR. Sixty-three MDD patients who were divided into PMR and non-PMR (NPMR) groups, and forty normal controls (NCs) were collected in this study. CBF throughout the whole brain was calculated based on the arterial spin labeling-magnetic resonance imaging (ASL-MRI) data. We hypothesized that COMT Val158Met polymorphism might influence the CBF changes of basal ganglia in MDD patients with PMR.
Materials and Methods
The experimental protocols were approved by the medical ethics committee for clinical research of ZhongDa Hospital affiliated to Southeast University (Number of the Ethical approval letter: 2018ZDSYLL154-P01). The methods were performed in accordance with the guidelines of the Declaration of Helsinki. All subjects gave written informed consent for participation.
Subjects
Sixty-three antidepressant-free MDD inpatients from the Second Affiliated Hospital of Xinxiang Medical University were recruited in the current study during August 2013 to December 2014. Forty NCs were recruited from the local community through advertising and contacts in the community. The details of the inclusion and exclusion criteria were described in the following sections. All subjects were of the Chinese Han population.
Diagnosis of MDD was confirmed using a Structured Interview according to the DSM-IV (SCID) by two trained psychiatrists (Yingying Yin and Hongxing Zhang). Depression severity was quantified using the 24-item Hamilton Depression Rating Scale (HDRS).26 The anxiety symptoms were assessed by Hamilton Anxiety Rating Scale (HARS).27 Physical, neurological and psychiatric examinations were also carefully performed to ascertain that the inclusion and exclusion criteria are strictly adhered to during the recruitment process. Fresh blood samples were collected in the early morning of the day after admission for genetic testing. MRI scanning of the whole brain was carried out within three days of admission for CBF calculation. Psychological assessments were performed on the same day with MRI scanning.
For the antidepressant-free MDD patients, the inclusion criteria included:
- age is from 18 to 60 years;
- right-handed;
- current duration of illness must be at least 2 weeks;
- HDRS-24 total score ≥18.
The exclusion criteria included:
- another major psychiatric illness along with MDD;
- current or long-term alcohol, drug dependence or smoking;
- Serious physical ailments, primary neurological illness, organic brain disease (eg, former stroke, cerebral vascular malformations, or epilepsy), former brain injury;
- endocrine disorders such as hypertension, diabetes, thyroid dysfunction, etc.;
- antidepressant treatment within 4 weeks prior to the beginning of study enrollment;
- Contraindications for MRI scanning such as pacemakers, defibrillators or other implanted electronic devices;
- cerebral infarction or cerebrovascular injury according to T2 weighted images.
For NCs, inclusion criteria included:
- age is from 18 to 60 years;
- right-handed;
- HDRS-24 total score <8.
Exclusion criteria included:
- onset history or family history of mental disorders such as schizophrenia, mood disorder, alcohol/smoking addiction, etc.;
- serious physical illness, organic brain disease (eg, former stroke, cerebral vascular malformations, or epilepsy), former brain injury;
- endocrine disorders such as hypertension, diabetes, thyroid dysfunction, etc.;
- contraindications for MRI scanning such as pacemakers, defibrillators or other implanted electronic devices;
- cerebral infarction or cerebrovascular injury according to T2 weighted images.
The PMR manifestation was measured using Salpetriere Retardation Rating Scale (SRRS),28,29 with a cut-off score of 20 defining the presence of PMR.30 The SRRS gauges different manifestations of retardation such as slowed gait, gross and facial movements, speech and thought. It has been validated by a wealth of studies.30–32 Specifically, the twenty-three MDD patients (N = 23) with SRRS scores ≥20 were considered as having PMR and grouped as such, while those patients (N = 40) with a SRRS score <20 were categorized as free from PMR and grouped as NPMR patients. In addition, Trail Making Test (TMT)-A and B, a frequently used neurocognitive drawing test, which can measure attentive and setshifting processes was also applied for evaluating the PMR. It is considered as a good indicator of PMR.33,34
MRI Acquisition
MRI was scanned using a Siemens 3.0 Tesla scanner with a 12-channel head coil in the Department of Clinical Magnetic Resonance Imaging at the Second Affiliated Hospital of Xinxiang Medical University. The head was stabilized with cushion to minimize head motion. Earplugs were used to reduce scanner noise. High-resolution 3-dimensional T1-weighted scans were recorded as magnetization prepared rapid gradient echo (MPRAGE) sequence (repetition time (TR) = 1900 ms, echo time (TE) = 2.48 ms; flip angle (FA) = 9°; acquisition matrix = 256 × 256; field of view (FOV) = 250 × 250 mm2; thickness = 1.0 mm, gap = 0; time = 4 minutes 18 seconds). ASL perfusion MRI was performed using the Siemens product Pulsed Arterial Spin Labeling (PASL) PICORE Q2T sequence (TR = 4000ms, TE = 12ms, TI1 = 600ms, TI2 = 1600ms, FA = 90°, matrix = 64 × 64, FOV = 220 × 220mm2, 27 axial slices, thickness = 4mm, gap = 1mm, time = 7 minutes 14 seconds). During R-fMRI, participants were instructed to lie still in the scanner, keep their eyes open, and refrain from falling asleep.
Image Quality Control and Processing
T1 images were manually checked by two experienced radiologists (Chunming Xie and Haisan Zhang) for quality controls, and artifacts were removed before preprocessing.
PASL data were preprocessed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm) based batch scripts in ASLtbx35 with the following steps: motion correction, temporal filtering, spatial smoothing with an isotropic Gaussian kernel (full width half maximum = 6mm), coregistration, and normalization. The amended algorithm was used for PASL image motion corrections.36 High pass filtering (cutoff = 0.5) was used for temporal PASL image denoising. Residual motions and global signal were regressed out from the control/label PASL images. CBF images were then generated using the model from an earlier published paper.37
Genotyping
Fresh blood samples were obtained from all subjects and DNA subsequently extracted. Genomic DNA was extracted by using the TIANamp genomic DNA kit. Genotyping was performed by using second-generation sequencing – HiSeq Sequencer (Illumina, Inc., San Diego, CA), following the manufacturer’s standard sequencing protocols. A 3.5Mb region containing 689 genes and 337 SNPs, which was associated with MDD, was sequenced. The sequenced region covered the COMT Val158Met loci. Based on COMT Val158Met genotype (Met-allele carriers (Met+) and Val/Val homozygote carriers (Met-)), PMR, NPMR and NCs groups were divided into two subgroups, respectively (ie, PMR Met-(n = 7); PMR Met+(n = 16); NPMR Met-(n = 20); NPMR Met+(n = 20); NCs Met-(n = 18); NCs Met+(n = 22)).
Statistical Analysis
Group differences displayed in Table 1 were assessed using SPSS 22.0 software (SPSS, Inc., Chicago, IL), and P values <0.05 were reported as statistically significant. Participants were divided into six groups according to the combination of genotype (Val/Val individuals and Met carriers) × disease (NCs, NPMR, and PMR). Continuous variables (age, body mass index (BMI), education, age of onset, total duration and current duration of disease, onset frequency and neuropsychological tests) were compared by analysis of variance (ANOVA), with post hoc tests using Tukey–Kramer analyses. Gender comparison was performed with Chi-square test. Hardy–Weinberg Equilibrium of the genotype frequencies was tested using Chi-square tests.
 |
Table 1 Demographic and Neuropsychological Data |
Main effects of PMR, COMT Met allele, as well as the interactions between COMT genotype and PMR on the CBF were analyzed in a voxel-wise manner throughout the whole brain using two-way factorial analysis of covariance (ANCOVA: PMR×COMT Met allele) with AlphaSim correction (single voxel P value = 0.01, FWHM = 6 mm, with 91 × 109×91 mm3 whole-brain mask, cluster sizes >1016 mm3), with age, BMI, education and HDRS score as the covariates.
Partial correlation analyses were also applied to evaluate the association between the CBF of significant brain regions and the PMR severity (SRRS and TMT scores), controlling age, BMI, education and HDRS score as the covariates.
Results
Demographic and Clinical Data
The demographic and clinical characteristics of 103 subjects in six groups are summarized in Table 1. Demographic characteristics such as gender, age, education, and BMI were well matched for the six groups. The clinical characteristics, such as age of onset, total duration, current duration, onset frequency, showed no significant difference between the four MDD groups (ie, PMR Met-, PMR Met+, NPMR Met-, NPMR Met+). The PMR group displayed more severe depression and retardation (ie, higher scores of HDRS and SRRS assessment) than the NPMR group.
Genotype and Allele Distribution
The COMT Val158Met genotypes were similarly distributed between PMR, NPMR and NCs subjects (χ2= 2.32, P = 0.31) and were in Hardy–Weinberg equilibrium (χ2= 0.31, P = 0.58 for PMR group; χ2= 0.38, P = 0.54 for NPMR group; χ2= 0.44, P = 0.50 for NCs group).
To eliminate the gender influence on the genotype distribution, the allele distribution was also compared between male and female subjects. As a result, there was no significant difference between male and female subjects in the distribution of COMT Val158Met genotypes (χ2= 0.06, P = 0.81).
Main Effects of PMR on the CBF of the Whole Brain
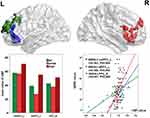
The main effect of PMR mainly influenced the CBF of bilateral PFC (Figure 1). The CBF of bilateral PFC in the PMR group was significantly increased when compared with the NCs and NPMR group (the histograms in Figure 1 and see Table 2 for details). Partial correlation analyses between the prefrontal CBF and PMR severity revealed a significant positive correlation (the scatter diagram in Figure 1).
 |
Table 2 The Influences of PMR, COMT Met allele and Their Interaction on the CBF of the Whole Brain |
Main Effects of COMT Met allele on the CBF of the Whole Brain
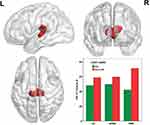
The main effect of COMT Met allele mainly influenced the CBF of bilateral thalamus (Figure 2). The CBF of bilateral thalamus in the Met allele carriers was significantly increased (the histograms in Figure 2 and see Table 2 for details). Partial correlation analyses between the CBF of thalamus and PMR severity revealed no significant correlation.
Interaction of PMR and COMT Met allele on the CBF of the Whole Brain
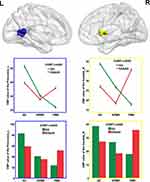
The interaction between PMR and COMT Met allele primarily influenced the CBF of left precuneus and right caudate (Figure 3). The Met allele leads to a deceased CBF in NCs and NPMR group but an increased CBF in PMR group (the histograms in Figure 3 and see Table 2 for details). Partial correlation analyses between the CBF of left precuneus, right caudate and PMR severity revealed no significant correlation.
Discussion
This study aimed to investigate the influence of COMT Val158Met polymorphism on the CBF in MDD patients with PMR. We hypothesized that COMT Val158Met polymorphism might influence the CBF of basal ganglia in MDD patients with PMR. The results demonstrated that the interaction of PMR and COMT Val158Met polymorphism primarily influenced the CBF of left precuneus and right caudate. The Met allele led to deceased CBF in NCs and NPMR group but increased CBF in PMR group. The finding supported our hypothesis, which might be interpreted in the following two aspects.
First, a previous study reported psychomotor slowing was negatively correlated with frontal perfusion,1 which was well replicated by subsequent studies.2–4 These researches consistently found that the CBF of PFC had a strong negative correlation with PMR in MDD patients. These concordant results demonstrate that the decreased frontal perfusion of MDD patients with PMR is steady and not influenced by other factors. A growing body of evidence supported that hypofrontality was associated with negative symptoms in MDD2 and indicated that frontal hypoperfusion or dysfunction was directly expressed as PMR. The current study did not find a role for the Met allele in the changes in frontal perfusion related to PMR. We also found that the main effect of PMR mainly influenced the CBF of bilateral PFC.
However, the prefrontal perfusion of PMR group was increased, and it is positively correlated with the PMR severity. Our results also conflicted with a previous study investigating the relationship between activity level and CBF of MDD patients using ASL-MRI method.38 Walther et al reported positive associations between activity level and CBF in the right orbitofrontal cortex (OFC), indicating negative associations between CBF in OFC and PMR severity. There may be several causes which led to the conflict results. The primary reason should be the different testing procedures. Most of the literature studies used PET or SPECT method, which was restricted to the region of interest analyses with low spatial resolution. The current study used MRI-based PASL with improved spatial resolution, which could get more accurate results. Although Walther et al also applied PASL technology, the method to measure their motor activity was different from ours. They recorded the activity level by wrist actigraphy for 24 h and entered the activity level into a whole-brain general linear model to investigate the association between objective motor activity and the CBF. However, we used SRRS score to evaluate the PMR severity, including different manifestations of retardation such as slowed gait, gross and facial movements, speech and thought. Another reason causing our results different from others might be the sample size. The sample size of previous studies was relatively smaller than ours. Due to the invasive side-effect from external contrast agents in PET or SPECT studies, the sample size was usually restricted to less than 20. Even in the MRI-ASL study of Walther et al, there were only 20 MDD patients. Thus, the sample size of previous studies was relatively smaller than ours, with of 63 diagnosed with MDD.
Last but not least, the reason is the complex regulatory mechanism of the PFC. Recent studies described how the sensorimotor network (SMN) and related motor function are modulated by default-mode network (DMN).39 Psychomotor function regulated SMN was oppositely modulated by DMN.40 Hyperactivity of DMN and hypoactivity of SMN could induce PMR.41 As the primary hubs of DMN, the increased activity of medial and lateral PFC in our study was just verified the recent discovery. The abnormal CBF in PFC was concordant. PFC is involved in the control of daily life activities42 and the generation of intentional and imitative motor acts.43 Anatomically, the PFC has white matter fiber connecting the primary motor area, supplementary motor cortex, and supplementary motor cortex44 to make up the motor circuit. Thus, the function of PFC is involved in many aspects of mental disorders, such as regulating mood, cognition, and movement, and others. It is pertinent to note that every neuroimaging study investigating mental disorders could find a dysfunction of PFC. Therefore, the concordant finding of structural and functional changes in PFC of previous studies and ours intensively demonstrated the primary role of PFC in various mental disorders.
Second, as a critical hub of the motor circuitry, basal ganglia is widely postulated to have functional or structural abnormalities in MDD patients with PMR. However, studies that found a negative correlation between PMR and the CBF of basal ganglia were not well replicated. We hypothesized that dopaminergic polymorphism might influence the CBF changes of basal ganglia, because the dopaminergic dysfunction resulted in some kinds of dyskinesia.45 In this study, we found that the COMT Val158Met polymorphism influenced the CBF of left precuneus and right caudate, which supported our hypothesis and demonstrated that genetic polymorphisms might modulate the CBF of basal ganglia and precuneus. The dopaminergic dysfunction in basal ganglia of MDD patients with PMR was well demonstrated in previous studies. For example, dopamine D5/6 ligand binding in the striatum was significantly correlated with both the measures of reaction time and verbal fluency.10 Besides, lower presynaptic dopamine in the left caudate11 and higher D2 binding potential in bilateral putamen12 have been described in patients with PMR. As the crucial enzyme modulating the degradation of synaptic dopamine, the COMT is thought to play a key role in clearing dopamine in the cortex. The G-to-A transition in codon 158 (Val158Met) is a functional polymorphism, which could modulate the cerebral COMT level and further influence brain structure and function. Specifically, Val homozygote carriers exhibited significantly smaller temporal lobe and hippocampal volumes,16,19 greater gray matter volume of the prefrontal cortex,17 and decreased prefrontal connectivities with the posterior cingulate/retrosplenial cortices.22
In contrast, Met carriers were associated with increased tissue volume of the hippocampus17 and amygdala,21 and thicker cortex in the right superior temporal sulcus as well as inferior prefrontal sulcus.18 Our study is the first to focus on the effect of COMT Val158Met polymorphism on the CBF of MDD patients with PMR, and we found that the Met allele led to a decrease in CBF of the left precuneus and right caudate in NCs and NPMR patients. Thus, this is a novel finding which supports the hypothesis of dopaminergic dysfunction in basal ganglia of MDD patients with PMR from the genetic view.
In addition, we found that the main effect of COMT Met allele primarily influences the CBF of the thalamus. The CBF of thalamus in the Met allele carriers was significantly increased. However, this study had no correlation between the thalamus and PMR. The thalamus is a critical component of the frontal cortical-basal ganglia-thalamic circuits that mediate motivation and emotional drive, planning and cognition to express goal-directed behaviors.46 Evidence suggests that dopamine signaling enhances thalamo-SMN coupling and SMN activity,8 which could interpret our findings perfectly. Another study found that psychomotor agitation exhibited increased thalamo-SMN connectivity,47 which suggested that the thalamus played an indirect role in psychomotor balance through regulating SMN rather than a direct component associated with psychomotor function.
There are several limitations in this study need to be mentioned. First of all, although the sample size of this study is moderate, we divided the participants into six groups according to the COMT Val158Met genotype, which resulted in a relatively small sample size in the statistical groups. For example, there are only 7 subjects in the PMR Met- group. Nevertheless, the minimum theoretical frequency for the six group is >5 and the total sample size is >40, so the statistical results are not biased, and the test’s power is tolerable. Second, although pASL can noninvasively measure blood flow and has gained increasing attention because of its straightforward implementation and the high stable tagging efficiency with respect to flow velocity, its signal-to-noise ratio (SNR) is relatively lower. A new ASL sequence, pseudocontinuous ASL (pcASL), could provide a better balance between tagging efficiency and SNR. Unfortunately, this technical limitation could not be solved in this batch of data, because our scanner would overload when we used pcASL sequences. It is hereby suggested that future studies with pcASL sequences need to be conducted to replicate our results. In addition, our previous study, which investigated the CBF changes underlying PMR from both cross-sectional and longitudinal comparisons, demonstrated that PMR was associated with decreased CBF in the right primary motor cortex (PMC). However, in our study, the main effect of PMR on CBF was found in the PFC rather than PMC. The discrepant results mainly come from the different statistical approaches. The two-way ANCOVA we used in this study considered two factors that might influence the CBF, and the main effect of every factor was extracted separately, so the main effect of PMR on CBF removed the impaction of another confounding factor. The CBF changes in PMC might be influenced by factors, such as different genomic methylation, which we have not considered. Therefore, further studies need to investigate more factors and refine the effect of every factor.
Conclusion
In conclusion, our findings indicate that the COMT Met allele could modulate the CBF changes of the left precuneus and right caudate in MDD patients with PMR, providing additional layer of information regarding earlier reports for different CBF changes of MDD patients with psychomotor retardation in the literature, which were assessed irrespective of polymorphisms among patients.
Ethics Approval and Consent to Participate
The study protocol was approved by the medical ethics committee for clinical research of ZhongDa Hospital affiliated to Southeast University. The methods were performed in accordance with approved guidelines. Written informed consent was available for all participants.
Acknowledgments
We wish to thank all the participants in this study as well as all those who provided financial support.
Funding
This work was supported by the National Natural Science Foundation of China (81971277, Y. Yuan; 81801349, Y. Yin) and the Program for one thousand Zhongyuan Talents (204200510020, H.X. Zhang) for data collection, the Natural Science Foundation of Jiangsu Province (BK20180373, Y. Yin) for the analysis and interpretation of data, and Jiangsu Provincial Key Research and Development Program (BE2019748, Y. Yuan) for the paper’s publication. The funding program provides capital for the researchers to do the data collection, analysis, interpretation, and the publication of the paper.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Mayberg HS, Lewis PJ, Regenold W, Wagner HN. Paralimbic hypoperfusion in unipolar depression. J Nucl Med. 1994;35(6):929–934.
2. Galynker II, Cai J, Ongseng F, et al. Hypofrontality and negative symptoms in major depressive disorder. J Nucl Med. 1998;39(4):608–612.
3. Videbech P, Ravnkilde B, Pedersen TH, et al. The Danish PET/depression project: clinical symptoms and cerebral blood flow. A regions-of-interest analysis. Acta Psychiatr Scand. 2002;106(1):35–44. doi:10.1034/j.1600-0447.2002.02245.x
4. Narita H, Odawara T, Iseki E, et al. Psychomotor retardation correlates with frontal hypoperfusion and the Modified Stroop Test in patients under 60-years-old with major depression. Psychiatry Clin Neurosci. 2004;58(4):389–395. doi:10.1111/j.1440-1819.2004.01273.x
5. Brody AL, Barsom MW, Bota RG, et al. Prefrontal-subcortical and limbic circuit mediation of major depressive disorder. Semin Clin Neuropsychiatry. 2001;6(2):102–112. doi:10.1053/scnp.2001.21837
6. Naismith S, Hickie I, Ward PB, et al. Caudate nucleus volumes and genetic determinants of homocysteine metabolism in the prediction of psychomotor speed in older persons with depression. Am J Psychiatry. 2002;159(12):2096–2098. doi:10.1176/appi.ajp.159.12.2096
7. Bench CJ, Friston KJ, Brown RG, et al. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychol Med. 1993;23(3):579–590. doi:10.1017/S0033291700025368
8. Martino M, Magioncalda P, Conio B, et al. Abnormal functional relationship of sensorimotor network with neurotransmitter-related nuclei via subcortical-cortical loops in manic and depressive phases of bipolar disorder. Schizophr Bull. 2020;46(1):163–174. doi:10.1093/schbul/sbz035
9. Conio B, Martino M, Magioncalda P, et al. Opposite effects of dopamine and serotonin on resting-state networks: review and implications for psychiatric disorders. Mol Psychiatry. 2020;25(1):82–93. doi:10.1038/s41380-019-0406-4
10. Shah PJ, Ogilvie AD, Goodwin GM, et al. Clinical and psychometric correlates of dopamine D2 binding in depression. Psychol Med. 1997;27(6):1247–1256. doi:10.1017/S0033291797005382
11. Martinot M, Bragulat V, Artiges E, et al. Decreased presynaptic dopamine function in the left caudate of depressed patients with affective flattening and psychomotor retardation. Am J Psychiatry. 2001;158(2):314–316. doi:10.1176/appi.ajp.158.2.314
12. Meyer JH, McNeely HE, Sagrati S, et al. Elevated putamen D 2 receptor binding potential in major depression with motor retardation: an [11 C] raclopride positron emission tomography study. Am J Psychiatry. 2006;163(9):1594–1602. doi:10.1176/ajp.2006.163.9.1594
13. Matsumoto M, Weickert CS, Akil M, et al. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003;116(1):127–137. doi:10.1016/S0306-4522(02)00556-0
14. Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51(4):593–628.
15. Massat I, Souery D, Del-Favero J, et al. Association between COMT (Val158Met) functional polymorphism and early onset in patients with major depressive disorder in a European multicenter genetic association study. Mol Psychiatry. 2005;10(6):598–605. doi:10.1038/sj.mp.4001615
16. Taylor WD, Züchner S, Payne ME, et al. The COMT Val158Met polymorphism and temporal lobe morphometry in healthy adults. Psychiatry Res. 2007;155(2):173–177. doi:10.1016/j.pscychresns.2007.01.005
17. Cerasa A, Gioia MC, Labate A, et al. Impact of catechol-O-methyltransferase Val(108/158) Met genotype on hippocampal and prefrontal gray matter volume. Neuroreport. 2008;19(4):405–408. doi:10.1097/WNR.0b013e3282f5f784
18. Cerasa A, Cherubini A, Quattrone A, et al. Met158 variant of the catechol-O-methyltransferase genotype is associated with thicker cortex in adult brain. Neuroscience. 2010;167(3):809–814. doi:10.1016/j.neuroscience.2010.02.040
19. Honea R, Verchinski BA, Pezawas L, et al. Impact of interacting functional variants in COMT on regional gray matter volume in human brain. Neuroimage. 2009;45(1):44–51. doi:10.1016/j.neuroimage.2008.10.064
20. Li J, Yu C, Li Y, et al. COMT val158met modulates association between brain white matter architecture and IQ. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):375–380. doi:10.1002/ajmg.b.30825
21. Ehrlich S, Morrow EM, Roffman JL, et al. The COMT Val108/158Met polymorphism and medial temporal lobe volumetry in patients with schizophrenia and healthy adults. Neuroimage. 2010;53(3):992–1000. doi:10.1016/j.neuroimage.2009.12.046
22. Liu B, Song M, Li J, et al. Prefrontal-related functional connectivities within the default network are modulated by COMT val158met in healthy young adults. J Neurosci. 2010;30(1):64–69. doi:10.1523/JNEUROSCI.3941-09.2010
23. Opmeer EM, Kortekaas R, van Tol M-J, et al. Influence of COMT val158met genotype on the depressed brain during emotional processing and working memory. PLoS One. 2013;8(9):e73290. doi:10.1371/journal.pone.0073290
24. Watanabe K, Kakeda S, Yoshimura R, et al. Relationship between the catechol-O-methyl transferase Val108/158Met genotype and brain volume in treatment-naive major depressive disorder: voxel-based morphometry analysis. Psychiatry Res. 2015;233(3):481–487. doi:10.1016/j.pscychresns.2015.07.024
25. Seok JH, Choi S, Lim HK, et al. Effect of the COMT val158met polymorphism on white matter connectivity in patients with major depressive disorder. Neurosci Lett. 2013;545:35–39. doi:10.1016/j.neulet.2013.04.012
26. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi:10.1136/jnnp.23.1.56
27. Schneider H, Esbitt S, Gonzalez JS. Hamilton Anxiety Rating Scale. Springer New York; 2013.
28. Widlăścher D, Hardy-Bayle M-C. Cognition and control of action in psychopathology. Curr Psychol Cogn. 1989;9(6):583–615.
29. Widlöcher D. Retardation: a basic emotional response. In: The Affective Disorders. Washington, DC: American Psychiatric Press; 1983:165–181.
30. Pier MP, Hulstijn W, Sabbe BG. No psychomotor slowing in fine motor tasks in dysthymia. J Affect Disord. 2004;83(2–3):109–120. doi:10.1016/j.jad.2004.05.002
31. Schrijvers D, Hulstijn W, Sabbe BG. Psychomotor symptoms in depression: a diagnostic, pathophysiological and therapeutic tool. J Affect Disord. 2008;109(1–2):1–20. doi:10.1016/j.jad.2007.10.019
32. Smith MJ, Brébion G, Banquet JP, et al. Retardation of mentation in depressives: posner’s covert orientation of visual attention test. J Affect Disord. 1995;35(3):107–115. doi:10.1016/0165-0327(95)00044-5
33. Buyukdura JS, McClintock SM, Croarkin PE. Psychomotor retardation in depression: biological underpinnings, measurement, and treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):395–409. doi:10.1016/j.pnpbp.2010.10.019
34. Gorwood P, Richard-Devantoy S, Baylé F, et al. Psychomotor retardation is a scar of past depressive episodes, revealed by simple cognitive tests. Eur Neuropsychopharmacol. 2014;24(10):1630–1640. doi:10.1016/j.euroneuro.2014.07.013
35. Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: aSLtbx. Magn Reson Imaging. 2008;26(2):261–269. doi:10.1016/j.mri.2007.07.003
36. Wang Z. Improving cerebral blood flow quantification for arterial spin labeled perfusion MRI by removing residual motion artifacts and global signal fluctuations. Magn Reson Imaging. 2012;30(10):1409–1415. doi:10.1016/j.mri.2012.05.004
37. Wang J, Zhang Y, Wolf RL, et al. Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: feasibility study. Radiology. 2005;235(1):218–228. doi:10.1148/radiol.2351031663
38. Walther S, Höfle O, Federspiel A, et al. Neural correlates of disbalanced motor control in major depression. J Affect Disord. 2012;136(1–2):124–133. doi:10.1016/j.jad.2011.08.020
39. Martino M, Magioncalda P, Huang Z, et al. Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc Natl Acad Sci U S A. 2016;113(17):4824–4829. doi:10.1073/pnas.1517558113
40. Northoff G, Heinzel A, Bermpohl F, et al. Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional-cognitive interaction. Hum Brain Mapp. 2004;21(3):202–212. doi:10.1002/hbm.20002
41. Northoff G, Hirjak D, Wolf RC, et al. All roads lead to the motor cortex: psychomotor mechanisms and their biochemical modulation in psychiatric disorders. Mol Psychiatry. 2021;26(1):92–102. doi:10.1038/s41380-020-0814-5
42. Krueger F, Moll J, Zahn R, et al. Event frequency modulates the processing of daily life activities in human medial prefrontal cortex. Cereb Cortex. 2007;17(10):2346–2353. doi:10.1093/cercor/bhl143
43. Babiloni C, Vecchio F, Bares M, et al. Functional coupling between anterior prefrontal cortex (BA10) and hand muscle contraction during intentional and imitative motor acts. Neuroimage. 2008;39(3):1314–1323. doi:10.1016/j.neuroimage.2007.09.043
44. Walther S, Hügli S, Höfle O, et al. Frontal white matter integrity is related to psychomotor retardation in major depression. Neurobiol Dis. 2012;47(1):13–19. doi:10.1016/j.nbd.2012.03.019
45. Calabresi P, Picconi B, Tozzi A, et al. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci. 2014;17(8):1022–1030. doi:10.1038/nn.3743
46. Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009;78(2–3):69–74. doi:10.1016/j.brainresbull.2008.09.013
47. Magioncalda P, Martino M, Conio B, et al. Intrinsic brain activity of subcortical-cortical sensorimotor system and psychomotor alterations in schizophrenia and bipolar disorder: a preliminary study. Schizophr Res. 2020;218:157–165. doi:10.1016/j.schres.2020.01.009
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.