Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Comprehensive Treatment of Severe Follicular Occlusion Triad: A Case Report
Authors Yang K, Shi M , Fu C, Huo R
Received 27 November 2021
Accepted for publication 9 March 2022
Published 30 March 2022 Volume 2022:15 Pages 541—546
DOI https://doi.org/10.2147/CCID.S351522
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Kun Yang,1 Mengdong Shi,1 Cong Fu,2,* Ran Huo1,2,*
1Department of Plastic Surgery, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Plastic Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Cong Fu; Ran Huo, Tel +86 15154101889 ; +86 15168889001, Fax +860531-68778153, Email [email protected]; [email protected]
Abstract: Follicular occlusion triad (FOT) is a chronic inflammatory skin disease that comprises hidradenitis suppurativa, acne conglobata, and perifolliculitis capitis abscedens et suffodiens and can seriously affect a patient’s quality of life. Currently, there is no consensus on the treatment plan for FOT. There are also only a few reports on the treatment of severe FOT. In July 2020, a male patient who was diagnosed with severe FOT was treated in our hospital and received comprehensive surgical treatment for 2 months. This treatment strategy was effective and the patient had no recurrence during a follow-up period of > 1 year. By retrospectively analyzing the clinical data of the patient, recording the patient’s condition during postoperative recovery, and reviewing relevant literatures, the clinical manifestations, diagnosis, choice of treatment methods, and prognosis of FOT were evaluated.
Keywords: chronic inflammatory skin disease, comprehensive treatment, surgical management
Introduction
Follicular occlusion triad (FOT) is a chronic inflammatory skin disease that refers to the simultaneous occurrence of hidradenitis suppurativa, acne conglobata, and perifolliculitis capitis abscedens et suffodiens in a patient. FOT was first named in 1956 by Pillsbury et al.1 In 1975, Plewig and Kilgman2 proposed to add pilonidal sinus to FOT and rename it as “follicular occlusion tetrad”, since these four diseases may all be associated with the pathogenesis of acne. In 1989, Plewig and Steger3 found that the common pathogenesis of these diseases were abnormalities in the epithelium of hair follicles; thus, they proposed the concept of acne inversa (AI) to replace the term FOT.4,5 As one of the diseases that comprise FOT, hidradenitis suppurativa (HS) is a recidivity chronic follicle occlusive disease that manifests as deep inflammatory nodules, sinuses, abscesses, and string-like scars. HS occurs mainly in intertriginous areas, such as the perianal, inguinal, axillary, and submammary regions, and usually affects both sides of the body.6 Acne conglobata (AC) is a severe nodular cyst that is prone to occur in young men and is generally not associated with systemic symptoms. These lesions are mostly located in the dorsal part of the chest and are characterized by pyogenic lesions, sinus tracts, and scar formation. Perifolliculitis capitis abscedens et suffodiens (PCAS) is a chronic suppurative skin disease that usually occurs on the heads of adult male patients. Skin lesions are initially characterized by inflammatory nodules and papules, and develop into cysts in the deeper part, with the formation of sinus tracts, and exudation of pus. This subsequently leads to scar formation and hair loss.7 A review of relevant literature revealed that the diagnosis of FOT should fully combine the medical history and clinical manifestations, supplemented by pathological examination of skin lesions. Pathological examination is used to assist in the differential diagnosis of diseases such as Langerhans histiocytosis, Crohn’s disease in the skin, proliferative pus skin disease, inguinal lymph granuloma, acne vulgaris, and scrofuliasis skin tuberculosis. Moreover, pathological examination can be used to exclude early malignant changes.5 In cases of FOT, skin lesions, including pustules, cysts, sinuses, and scars, along with the pain and unpleasant odor caused by the exudation of pus, greatly affect the patients’ physiological and mental health. Currently, there is no consensus on the optimum treatment strategy for FOTs. Herein, we report a case of severe FOT in a 21-year-old male patient who received comprehensive surgical treatment for 2 months and did not experience recurrence after a follow-up period of >1 year.
Case Report
A 21-year-old male patient was admitted to the hospital with multiple purulent sinus tracts and scar hyperplasia in the buttocks and bilateral axilla for more than 5 years. He was a Chinese student with a BMI of 28.1 kg/m2, and denied any history of smoking, drug use, and comorbid diseases. Since the age of 15 years, the patient had multiple purulent sinus tracts in the buttocks and bilateral axilla, and the wound ulcerated repeatedly. He had undergone debridement and skin grafting in the bilateral axillary region 4 months prior. Physical examination revealed multiple abscesses, sinus tract formation, and scar hyperplasia in the buttocks and perineum. The skin of the lesion was red and swollen with a hard texture, as shown in Figure 1A. When the skin around the sinus tracts was palpated, purulent yellow secretions ooze out with obvious tenderness. There were a hyperplastic scars in the axillary region bilaterally, and scar contracture caused an obvious limitation in the range of abduction of the upper limbs, as shown in Figure 1B. Multiple nodular cystic acne can be seen on the chest, along with the formation of keloids; the larger one is about 1.0×1.0 cm, as shown in Figure 1C. Inflammatory nodules and papules were observed on the forehead and occipital region of the patient, while scar hyperplasia was not obvious, as shown in Figure 1D. The abnormal laboratory examinations were as follows: white blood cell count, 10.2×109/L [normal (3.5~9.5) ×109/L]; absolute neutrophil count, 7.58×109/L [normal (1.8~6.3) ×109/L]; hemoglobin, 120 g/L [normal 130–175 g/L]; albumin, 37.2 g/L [normal 40–55 g/L]; Gamma 34.3% [normal 11.1–18.8%]; uric acid, 525 µmol/L [normal 208–428 µmol/L]; and D-dimer, 0.91 mg/L [normal 0–0.5 mg/L]. The bacterial culture of secretions from the buttock wounds revealed a mixed growth of Proteus, Escherichia coli, and Enterococcus. An electrocardiogram showed sinus arrhythmia. The pathological examination of specimens taken from the skin and subcutaneous tissue of the left buttock showed acute and chronic inflammation with granulation tissue hyperplasia.
The patient underwent debridement and negative pressure drainage five times during the first stage of treatment. When the drainage volume was reduced and fresh granulation tissue had grown, as shown in Figure 2, the patient underwent a second-stage treatment including debridement and skin grafting, as well as debridement and dressing change. Postoperative images are shown in Figure 3. The patient received an intravenous infusion of levofloxacin and oral acitretin (25 mg daily for 2–4 weeks) simultaneously. After discharge, the patient was counselled and instructed on wound and skin care, clean and healthy living habits, avoidance of external irritation, and weight management. The patient is content with the effect of treatment, and there has been no sign of recurrence so far.
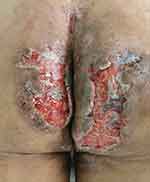 |
Figure 2 Fresh granulation tissue after debridement and negative pressure drainage. |
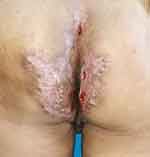 |
Figure 3 Postoperative of the wounds on the buttocks. |
Discussion
Pathogenesis
The major pathogenesis of FOT is follicle atresia, which is caused by hyperkeratosis of the epithelium of hair follicles. Sebum secreted by the follicle wall and exfoliated epithelial cells cannot be properly eliminated because of sebaceous duct atresia in hair follicles, which heaps up in the hair follicle and forms keratin plugs. When a secondary bacterial infection occurs, keratin plugs can lead to inflammation around and deep inside the hair follicle, with extensive infiltration of lymphocytes, neutrophils, and tissue cells. A series of inflammatory pathological changes occur, including recurrent inflammatory nodules, abscesses, fistulas, and sinus tracts. Genetic factors play an important role in the pathogenesis of FOT,8 and 35–40% of patients with FOT have a positive family history.9 Studies on the family lineage of patients with FOT have shown that the disease is inherited in an autosomal dominant pattern.10,11 Von der Werth and Williams proposed that the onset of FOT may be related to multiple genes, which is consistent with genetic heterogeneity.12 Moreover, changes in hormone levels, poor living habits including smoking and drinking, as well as external stimuli, such as mechanical stimulation, local dampness, and dirt on the skin have also been shown to have a certain impact on the incidence of FOT.13,14
Prognosis
Repeated wound rupture in patients with FOT may result in squamous cell carcinoma.18 Swedish scholars, Lapins J et al, followed up patients with hidradenitis suppurativa for 22 years and found that they had a higher incidence of malignant skin tumors compared to the general population.19 The findings of these studies suggest that careful attention should be paid to patients with chronic repeatedly ruptured wounds, and complete pathological examinations should be performed to detect malignant changes early.
Clinical Classification and Treatments
Currently, the clinical classification of FOTs is mainly based on the Hurley classification used for hidradenitis suppurativa,15 which can be divided into three types according to clinical manifestations and prognosis, namely, mild, moderate, and severe, as shown in Table 1. Different treatments are usually chosen according to the classification of the lesion. The treatment of patients with mild cases mainly involves the use of topical antibiotics for the treatment of skin lesions and counselling patients on skin care techniques and health education. Patients are supposed to maintain a regular schedule, quit smoking and alcohol, control their weight, and avoid friction and stimulation of the intertriginous areas.16 Patients with moderate FOT should be treated primarily with internal medications. Effective antibiotics, anti-androgen drugs, and retinoid drugs can be used systematically.17 Although previous studies have shown that antibiotics have a limited therapeutic effect on FOT, their use can partially alleviate local abscess formation and infection of ulcers. Anti-androgen drugs are only suitable for women of childbearing age, and there is still considerable controversy regarding the efficacy of anti-androgen drugs in the treatment of FOTs. Retinoid drugs can lead to the contraction of sebaceous glands, reduce sebaceous secretion, and promote keratinocyte differentiation, making them the first choice for the treatment of acne. However, teratogenicity, liver toxicity, abnormalities of the skin and mucous membranes, idiopathic increase in intracranial pressure, and other side effects should be considered fully. Furthermore, these drugs are strictly contraindicated in pregnant women. They should also be used with caution in adolescents patients and the families of such patients should be fully informed of the side effects of this drug.
 |
Table 1 Classification of FOT |
For patients with severe FOT, a multi-step and comprehensive treatment based on surgery supplemented by medical treatment is recommended. Systematic use of effective antibiotics and retinoids does not only alleviate the clinical symptoms in patients, but also reduce the scope of the lesions. For patients who fail to respond to these drugs, adalimumab, infliximab, oral retinoids, and dapsone may be beneficial.20 Adalimumab is the only licensed drug for the treatment of HS. For adult patients, the dosing schedule is recommended as 160 mg subcutaneous dose at the beginning, 80 mg on day 15, and 40 mg once a week starting on day 29.22 The common side effects of adalimumab include an increased risk of infection and injection site reactions.
Surgical treatment is a multi-step procedure. In the first stage, the ulcerated wounds were completely removed, and a VAC negative pressure dressing was placed deep in the wound to completely remove the abnormal follicles, which can effectively inhibit recurrence. Second-stage surgical treatment can be performed until the drainage volume is reduced, the drainage fluid color becomes clear, or fresh granulation tissue grows. Flap transfer or skin grafting is performed according to the location and acreage of the wound to cure residual wounds. However, the scar contractures after axillary skin grafting in the patient mentioned above suggests that a thicker and sufficiently large skin flap should be used for skin grafting, and a split-thickness skin graft or full-thickness skin graft is recommended.21 Moreover, strict anti-scar treatment should be performed after surgery, including the regular application of scar creams, laser treatment, and functional exercise. Because the coverage of defects in the axilla is always large, skin grafting for the axilla should be used with caution. Throughout the treatment process, patient education and support are essential management components. Providing patients with guidance on wound and skin care, dietary guidance, clean and healthy living habits, avoidance of external irritation, and weight management21 is beneficial.
Conclusion
The characteristics of recurrent attacks, long course of disease, and involvement of the whole body in FOT make its treatment difficult. Different treatment options can be selected according to the clinical symptoms and the location of the lesion. For patients with severe clinical symptoms, a multi-step and comprehensive treatment based on surgery supplemented by medical treatment is often adopted. However, the patient in this report was followed up for a short period of time, and the long-term effects of the treatment remain to be confirmed. Clinicians should pay more attention to this disease and simultaneously provide patients with more education on the disease. Early diagnosis and intervention can effectively delay disease progression, prevent disfiguring injuries, reduce the impact of the disease on the physiological and mental health of patients, and reduce the economic burden.
Ethics Approval and Informed Consent
The patient consented to the publications of his images in this case report.
Consent for Publication
Approval for the publication of the patient’s case details was obtained from Shandong Provincial Hospital Affiliated to Shandong First Medical University.
Acknowledgments
We would like to thank Editage for English language editing.
Disclosure
Cong Fu and Ran Huo are co-correspondence authors for this study. The authors report no conflicts of interest in this work.
References
1. Pillsbury DM, Shelley WB, Kligman AM. Dermatology.
2. Plewig G, Kligman AM. Acne. Morphogenesis and Treatment. Berlin: Springer; 1975:192–193.
3. Plewig G, Steger M. Acne inversa (alias acne triad, acne tetrad or hidradenitis suppurativa). In: Marks R, Plewig G, editors. Acne and Related Disorders. London: Martin Dunitz; 1989:345–357.
4. Yaqi D, Han L, Yongyan F, et al. Photodynamic therapy of 5-aminolevulinic acid in the treatment of 1 case of abnormal acne. J Clin Dermatol. 2016;045(004):305–307.
5. Yun H, Qian Z, Haoxiang X, et al. Abnormal acne. J Clin Dermatol. 2014;1:47.
6. Anargyros K. Quality of Life and Psychosocial Implications in Patients with Hidradenitis Suppurativa. Dermatology. 2016;2:34–54.
7. Ping X, Shuping G. Research progress of head abscess penetrating folliculitis. Chine J Clin. 2017;11(001):148–152.
8. Yuan L. Research progress on the naming, pathogenesis and etiology of reverse acne. Chine Journal of Dermatovenereol. 2014;8:849–851.
9. Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28(4):779–793. doi:10.1016/j.det.2010.07.003
10. von der Werth JM, Williams HC, Raebrun JA. The clinical genetics of hidradenitis suppurativa revisited. J Dermatol. 2000;142(5):947–953.
11. Yuanhua C. The diagnosis and treatment of triad of follicle atresia. Zhonghua J Dermatol. 2008;41(4):275–278.
12. Von der werth JM. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389.
13. Gasparic J, Theut Riis P, Jemec GB. Recognizing syndromic hidradenitis suppurativa: a review of the literature. J Eur Acad Dermatol Venereol. 2017;31(11):1809–1816.
14. Miller IM, McAndrew RJ, Hamzavi I. Prevalence, risk factors, and comorbidities of hidradenitis suppurativa. Dermatol Clin. 2016;34(1):7–16.
15. Sabat R, Jemec GBE, Matusiak Ł, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6(1):18. doi:10.1038/s41572-020-0149-1
16. Lam J, Krakowski AC, Friedlander SF. Hidradenitis suppurativa (acne inversa): management of a recalcitrant disease. Pediatr Dermatol. 2007;24(5):465–473.
17. Vallerand IA, Lewinson RT, Farris MS, et al. Efficacy and adverse events of oral isotretinoin for acne: a systematic review. Br J Dermatol. 2018;178:76.
18. Ben AJ, Bouasker I, Najah H, et al. Squamous cell carcinoma arising in Verneuil’ s disease. Tunis Med. 2008;86(2):169–170.
19. Lapins J, Ye WM, Nyrén O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137(6):730–734.
20. Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa. Cochrane Database Syst Rev. 2015;2015(10):CD010081. doi:10.1002/14651858
21. Grishkevich VM. Shoulder adduction contracture after burn: anatomy and treatment with quadrangular local scar subcutaneous pedicled flap, a new approach. Burns J Int Soc Burn Injuries. 2013;39(7):1423–1429.
22. Kimball AB, Okun MM, Williams DA, et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med. 2016;375(5):422–434.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

