Back to Journals » Drug Design, Development and Therapy » Volume 17
Computational Systems Pharmacology and Molecular Docking Reveal an Anti-Apoptosis and Anti-Inflammatory Mechanism of Compound Angelica Ligusticum Wallichii Granules in the Treatment of Endometriosis
Received 6 October 2022
Accepted for publication 27 January 2023
Published 9 March 2023 Volume 2023:17 Pages 743—759
DOI https://doi.org/10.2147/DDDT.S392500
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Yueyan Li, Jialei Zhu, Jing Tang
Department of Pharmacy, Obstetrics and Gynecology Hospital of Fudan University, Shanghai, 200090, People’s Republic of China
Correspondence: Jing Tang, Department of Pharmacy, Obstetrics and Gynecology hospital of Fudan University, 128 Shenyang Road, Yangpu District, Shanghai, 200090, People’s Republic of China, Tel/Fax +86 21 33180593, Email [email protected]
Background: Traditional medicine is a common treatment option for endometrioid-related symptoms. In the past few decades, Guixiong Xiaoyi formula has been widely used as a traditional medicine for the treatment of endometriosis.
Purpose: This study aimed to prepare compound Angelica Ligusticum wallichii granule (CALG) by modern technological methods and to study its pharmacodynamics and mechanisms of treating endometriosis.
Methods: The ingredients of CALG were determined by UPLC-Q-TOF/MS. Target prediction of compounds and diseases was performed using databases, and the mechanisms of CALG were predicted by Gene Ontology and Kyoto Encyclopedia of Genes and Genomes and verified by molecular docking. Furthermore, a rat model of endometriosis was established to study the effects of CALG on endometriosis in vivo.
Results: CALG with good specificity, durability, and stability was obtained following a detailed preparation process and quality control standard. Using network systems pharmacology, 109 chemical compositions and 104 core targets were identified for the treatment of endometriosis. The composition-target-channel-disease network topology analysis of the top 15 chemical compositions of CALG showed that the beneficial effect of CALG on endometriosis was attributed to phenolic compounds. In addition, CALG treatment reduced the volume of ectopic uterine lesions, promoted apoptosis, inhibited the secretion of inflammatory cytokines, and increased HIF-1 expression in rats with endometriosis.
Conclusion: CALG induces apoptosis and inhibits inflammation and is a promising drug for the treatment of endometriosis.
Keywords: endometriosis, Angelica Ligusticum wallichii granule, network systems pharmacology, molecular docking, traditional Chinese medicine
Graphical Abstract:
Introduction
Endometriosis is a common chronic disease in women of childbearing age, characterized by the presence of functional endometrial tissues outside the uterine cavity, especially in the ovaries, peritoneum, uterine and cervical ligaments, and rectovaginal sac.1 Approximately 20% to 50% of infertile women reportedly suffer from endometriosis, and approximately 30% to 50% of women with endometriosis are sub-fertile or infertile.2,3 Many factors, such as retrograde menstruation, estrogen, and inflammation contribute to the etiology of endometriosis.4 Conventional treatment for endometriosis is to lower estrogen levels throughout the body5,6 using drugs, including progesterone, androgen, gonadotropin-releasing hormone (GnRH) agonists, and aromatase inhibitors.7,8 However, these treatments do not produce satisfactory therapeutic effects, and are associated with considerable side effects.
In recent years, traditional medicine, also known as complementary and alternative medicine (CAM), has become a common treatment option for endometrioid-related symptoms.9 The diversity of chemical components in traditional Chinese medicine (TCM) provides a great advantage for the treatment of multi-factor diseases such as endometriosis by adopting the unique therapeutic principle of “Multi-component and multi-target”. In the past few decades, Guixiong Xiaoyi formula has been widely used as a traditional medicine for the treatment of endometriosis. Clinical studies have shown that Guixiong Xiaoyi formula is effective in relieving pelvic pain in patients with moderate-to-severe endometriosis, preventing the recurrence of endometriosis, and improving the pregnancy rate.10 Animal studies have shown that Guixiong Xiaoyi formula significantly inhibits cell proliferation, promotes ectopic endometrial cell apoptosis, and reduces the area of ectopic foci in rats.11
However, TCM is associated with some disadvantages, such as unstable quality, inconvenient decocting for patients, unreasonable simple decocting methods, incomplete extraction of effective components, and uncertain quality of decocting liquids. Therefore, performing quality control analysis qualitatively and quantitatively is important to ensure the safety and effectiveness of TCM.12 Guixiong Xiaoyi formula is the TCM prescription composed of several kinds of herbs. Compound Angelica Ligusticum wallichii granule (CALG) is a granule prepared by Guixiong Xiaoyi formula after extraction through standardized process. The drug dosage forms of the two are different, but the efficacy is the same. In order to ensure the standardization and consistency of the experimental medication, we subsequently conducted experiments with CALG. In this study, using modern pharmaceutical and quality control technology, we carried out a systematic study to establish a detailed pharmaceutical preparation process and quality control standards for the safety and effectiveness of CALG. We further investigated the mechanism of action underlying the effects of CALG on endometriosis using network systems pharmacological analysis, and in an experimental rat model of endometriosis.
Materials and Methods
Chemicals and Reagents
CALG was produced by the National Engineering and Research Center for TCM, Shanghai Traditional Chinese Medicine Technology Co. Ltd. Acetonitrile and methanol were from Merck (Darmstadt, Germany), and formic acid was from CNW (New York, USA). Reference substances of ferulic acid, paeoniflorin, and salvianolic acid B were from China National Institute for Food and Drug Control. Diphereline was from IPSEN pharma biotech (Paris, France), and Gestrinone capsules were from China Resources Zizhu Pharmaceutical Co., Ltd (Beijing, China). Estradiol (E2), tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF) enzyme-linked immunosorbent assay (ELISA) assay kit were from MeiMian Biotech Co., Ltd (Jiangsu, China). SD rats were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). Rat chow was from Jiangsu Synergy Medical Bioengineering Co., Ltd (Jiangsu, China).
Preparation and Quality Standards of Compound Angelica Ligusticum Wallichii Granules
Preparation
CALG was composed of Angelica sinensis (Oliv). Diels 10g, Ligusticum chuanxiong Hort. 6g, roasted Astragalus membranaceus 10g, Salvia miltiorrhiza Bge. 10g, Whitmania pigra Whitman 3g, Paeonia veitchii Lynch 10g, Cynanchum paniculatum (Bunge) Kitagawa 10g, Gleditsia sinensis Lam 6g, Epimedium brevicornum Maxim. 10g, Prunus davidiana (Carr). Franch. 10g, Eupolyphaga sinensis Walker 10g, vinegar Cyperus rotundus L. 10g, vinegar Corydalis yanhusuo W.T.Wang 10g, and Daemonorops draco Bl. 1g. The characteristics of herbs are shown in Table 1. All reagents were purchased from Jiangyin Tianjiang Pharmaceutical Co., Ltd. Deionized water (10 times) was added, and the mixtures were then boiled at 100°C for 1.5 h, under a pressure of 0.07 MPa. This process was repeated 3 times with filtration steps in between each decoction. The combined extracts were filtered and concentrated under reduced pressure to a relative density of 1.1–1.2, vacuum dried at 60°C, and crushed to obtain a fine powder. The fine powder was then mixed with maltodextrin and dextrose at a ratio of 9:2:1, respectively. A stable compound granule preparation with a moderate viscosity was obtained by dry granulation after 30 minutes of full mixing in a multi-motion mixer.
 |
Table 1 Components of Compound Angelica Ligusticum Wallichii Granules |
Qualitative Identification of CALG by Thin-Layer Chromatography
To prepare the test solution, 9 g of powdered CALG was dissolved in 50 mL diethyl ether using an ultrasonic treatment for 10 minutes. After filtration, the filtrate was evaporated, and the residue was dissolved in 1 mL methanol. In addition, the reference medicinal materials were separated using the same method for preparing the test material solution. According to the thin-layer chromatography (TLC) test (General rule 0502), 10 µL of each solution was absorbed on the same silica gel G thin layer plate, using n-hexane-ethyl acetate (4:1) as the developing agent, and was expanded, removed, dried, and placed under an ultraviolet lamp (365 nm) for inspection. Fluorescent spots of the same color were found in the corresponding positions of both samples.
Quantitative Identification of Ferulic Acid, Paeoniflorin, and Salvianolic Acid B of CALG by High-Performance Liquid Chromatography
According to the results of a pre-extraction experiment, extraction technology, and preparation study, paeoniflorin, and salvianolic acid B were selected as the detection indexes. The content measurement experiment was performed by weighing 0.3 g of compound, dissolving it in mobile phase (Acetonitrile-0.1% phosphoric acid solution (14:86) for paeoniflorin, Methanol-water (8:2) for salvianolic acid B), filtering, and using the filtrate as the test solution. An appropriate amount of reference substance was dissolved with the mobile phase, shaken, and used as the reference substance solution. According to high-performance liquid chromatography (HPLC) (General rule 0512), 10 µL of each solution was absorbed and injected into the liquid chromatography column for determination. The content in CALG was determined using an Agilent 1200, MWD detector, Agilent 1200 workstation, and chromatographic column (waters T3, 4.6 mm × 250 mm, 5μm). The following parameters were used: filler, octadecyl silane bonded silica gel; mobile phase, 0.1% acetonitrile 1% phosphoric acid solution (14:86); detection wavelength, 230 mm.
Quantitative Analysis of CALG by UPLC-Q-TOF/MS
Quantitative component analysis was performed using the Agilent 1290 Infinity ultra-high performance liquid system (Agilent Technologies, Santa Clara, California, USA) equipped with a photodiode array detector and passed through the Waters ACQUITY UPLC HSS T3 column (2.1 mm × 100 mm) (1.8 µm, Waters, Milford, MA, USA) for separation. The mobile phase was composed of mobile phase A (0.1% formic acid aqueous solution, volume/volume) and mobile phase B (acetonitrile). The gradient elution steps were as follows: 0–10 min, 0% B; 10–15 min, 0–3% B; 15–20 min, 3–5% B; 20–32 min, 5–8% B; 32–42 min, 8–10% B; 42–50 min, 10–18% B; 50–65 min, 18–22% B; 65–72 min, 22–30% B; 72–82 min, 30–50% B; 82–97 min, 50–95% B; 97–100 min, 95–0% B. The column temperature was maintained at 30°C, the injection volume was 1 μL, and the detection wavelength was 254 nm.
The Agilent ultra-high performance liquid system was coupled with triple quadrupole mass spectrometry (Triple TOF® 4600, AB Sciex, Massachusetts, USA), a high-resolution mass spectrometer equipped with an electrospray ionization (ESI) interface. Data were collected in two ways: negative and positive ion modes. The operating parameters of the instrument were as follows: spray positive/negative ion source voltages, 5000 V and −4500 V, respectively; ion source temperature, 500°C; atomizing gas, N2; atomizing gas pressure (GS1), 50 psi; heating gas pressure (GS2), 50 psi; curtain gas pressure, 35 psi; declustering voltage, 100 V; collision energy, 10V; mass range (m/z), 50~2000; mass spectrum range (m/z), 50 ~ 1250; declustering voltage, 100 V; collision voltage, ±40 eV; collision voltage swing, 20 eV; ion release delay, 30 ms; and ion beam width, 15 ms.
Network Systems Pharmacology Analysis
The high-resolution database, Natural Products HR-MS/MS Spectral Library 1.0, was used to identify the MS/MS spectrometry results, and relevant literature was searched for comparison to infer compound information. All compounds detected in CALG and compounds inferred from the literature were then included in the analysis, and three databases were used for drug target prediction, including the Swiss Target Prediction database (http://www.swisstargetprediction.ch/), PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and Stitch database (http://stitch.embl.de/). With human genes as background, endometriosis was used as a keyword to search for endometriosis-related genes in the therapeutic target database (TTD, http://db.idrblab.net/ttd/), the disease gene search engine with evidence sentences database (DiGSeE, http://www.disgenet.org/) and the Online Mendelian Inheritance in Man database (OMIM, https://omim.org/). All targets were standardized to gene symbols through the bioDBnet database, and duplicate values were removed.
The retrieved genes of candidate compounds and endometriosis were imported into the STRING database (http://string-db.org/) to construct protein–protein interaction (PPI) networks. Interactions with a confidence score ≥0.9 were selected, and networks were merged using Cytoscape 3.6.1 software (https://cytoscape.org/). Nodes with a greater degree value than the median were screened as core targets with topological analysis.
The core targets were input into the database for annotation visualization and integrated discovery (DAVID, https://david.ncifcrf.gov/). The identifier “Gene official symbol” and species “Homo sapiens” were selected for Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotations, and the enrichment pathways (p ≤ 0.05) were visualized using the R language (Version 3.5.3) ggplot2 package.
To analyze the association among endometriosis, compounds, the candidate targets, and related pathways, a Component-Target-Pathway-Disease (C-T-P-D) network was constructed by Cytoscape 3.6.1. The corresponding nodes were ranked according to the degree value by using the plug-in.
Molecular Docking and Analysis
After obtaining candidate targets from the PPI network, they were imported into the protein database (PDB) (https://www.rcsb.org/) and proteins were downloaded in PDB format to improve the reliability of the molecular docking results. The 3-dimensional (3D) structures of the components downloaded from PubChem database (https://pubchem.ncbi.nlm.nih.gov/) were rendered with the ChemDraw software. The processed structures were then imported into AutoDockTools-1.5.7 to be hydrogenated and charged, and the rotatable bond number was calculated. Then, the ligands and non-protein molecules in the genes were removed by PyMOL software, and the AutoDockTools-1.5.7 software was again used to add hydrogen and calculate the total charges. Through the clustering tool in AutoDockTools-1.5.7, the lowest energy pose was chosen as the research object. Via PyMOL, the high-quality 3D and 2D structures of small molecules and proteins were created, and the corresponding protein residues and binding bonds were displayed.
Sprague Dawley Rats as an Endometriosis Model for Pharmacodynamic Verification
Construction of a Rat Model of Endometriosis
All rats (weighing 210 ± 20 g at the time of surgery) were housed under controlled conditions of temperature 22 ± 3 °C, relative humidity 55 ± 15%, and a 12-hour light/dark cycle with access to food and water. A total of 50 rats were divided into two groups: the sham-operated group (N = 8) and the endometriosis model group (N = 42). Generally, animals were anesthetized with pentobarbital sodium (65 mg/kg) before a roughly 3 cm midline incision was made in the lower abdomen, then the opened abdominal cavity of sham-operated rats was sutured, while the remaining 42 rats were used for endometriosis model development. After the right side of the uterus was ligated, the right uterine horn was excised and placed in phosphate buffered saline (PBS). The horn was incised longitudinally, cut into 5 × 5 mm sections, and transplanted on the outer surface of the right abdominal wall, and finally the opening was sutured. After surgery, each rat was injected with gentamicin (400,000 units) for 3 consecutive days. Two days following the operation, they were injected with 0.1 mg/kg/d of E2, every 4 days for 2 weeks to promote the growth of ectopic endometrium.11,13 All animal procedures were performed in strict accordance with the guideline of the Institutional Animal Care and Use Committee of Fudan University. The ethics approval has been obtained from Ethics Committee of Obstetrics and Gynecology hospital of Fudan University (2022120031Z).
Treatments
Rats were checked after 28 days of surgery, by observation and measurement of cystic formations on ectopic surfaces. Thirty-six rats with successful establishment of endometriosis and a similar size of endometriotic lesions were randomly divided into 6 groups (N = 6 per group) as follows: model group, diphereline group, gestrinone group, CALG low-, middle- and high-dose groups. We used the body surface area calculation method of humans and animals to convert the drug dose. We calculated the dose of diphereline in mice using Meeh Rubner’s formula according to the dose of diphereline used by clinical patients. Rats in the diphereline group received intramuscular administration of diphereline at 0.05 mg/kg/day for 7 consecutive days and were then maintained at 1/5 of the initial dose for 21 days. Rats in the gestrinone group received intragastric administration of gestrinone at 0.28 mg/kg twice per week. Rats in the CALG low-dose, medium-dose, and high-dose groups received CALG by oral gavage for 28 days, at a dose of 13 g/kg/d, 26 g/kg/d, or 52 g/kg/d, respectively. The lowest dose of CALG was calculated using a dose conversion formula for different species based on body surface area.14 The sham operated group and the model group were given the same volume of distilled water intragastrically. After 28 days of drug administration, the length, width, and height of the lesions were measured by vernimetric caliper, and the volume of endometriosis lesions was calculated by length × width × height.
Sample Collection
After 28 days of treatment, the rats in each group were sacrificed, and blood was collected immediately and processed to plasma. The size of the lesions (the longest diameter and the volume of the lesion) was measured, and each lesion was immediately fixed in 4% paraformaldehyde solution. For the sham operated group the endometrium was isolated and fixed in the same manner. The samples were then embedded in paraffin for immunohistochemical study and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining analysis.
TUNEL Stain
The paraffin sections were washed in xylene for 10 minutes two times and then with 100% ethanol, 95% ethanol, and 75% ethanol for 5 minutes each, followed by two washes with PBS. The sections were then incubated with 0.1% Triton X-100 and 0.1% sodium citrate for 8 minutes and rinsed twice with PBS for 5 minutes each. TUNEL reaction solution (25 μL) was added to each sample and incubated in a humid environment at 37 °C for 60 minutes in darkness. The slides were rinsed with PBS 3 times for 10 minutes each and then incubated at room temperature with 4’,6-diamidino-2-phenylindole for 2 minutes to stain the nuclei. The slide was rinsed in PBS 3 times and placed on a cover slide with a fade-resistant mount. The apoptotic cells were observed and photographed under a fluorescence microscope.
Enzyme Linked Immunosorbent Assay
Blood samples from each group of rats were collected from the carotid artery and left at room temperature for 30 minutes. The samples were centrifuged at 4 °C at 3800 rpm for 10 minutes and the serum collected. The levels of VEGF, TNF-α and E2 in the serum of each group were detected using the Meimian (Shanghai, China) ELISA kit.
Statistical Analysis
All data are expressed as mean ± standard deviation. One-way analysis of variance was used to compare the difference among groups, followed by post-hoc Tukey multiple comparison tests. A p value of <0.05 was considered to be statistically significant. All data were analyzed using GraphPad Prism 9.0 (GraphPad Software Inc, CA, USA).
Results
Preparation and Quality Control of CALG
The quality standard of CALG was carried out according to the Technical Guidelines for Research on Quality Standards of Traditional Chinese Medicine New Drugs (Draft for Comments). As shown in Figure S1, this product consisted of light brown to tan granules with a slightly bitter taste. The particle size, moisture content, melting property, filling quantity difference and microbial limit test met the requirements of General Rule 0104 of Chinese Pharmacopoeia (2020 edition).
TLC was used to investigate the specificity and durability of the preparation by referring to the identification items of various substances in Pharmacopoeia. The quality standard study showed that the main spots of flos angelicae sinensis and ligusticum chuanxiong exhibited the same color fluorescence at the corresponding positions as the main spots of the control herbs. The negative control did not show spots in the corresponding chromatographic positions (Figure 1A). In addition, the effect of different experimental temperatures on the TLC identification of CALG was investigated. As shown in Figure 1B, there is a significant change in the Rf value between the low temperature (4°C) and room temperature regions, which indicated that temperature had a certain influence on the unfolding effect. Therefore, subsequent expansion at room temperature was selected. In addition, three pilot batches were examined by TLC, and no significant differences were found between the samples of different batches (Figure 1C), suggesting that the standard preparation was stable.
The content of paeoniflorin and salvianolic acid B in the CALG was systematically studied using HPLC, which showed that the peak times of the target peaks on the chromatogram were appropriate with good resolution and there was no negative interference (Chromatograms of paeoniflorin and salvianolic acid B are shown in Figure 1D and E, accordingly). The validation of accuracy, specificity, repeatability, intermediate precision, and linearity was good. As a result, to ensure the quality of the product, 80% of the average value is used as the lower limit of content. The content of salvianolic acid B (C36H30O16) in CALG should not be less than 0.30%, while paeoniflorin not be less than 0.27%.
Identification of the Main Ingredients and Contents of CALG in vitro
This study used a high-quality chemical compound database of Shanghai Standard Technology Co., Ltd, which currently contains more than 1,300 natural compounds based on nearly 500 Chinese herbal pieces and covers common structural types of compounds such as flavonoids, saponins, alkaloids, organic acids, and phenylpropanoids. The database includes the Chinese and English names, molecular formulas, structural formulas, chemical categories, plant origins, CAS numbers, ChemSpider numbers, KEGG numbers and other information on each compound. Most importantly, it contains more than 20,000 high-resolution, accurate, high-quality secondary mass spectrograms, which helped us identify the ingredients of compounds efficiently and accurately.
To generate an overall chemical profile of CALG, a UPLC-Q-TOF/MS method was performed. A total of 121 compounds from CALG were identified or preliminarily characterized according to the accurate mass spectrometry measurements combined with related literature. Among them, 27 were from salvia miltiorrhiza, 13 were angelica, 25 were prepared radix astragalus, 27 were red peony root, 26 were chuanxiong, 10 were vinegar xiangfu, 34 were vinegar yanhu, 11 were saponin, 22 were herba epimedii, 11 were Xuchangqing, 12 were soil trionyx, 11 were scalded leeches, 13 were blood powder, and 18 were peach nuts. Detailed data of each compound are shown in Table S1. The base peak chromatograms of CALG in the positive and negative ion modes are shown in Figure 2A and B, respectively.
 |
Figure 2 Base peak chromatograms of CALG in (A) negative ion mode, (B) positive ion mode. |
Network Systems Pharmacology-Based Prediction of the Potential Mechanisms of Action of CALG in Endometriosis
A total of 121 compounds were analyzed, and 109 were selected as candidate compounds by eliminating the repetitions. Using these databases, 1381 targets of compounds (Table S2) were obtained, and 753 disease-related targets were selected by similar steps (Table S3). The protein–protein interaction (PPI) network analysis of targets was carried out using the STRING interaction database, A PPI network of core targets was constructed by choosing the degree value of the intersection network larger than the median, which consisted of 104 nodes and 809 edges (Figure 3), and degrees of core nodes were shown in Table S4.
 |
Figure 3 (A) The core targets of CALG in the treatment of endometriosis. |
GO enrichment analysis was then performed on 104 core targets using DAVID database. There were 133 GO terms (FDR < 0.05), of which 88 items were related to biological processes, mainly including positive regulation of transcription from the RNA polymerase II promoter, positive regulation of transcription, and positive regulation of cell proliferation (Figure 4A). Twenty-nine items were related to molecular functions, generally related to transcription factor binding, enzyme binding, and identical protein binding (Figure 4B). And 16 items were related to cellular components, mostly in the nucleus, nuclear chromatin, and nucleoplasm (Figure 4C). In addition, a total of 119 pathways were obtained from 104 core targets, and 74 pathways were screened with FDR < 0.05. Among them, the top 20 related pathways are shown in Figure 4D, and the degree of which is listed in Table S5.
Based on topological analysis of the composition-target-pathway-endometriosis network, it was found that among the top 15 chemical components, the beneficial effects on endometriosis were attributed to phenolic compounds, including flavonoids and phenolic acids such as caffeic acid, ferulic acid, citric acid, gallic acid, and chlorogenic acid (Table 2). Target prediction and pathway enrichment showed that caffeic acid (Chuanxiong) and ferulic acid (Angelica sinensis, Chuanxiong) in CALG acted on the HIF-1 signaling pathway and PI3K-Akt signaling pathway, suggesting that the HIF-1 signaling pathway and PI3K-Akt signaling pathway may be involved in the beneficial effects of CALG in the treatment of endometriosis (Table 3 and Figure 5).
 |
Table 2 Top 15 Degree of Potentially Active Components |
 |
Table 3 Top 12 Related Pathways in Endometriosis |
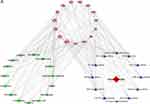 |
Figure 5 (A) Network of top 15 targets -top 15 potential active compounds - 12 related pathways in endometriosis. |
Molecular Docking
Molecular docking is an approach used for drug design by exploring the interaction and recognition between receptors and ligands. It is a theoretical simulation approach that focuses on the study of intermolecular interactions and the prediction of their binding patterns and affinity. In recent years, molecular docking has become an important technology in the field of computer-aided drug research.15
Table 4 shows the results derived from the molecular docking software (Autodock vina). Following processing by the Autodock tools software, the best-docked complexes are shown of the best docking of the receptor and ligand. Generally, the binding capacity is believed to be stronger when the docking binding free energy is lower than −5 Kcal/mol.
 |
Table 4 Molecular Docking Results |
As shown in Table 4, the binding energies of the five active ingredients to the top five ranked targets (STAT3, MAPK1, PIK3CA, EGFR and NFKB1) were all less than −5 kcal/mol, indicating a high affinity between the compounds and the core target proteins.
Identification of Pharmacology and Mechanisms of CALG in vivo
CALG Reduces Lesion Volume in Rats with Endometriosis
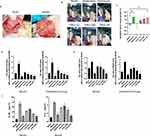
A second exploratory laparotomy was performed to examine the endometriosis implants and to ensure that all rats presented similar stages of endometriosis, and a total of 36 rats were selected (Figure 6A). Compared with the model group, the diphereline group and the CALG high-dose group showed significant inhibition of ectopic vesicle growth (p < 0.05), while there was no statistically significant difference between the other groups (p > 0.05, Figure 6B and C).
Serum Levels of Inflammatory Cytokines in Rats with Endometriosis
Many inflammatory cytokines are involved in the pathogenesis of endometritis.4,16,17 Hence, we investigated the levels of TNF-α, E2, and VEGF in the serum of rats with endometritis. As shown in Figure 6D and E, ELISA assays showed that the levels of E2 and VEGF in serum and endometrial tissues in model group were significantly higher than those in the sham group (p < 0.05), while the level of E2 in the CALG high-dose group was lower than the endometriosis group (p < 0.05). In addition, the level changes of pro-inflammatory cytokines IL-1β and IL-6 are also similar to E2 and VEGF (Figure 6F), suggesting that CALG decreased the expression levels of pro-inflammatory cytokines.
CALG Increased Apoptosis of Cells in Rats with Endometriosis
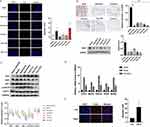
As shown in Figure 7A and B, compared with the control group, diphereline significantly increased the apoptosis of ectopic endometrium (p < 0.05), and high-dose CALG also increased the apoptosis of ectopic endometrium (p < 0.01). These findings suggest that CALG achieved a therapeutic effect on endometriosis by inducing apoptosis of endometrial cells.
Expression of STAT3 and HIF1A in Ectopic Endometrium
Western blot analysis was used to analyze the levels of total STAT3 and phosphorylated STAT3 (pSTAT3) in ectopic endometrium in rats. Compared with the control endometriosis group, the level of p-STAT3 in the ectopic endometrium of the CALG-treated groups was significantly lower (Figure 7C). HIF1A is a known target of STAT3 and a key mediator of angiogenesis, inflammation, proliferation, and extracellular invasion.18 HIF1A levels in the ectopic endometrium of CALG-treated groups were significantly higher than those in the control group.
Expression of STAT3, MAPK1, PIK3CA, EGFR and NFKB1 in Ectopic Endometrium
The expression levels of the top five ranked targets (STAT3, MAPK1, PIK3CA, EGFR and NFKB1) were detected in ectopic endometrium by Q-PCR. As shown in Figure 7D, compared with the control group, CALG decreased the mRNA expression levels of the top five ranked targets in ectopic endometrium in rats (p < 0.05).
Identification of Pharmacology of CALG in vitro
CALG Increased Apoptosis in hEM15A Cells
hEM15A cells are human immortalized endometrial stromal cell lines. As shown in Figure 7E, compared with the control group, CALG increased the apoptosis in hEM15A cells (p < 0.05). The finding proved that CALG achieved a therapeutic effect on endometriosis by inducing apoptosis of endometrial cells in vitro.
Discussion
Endometriosis is an estrogen-dependent disease with inflammatory lesions outside the uterus, causing pelvic pain and fertility.19 TCM, such as Guixiong Xiaoyi formula, has been widely used to treat endometriosis but is associated with some shortcomings, such as the lack of quality control standards. Therefore, carrying out systematic research to establish detailed drug preparation technology and quality control standards is important to ensure quality for clinical use and to expand the clinical use of TCM. In the present study, we used modern pharmaceutical and quality control technology to develop CALG, ranging from the source and quality control to the selection and optimization of the extraction and purification process and the formulation of the quality standard of preparation. Furthermore, we performed computational systems pharmacology in combination with in vivo animal studies to identify the mechanisms underlying the effects of CALG on endometriosis and found that CALG alleviated endometriosis by inhibiting apoptosis and inflammation.
To explore the specific mechanism of CALG effects on endometriosis, network systems pharmacology analysis was initially used. Using UPLC-QTOF-MS, we identified 121 chemical components, and the analysis of the top 15 potential active ingredients in CALG revealed that the most beneficial compounds were attributed to phenolic compounds, including caffeic acid, ferulic acid, citric acid, gallic acid and chlorogenic acid. Target prediction and pathway enrichment showed that caffeic acid (Chuanxiong) and ferulic acid (Angelica sinensis, Chuanxiong) in Guixiong xiaoyi preparation acted on the HIF-1 signaling pathway. Jamali20 reported that caffeic acid reduced oxidative stress and improved the antioxidant state in ectopic endometrial cells. In addition, Tang21 found that the combined application of ferulic acid, ligustrazine and tetrahydropalmatine inhibited the growth of ectopic endometrial tissues and reduced the volume of ectopic tissues in rats with endometriosis. In addition to phenolic compounds, pistil isoflavone in CALG has the effect of phytoestrogen, which has been reported to inhibit the growth and development of ectopic endometrium in the rat model of endometriosis.22
In this study, a rat model of endometriosis was used to further verify the pharmacological effects and mechanism of CALG on endometriosis. CALG at a dose of 52 g/kg/d for 28 days significantly reduced the volume of ectopic uterine lesions. Apoptosis, cell proliferation, and cell-mediated immune responses are known to contribute to the growth of endometriosis.23 We therefore performed TUNEL staining to examine the apoptosis of endometrial cells in rats. The TUNEL results showed that CALG at a dose of 52 g/kg/d induced apoptosis of endometrial cells in rats with endometriosis. Apoptosis (programmed cell death) can effectively eliminate cells without inflammatory reactions.24 Endometriosis may be caused by decreased apoptosis.13 Compared with healthy women, women with endometriosis are reported to have decreased expression of pro-apoptotic factors, such as Bax, and increased expression of anti-apoptotic factors, such as Bcl2, in endometrial tissue.25 Consistent with these results, CALG treatment increased Bax expression and decreased Bcl2 expression in ectopic endometrium. Besides, CALG treatment decreased the secretion of inflammatory factors. In addition, the levels of p-STAT3 in ectopic endometrium were significantly lower, and HIF1A expression was significantly higher in the CALG groups compared with the control group, suggesting that CALG inhibited the STAT3 signaling pathway and activated the HIF1A signaling pathway. The expression levels of the top five ranked targets were also decreased after the treatment of CALG. The results of network pharmacology analysis showed the correspondence to the animal experiments.
At present, many studies have shown that TCM has a synergistic effect on the treatment of endometriosis.26,27 The mechanisms involved include regulating the immune response, apoptosis, and angiogenesis,28 which are consistent with our findings. However, many studies use Chinese herbal medicine directly, and the quality control may be unstable.29,30 Before this experiment, we first prepared Guixiong Xiaoyi Formula into CALG with the same efficacy, which ensured the accuracy of the experiment. This study is conducive to the development of Chinese patent medicine produced by modern technology. It is the characteristics and advantages of this study.
Conclusion
Our study revealed that CALG produced a better therapeutic effect on endometriosis than gestrinone, a commonly used hormone for the treatment of endometriosis in clinical practice and had a therapeutic effect comparable to the GnRH agonist Daphne. Due to less adverse reactions and low prices, CALG may be used as a substitute or supplement for Daphne in clinical application. However, our study has some limitations. Although the biological behavior of autogenous endometrial explants is similar to that of human lesions in vivo, it may be more convincing to evaluate the effects of CALG in vivo and in vitro. In addition, we only focused on the effects of cell proliferation, apoptosis, and inflammatory factors. Further studies could evaluate other areas, such as immunity and oxidative stress. In general, our findings confirm the clinical effectiveness of CALG and also provide a foundation for further experimental studies in endometriosis.
Abbreviations
CALG, compound Angelica Ligusticum wallichii granules; CAM, complementary and alternative medicine; E2, estradiol; GnRH, gonadotropin-releasing hormone; GO, Gene Ontology; HPLC, high-performance liquid chromatography; KEGG, Kyoto Encyclopedia of Genes and Genomes; TCM, traditional Chinese medicine; TLC, thin-layer chromatography; TNF-α, tumor necrosis factor-α; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; UPLC-Q-TOF/MS, ultra-performance liquid chromatography-tandem quadrupole time of flight mass spectrometry; VEGF, vascular endothelial growth factor.
Acknowledgments
This research was supported by the Scientific Research Program of Science and Technology Commission of Shanghai Municipality (19401900900); thanks for the participation of miss Lingjun Kong and Yuanyuan Qiu. Thanks for the technical support of Shanghai Fangxin Health Technology Development Co., Ltd.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest for this work.
References
1. Piessens S, Edwards A. Sonographic evaluation for endometriosis in routine pelvic ultrasound. J Minim Invasive Gynecol. 2020;27(2):265–266. doi:10.1016/j.jmig.2019.08.027
2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. doi:10.1056/NEJMcp1000274
3. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4(1):9. doi:10.1038/s41572-018-0008-5
4. Zhao Y, Gong P, Chen Y, et al. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci Transl Med. 2015;7(271):271ra9. doi:10.1126/scitranslmed.3010626
5. Kotlyar A, Taylor HS, D’Hooghe TM. Use of immunomodulators to treat endometriosis. Best Pract Res Clin Obstet Gynaecol. 2019;60:56–65. doi:10.1016/j.bpobgyn.2019.06.006
6. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–275. doi:10.1038/nrendo.2013.255
7. Lin Y-H, Chen Y-H, Chang H-Y, Au H-K, Tzeng C-R, Huang Y-H. Chronic niche inflammation in endometriosis-associated infertility: current understanding and future therapeutic strategies. Int J Mol Sci. 2018;19(8):2385. doi:10.3390/ijms19082385
8. Tomassetti C, D’Hooghe T. Endometriosis and infertility: insights into the causal link and management strategies. Best Pract Res Clin Obstet Gynaecol. 2018;51:25–33. doi:10.1016/j.bpobgyn.2018.06.002
9. Bina F, Soleymani S, Toliat T, et al. Plant-derived medicines for treatment of endometriosis: a comprehensive review of molecular mechanisms. Pharmacol Res. 2019;139:76–90. doi:10.1016/j.phrs.2018.11.008
10. Huang S, Min S, Zhu Z. Effect of Guixiong Xiaoyi Fang on recurrence and pregnancy of endometriosis patients after operation. Fudan Xuebao. 2017;44(1):42–46. doi:10.3969/j.issn.1672-8467.2017.01.007
11. Jin Z, Wang L, Zhu Z. Effect of GuiXiong Xiaoyi Wan in treatment of endometriosis on rats. eCAM. 2015. doi:10.1155/2015/208514
12. Zhao J, Ma S-C, Li S-P. Advanced strategies for quality control of Chinese medicines. J Pharm Biomed Anal. 2018;147:473–478. doi:10.1016/j.jpba.2017.06.048
13. Hu C, Wang Z, Pang Z, et al. Guizhi fuling capsule, an ancient Chinese formula, attenuates endometriosis in rats via induction of apoptosis. Climacteric. 2014;17(4):410–416. doi:10.3109/13697137.2013.876618
14. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi:10.1096/fj.07-9574LSF
15. Chen G, Seukep AJ, Guo M. Recent advances in molecular docking for the research and discovery of potential marine drugs. Mar Drugs. 2020;18(11):545. doi:10.3390/md18110545
16. Zhang A, Wang G, Jia L, Su T, Zhang L. Exosome-mediated microRNA-138 and vascular endothelial growth factor in endometriosis through inflammation and apoptosis via the nuclear factor-κB signaling pathway. Int J Mol Med. 2019;43(1):358–370. doi:10.3892/ijmm.2018.3980
17. Samimi M, Pourhanifeh MH, Mehdizadehkashi A, Eftekhar T, Asemi Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: basic science and new insights based on gene expression. J Cell Physiol. 2019;234(11):19384–19392. doi:10.1002/jcp.28666
18. Henze A-T, Acker T. Feedback regulators of hypoxia-inducible factors and their role in cancer biology. Cell Cycle. 2010;9(14):2749–2763. doi:10.4161/cc.9.14.12249
19. Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15(11):666–682. doi:10.1038/s41574-019-0245-z
20. Jamali N, Mostafavi-Pour Z, Zal F, et al. Combination effect of caffeine and caffeic acid treatment on the oxidant status of ectopic endometrial cells separated from patients with endometriosis. Iran J Med Sci. 2019;44(4):315–324. doi:10.30476/IJMS.2019.44970
21. Tang Q, Shang F, Wang X, et al. Combination use of ferulic acid, ligustrazine and tetrahydropalmatine inhibits the growth of ectopic endometrial tissue: a multi-target therapy for endometriosis rats. J Ethnopharmacol. 2014;151(3):1218–1225. doi:10.1016/j.jep.2013.12.047
22. Chen Y, Chen C, Shi S, et al. Endometriotic implants regress in rat models treated with puerarin by decreasing estradiol level. Reprod Sci. 2011;18(9):886–891. doi:10.1177/1933719111398500
23. Cho YJ, Lee JE, Park MJ, O’Malley BW, Han SJ. Bufalin suppresses endometriosis progression by inducing pyroptosis and apoptosis. J Endocrinol. 2018;237(3):255–269. doi:10.1530/JOE-17-0700
24. Takai E, Taniguchi F, Nakamura K, Uegaki T, Iwabe T, Harada T. Parthenolide reduces cell proliferation and prostaglandin E2 [corrected] in human endometriotic stromal cells and inhibits development of endometriosis in the murine model. Fertil Steril. 2013;100(4):1170–1178. doi:10.1016/j.fertnstert.2013.06.028
25. Harada T, Taniguchi F, Izawa M, et al. Apoptosis and endometriosis. Front Biosci. 2007;12:3140–3151. doi:10.2741/2302
26. Wu Y, Liu Y, Jia H, et al. Treatment of endometriosis with dienogest in combination with traditional Chinese medicine: a systematic review and meta-analysis. Front Surg. 2022;9:992490. doi:10.3389/fsurg.2022.992490
27. Dong P, Ling L, Hu L. Systematic review and meta-analysis of traditional Chinese medicine compound in treating infertility caused by endometriosis. Ann Palliat Med. 2021;10(12):12631–12642. doi:10.21037/apm-21-3425
28. Wu Y, Zhu Y, Xie N, et al. A network pharmacology approach to explore active compounds and pharmacological mechanisms of a patented Chinese herbal medicine in the treatment of endometriosis. PLoS One. 2022;17(2):e0263614. doi:10.1371/journal.pone.0263614
29. Shan J, Cheng W, Zhai DX, et al. Meta-analysis of Chinese traditional medicine bushen Huoxue prescription for endometriosis treatment. Evid Based Complementary Altern Med. 2017;2017:5416423. doi:10.1155/2017/5416423
30. Lin Y, Hou R, Zhang T, et al. Efficacy and safety of Chinese Herbal Medicine for endometriosis associated pain. Am J Chin Med. 2022;50(4):1095–1111. doi:10.1142/S0192415X22500446
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.