Back to Journals » International Journal of General Medicine » Volume 15
Comprehensive Assessment of the Left Ventricular Systolic Function in the Elderly with Acute Myocardial Infarction Using Echocardiography
Authors Huang X, Liu Y, Guan B, Yang W, Sun S, Luo J, Luo Y, Cao J, Deng Y
Received 17 November 2021
Accepted for publication 25 January 2022
Published 11 February 2022 Volume 2022:15 Pages 1437—1445
DOI https://doi.org/10.2147/IJGM.S348594
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Xin Huang,1,* Yuan Liu,2,* Bo Guan,1 Wenyi Yang,1 Shasha Sun,1 Jiakun Luo,1 Yukun Luo,3 Jian Cao,1 Yujiao Deng3
1Department of Cardiology, The Second Medical Center & National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China; 2Department of Emergency, The Second Medical Center & National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China; 3Department of Ultrasound, The First Medical Center of Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yujiao Deng, Department of Ultrasound, The First Medical Center of Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China, Email [email protected] Jian Cao, Department of Cardiology, The Second Medical Center & National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China, Email [email protected]
Aim: To evaluate the left ventricular (LV) systolic function in elderly with non-ST elevation myocardial infarction (NSTEMI) and ST elevation myocardial infarction (STEMI) using real-time three-dimensional echocardiography (RT-3DE) and two-dimensional speckle tracking imaging (STI).
Methods: Forty NSTEMI and forty STEMI patients after undergoing percutaneous coronary artery intervention (PCI) were enrolled. The myocardial segments were supplied by the infarct-related artery (Myo-IRA) which were indicated by the selective coronary arteriography (SCA). The LV end-diastolic volume (LVEDV), end-systolic volume (LVESV), stroke volume (LVSV) and ejection fraction (LVEF) were acquired by 4D LV Volume Tom Tec. LV longitudinal peak systolic strain (LPSS), radial peak systolic strain (RPSS), circumferential peak systolic strain (CPSS) of Myo-IRA segments, LV rotational peak degree in the base (rot-base) and in the apex (rot-apex), and twist were acquired by strain analysis software. Forty older healthy individuals were included as normal controls.
Results: The LVEF of the NSTEMI and STEMI patients at 1 week after PCI were significantly lower (P< 0.05), then, this parameter was improved in both groups after 3 months, but was still significantly lower than that of the controls (P< 0.05). The LPSS, RPSS, CPSS of the Myo−IRA segments, rot−Base, rot−Apex and twist in both groups were significantly lower than those in the controls. The LPSS and CPSS of the Myo-IRA segments, rot−Base, rot−Apex and twist in NSTEMI patients were obviously higher than those in STEMI patients in 1 week and 3 months after PCI (P< 0.05). After 3 months, the RPSS of NSTEMI patients was improved notably and was obviously higher than that of STEMI patients (P< 0.05). All these values in STEMI and NSTEMI patients were improved after 3 months, apart from LPSS in STEMI patients (P> 0.05), but were still significantly lower than those in the controls (P< 0.05).
Conclusion: RT-3DE and STI can sensitively assess LV systolic function with different extents of transmural damage.
Keywords: echocardiography, acute myocardial infarction, left ventricular systolic function, elderly
Introduction
Over the last decade, China has experienced a four-fold increase in hospital admissions for acute myocardial infarction (AMI), and 60% of the patients are 65 years and older1,2 (The World Health Organization defines the elderly as “over 65 years old”). For better treatment and improved prognosis in patients with acute myocardial infarction (AMI), clinicians have emphasized the division of AMI into non-ST elevation myocardial infarction (NSTEMI) and ST elevation myocardial infarction (STEMI).3 In NSTEMI, the myocardial ischemic damage has not yet spread throughout the full thickness, and the electrocardiogram (ECG) shows ST-segment depression or T wave inversion. In STEMI, the ECG shows ST-segment elevation or wide and deep Q wave, corresponding to severe stenosis or occlusion in the coronary arteries, which causes full thickness transmural myocardial damage and can progress to a larger area of myocardial infarction that is indicated by pathological Q waves. Conventional echocardiography is not sensitive enough to assess the mild acute myocardial infarction (AMI), especially the non-ST elevation AMI (NSTEMI), and the myocardial motion is often normal or mildly reduced in such cases, leading to a higher rate of missed diagnosis.4,5 It is therefore necessary to explore more sensitive ultrasound techniques to assess such patients. In this study, quantitative assessment of left ventricle (LV) systolic function was performed in the elderly patients with NSTEMI and STEMI after percutaneous coronary intervention (PCI) by real-time three-dimensional echocardiography (RT-3DE) and two-dimensional speckle tracking imaging (STI). This study was performed to explore the different transmural extent of AMI lesion, to determine the effects of therapeutic measures, and provide more useful and accurate reference information for the comprehensive analysis of the condition and patient prognosis.
Materials and Methods
Study Population
Eighty elderly patients diagnosed with AMI were prospectively enrolled in our hospital. These patients were divided into the NSTEMI group (n = 40, 27 men, 13 women, mean age 70.45 ± 4.08 years, median age 70 years, age range 65–79 years) and the STEMI group (n = 40, 28 men, 12 women, mean age 71.07 ± 4.15 years, median age 71 years, age range 65–79 years). All patients had chest pain and increased levels of myocardial injury markers. Selective coronary arteriography (SCA) showed that at least one coronary artery had subtotal or total occlusion. All patients were suitable candidates for PCI and with optimal recanalization, reperfusion of the occluded vessel and side branches (if any) with TIMI (Thrombolysis in Myocardial Infarction) flow grade 3. All patients were performed echocardiography in 1 week and 3 months after PCI. The myocardial segments were supplied by the infarct-related artery (Myo-IRA) which were indicated by the SCA. The Myo-IRA segments were enrolled conforming the situation that they were the blood supply scope of coronary artery stenosis. There were 77 basal Myo-IRA segments, 75 middle Myo-IRA segments, and 59 apical Myo-IRA segments in NSTEMI patients, and 65 basal Myo-IRA segments, 67 middle Myo-IRA segments, and 74 apical Myo-IRA segments in STEMI patients. The exclusion criteria were as follows: (1) patients with congenital heart disease, cardiomyopathy, severe valvular heart disease, severe arrhythmia, and multiple organ complications caused by severe hypertension and diabetes; (2) patients with suboptimal recanalization (persistence of significant side branch occlusion, final TIMI flow grade 1 or 2, or residual percentage diameter stenosis >30%); (3) patients with procedural failure (failure to cross a lesion with a balloon angioplasty catheter); (4) patients with low-quality RT-3DE or STI image; (5) patients with loss to follow-up. In addition, a control group was added, composed of randomly selected healthy volunteers (n = 40, 25 men, 15 women, mean age 70.53 ± 4.11, median age 70 years, age range 65–79 years).
Echocardiographic Imaging and Analysis
Echocardiography was performed by using a Vivid 7 Dimension ultrasound scanner (GE, USA) with 3V volume phased array transducer (frequency range of 1.5–3.2 MHz) and M3S volume phased array transducer (frequency range of 1.7–3.4MHz); Echo PAC workstation, equipped with 4D LV Volume Tom Tec and 2D strain analysis software. All patients were placed in the left lateral decubitus position, and echocardiography were acquired with a simultaneous ECG signal. The 3V probe was placed at the apical four-chamber view of the LV until the ideal image appeared. RT-3DE data sets were acquired when the patient held their breath, by using a 60°×60° wide-angle acquisition mode in 4 consecutive cardiac cycles. The data sets were transferred to the Echo PAC workstation. The LV end-diastolic volume (LVEDV), end-systolic volume (LVESV), stroke volume (LVSV), and ejection fraction (LVEF) were acquired by 4D-LV analysis software, and three times measurements were averaged.6
Two-dimensional high frame rates (60–80 frames/s) images were obtained from the apical long-axis, four-, two-chamber, and the short-axis mitral, papillary muscle, and apical level of the left ventricle, respectively, during end-expiratory breath hold using M3S probe. Three consecutive cardiac cycles were acquired at each view and saved in a cine-loop format for offline analysis with the support of a digitized software package. LV endocardial borders were manually traced at end-systolic phase for strain analysis and the regions of interest were chosen to fit the whole myocardium.7 The longitudinal peak systolic strain (LPSS), radial peak systolic strain (RPSS), and circumferential peak systolic strain (CPSS) of all Myo-IRA segments were acquired by the 2D strain analysis software.8 The average LPSS, RPSS and CPSS of apical, middle, basal Myo-IRA segments were calculated. The LV rotational peak degree in the base (rot-base) and the apex (rot-apex) were acquired also by the 2D strain analysis software. The LV twist values were calculated (LV twist = rot-apex–rot-base).9 All the values were measured over three cardiac cycles and the average was calculated.
Statistical Analysis
Data analysis was performed by the standard software (SPSS, Version 20.0). Continuous variables were presented as mean ± standard deviation (SD). The normality test was used to compare continuous variables, and one-way analysis of variance (ANOVA) was used to compare echocardiographic values of the NSTEMI and STEMI patients in different times (1 week and 3 months after PCI) with those of the controls. Continuous variables from the different groups were compared by using Fisher’s least significant test. All P values were two-sided, and P values less than 0.05 were considered statistically significant.
Results
Baseline Characteristics
Baseline characteristics of the NSTEMI and STEMI patients are shown in Table 1. There were no statistical differences (all P>0.05) between the two groups in the treatment details (ACEI or ARB, Aspirin, Statin, Bata-blocker) and the coronary angiogram (LAD disease, LCX disease, RCA disease, Multiple coronaries disease).
 |
Table 1 Baseline Characteristics of the NSTEMI and STEMI Patients |
LV Volume Parameters
In 1 week after PCI, compared with the controls, the LVESV and LVEDV in the NSTEMI and STEMI patients were significantly higher than those in the controls (P<0.05), and the LVEF and LVSV were significantly lower in both groups (P<0.05). After 3 months, the LVESV and LVEDV were significantly decreased in both groups compared with 1 week after PCI (P<0.05), the reductions were particularly evident in the NSTEMI group, and even no statistical differences were seen when compared with the controls (P>0.05). The LVEF and LVSV were improved in both groups, and showed a significant difference in the NSTEMI group, compared with 1 week after PCI (P<0.05). However, the LVEF in both groups were still significantly lower than that in the controls (P<0.05) (Table 2).
 |
Table 2 Left Ventricular Volume Function in Different Times |
 |
Table 3 The LV Multi-Dimensional Strain in the Elderly STEMI and NSTEMI Patients |
LV Multi-Dimensional Strain
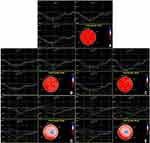
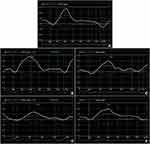
The LPSS and CPSS of the apical segments were higher than those of the basal and middle segments, the RPSS of the middle segments were higher than that of the basal and apical segments, and strain-time curves were distributed orderly in the controls. The LV apex expressed counterclockwise rotation with positive values, and the base clockwise rotation with negative values. The rot−Apex values were greater than the rot−Base, and a gradient decreasing trend was shown in the controls. While these regularities were not obvious, strain-time curves were distributed disorderly in the NSTEMI and STEMI patients (Figure 1). The LPSS, RPSS and CPSS of the Myo-IRA segments, rot−Base, rot−Apex and twist in the NSTEMI and STEMI patients were all significantly lower than those in the controls, especially in the STEMI patients (P<0.05). The LPSS and CPSS of the Myo-IRA segments, rot−Base, rot−Apex and twist in NSTEMI patients were obviously higher than those in STEMI patients in 1 week and 3 months after PCI (P<0.05). After 3 months, the RPSS of NSTEMI patients was improved notably and was obviously higher than that of STEMI patients (P<0.05). All these values in STEMI and NSTEMI patients were improved after 3 months, apart from LPSS in STEMI patients (P>0.05), but still significantly lower than those in the controls (P<0.05) (Table 3, Figure 2).
Discussion
LVEF and LV Volume Measured by RT-3DE
Echocardiography plays an indispensable role in cardiovascular diagnosis and treatment. One of the most common causes to take echocardiography is assessing heart function. LVEF is the most-used parameter and its measurement is based on cardiac morphology. RT-3DE can fully display the three-dimensional shape of the heart in different phases. It can measure volume without relying on assumption of the cardiac geometry, and the accuracy is not affected by the deformation of the heart chamber, segmental wall motion abnormalities, or other pathological states.10 Previous studies have shown that the LV volume measured by RT-3DE had a high degree of similarity with cardiac magnetic resonance.6,11
In this study, all patients underwent RT-3DE, and the left ventricular contractile dysfunction was associated with regional wall motion abnormality and a larger LV volume. The LVESV and LVEDV in the NSTEMI and STEMI patients were significantly increased (P<0.05), LVEF and LVSV were significantly lower in both groups (P<0.05), in 1 week after PCI, compared with the controls. The coronary artery serious lesions caused myocardium injury and necrosis. The necrotic myocardium was gradually replaced by fibrous tissue, and resulted in necrotic myocardium cell elongation, destruction of muscle fiber, thinning of connective tissue structure and stretching of the regional wall. This resulted in an increase of LV internal diameter and capacity, then the increased LV volume amplified the systolic wall shear stress, leading to myocardial necrosis bundle fracture, further expansion of the infarct area, wall thinning, and ultimately severe cardiac dysfunction.12,13
PCI has played an important role in minimally invasive vascular recanalization surgery, and has been used widely in clinical practice. By this way, the occluded vessels were recanalized successfully in order to reduce the myocardial necrosis area, limit the infarct size and transmural extent, effectively terminate the vicious cycle of ischemic damage and systolic dysfunction, and finally provided a firm foundation for LV functional recovery.14 In this study, the IRA was recanalized early and with optimal recanalization. However, LV systolic dysfunction was still found in NSTEMI and STEMI patients 1 week after PCI. Then, LVEDV, LVESV, LVSV and LVEF in both groups recovered partially after 3 months. Clearly, the improvement of blood supply can only better myocardium ischemia but cannot rescue necrotized myocardium. The viable myocardium, including hibernating myocardium, stunned myocardium, injured myocardium, and so on, need a long time to recover.15,16
LV Multi-Dimensional Deformation Assessed by STI
STI is a powerful ultrasonic technology for quantifying myocardial multi-dimensional strain, and it is independent of angle dependency and the surrounding tissue movement.17,18 There are some rules for LV multi-dimensional deformation in normal controls. The LPSS and CPSS of the apical segments were higher than those of the basal and middle segments. This phenomenon is in line with the geometry-class rules, moving from cardiac base to apex. Furthermore, mitral annular influence also is not obviously neglectable. While RPSS of the middle segments is greater than that of the base and apex in the normal controls, possibly due to the presence of papillary muscle and lead to middle radial strain dominance.
LV multi-strain gradient was not obvious in the NSTEMI and STEMI patients. Segmental wall motion abnormality was noted, LPSS, RPSS, and CPSS of all the Myo-IRA segments were significantly lower, more so in the STEMI patients. It is widely known that myocardium will hit grief once patients are caught in coronary artery stenosis or occlusion. Under such circumstances, myocardial metabolism is in short supply, coupled with the calcium overload, acidosis, and other factors, resulting in myocardial cell edema, necrosis, rupture, and intercellular narrowing, which reduces strain significantly.19 In NSTEMI patients, coronary subtotal occlusion is thought to involve the formation of platelet-rich thrombi and causes subendocardial myocardial necrosis, rarely involving the entire myocardium.20,21 Coronary occlusion of STEMI patients mainly relates to fibrin thrombosis and usually leads to severe myocardial transmural necrosis.22 So, the myocardium multi-dimensional strains have particularly declined, especially in the STEMI group.
PCI is an effective treatment that unblocks narrowed coronary arteries by reason of atherosclerosis or atherothrombosis. The effect may be limited for the infarcted myocardium, especially the transmural extent of infarction. And, there may be bequeathed different degrees impairment of myocardial ischemic reperfusion injury after AMI, such as production of oxygen-free radicals, inflammatory reaction, endothelial cell injury, and so on.23,24 Thus, in this study, LPSS and CPSS were significantly improved in the NSTEMI and STEMI patients after 3 months, but all those values were still significantly lower than those in the controls. It should be noted that LPSS of the Myo-IRA segments showed no significant improvement in STEMI patients in 3 months compared with 1 week after PCI. The coronary vasculature is from outward to inward to accomplish the blood supply. The micrangium of subendocardial myocardium is small and highly resistive, and it is most liable to suffer ischemic damage.25,26 Generally, the subendocardial myocardium is easily and badly damaged, the blood supply is difficult to restore even opening infarction-related artery in time and with prolonged treatment. PCI can effectively improve perfusion of the non-infarction myocardial cells, for example the epi- and mid-myocardium of the NSTEMI patients, and the border of the myocardial ischemic area. The myocardium may be in a state of hibernated, stunned or injured condition and would take a long time to restore,15,16 especially in elderly patients.
The base resides in a clockwise while the apex is in a counter-clockwise viewing from the apex to the bottom of the heart, and the interaction of these two counter-rotating forces produces torsional motion. In this study, rot-apex was greater than the rot-base, showing a decreasing trend, and twist was in a counterclockwise motion in the normal controls. This is possibly because the myocardial fibers from inside to outside show a gradual decline in stress gradient,27 and from apex to base, the stress gradient also progressively decreases. The apical rotation contributes more to LV twist than the basal rotation. However, in the pathological state, myocardium injury or necrosis which is caused by coronary arterial lesions, the regularity of rotation and twist is lost. The rot-base, rot-apex, and twist were significantly lower in the NSTEMI and STEMI patients, especially in the STEMI patients. The rot gradient from apex to base was not obvious, the apex rotation movement that plays a decisive role in the twist motion was gravely impaired, which is related to the fact that the apex is the thinnest part in the left ventricle and is more sensitive to ischemic injury.28,29 After three months, the rot-base, rot-apex, and Twist were obviously improved, though they were still significantly lower than those of normal. It is suggested that the opening of IRA can better ischemia region but cannot rescue necrotic myocardium. And, the establishment of the collateral circulation and the recovery of myocardium function also are conducive to the improvement of rotation and twist.
Limitations
There were several limitations in this study. Firstly, the major limitation was that the estimate was based on a small sample. In further research, we should do more work to verify and confirm the results. Secondly, we only focused our attention on short-term (3 months) follow-up rather than on longer follow-up. More high-quality studies with large samples and longer following-up should be proposed. Last but not least, multi-modality fusion is one of the hottest discussed issues in the current research of medical image processing and it has a deep impact on clinical treatment. Although echocardiography played a great role with its advantages in safety, speed and real-time, it would benefit more from multi-modality images in clinical medicine.
Conclusions
The LV volume functions, longitudinal, radial, circumferential, and twist movement in the elderly NSTEMI and STEMI patients can be objectively and sensitively revealed by using RT-3DE and STI respectively. All the Myo-IRA segments suffered severe damage, especially in the STEMI. Optimal recanalization can be better for the elderly NSTEMI and STEMI patients, especially NSTEMI, but it would take a long time to restore.
Ethics Approval and Consent to Participate
This study has been approved by the Chinese PLA General Hospital Medical Ethics Committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study.
Disclosure
The authors declare that they have no conflict of interests.
References
1. Dreyer RP, Zheng X, Xu X, et al. Sex differences in health outcomes at one year following acute myocardial infarction: a report from the China Patient-Centered Evaluative Assessment of Cardiac Events prospective acute myocardial infarction study. Eur Heart J Acute Cardiovasc Care. 2019;8(3):273–282. doi:10.1177/2048872618803726
2. Li J, Li X, Wang Q, et al. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet. 2015;385(9966):441–451. doi:10.1016/S0140-6736(14)60921-1
3. Liang JJ, Fender EA, Cha YM, Lennon RJ, Prasad A, Barsness GW. Long-term outcomes in survivors of early ventricular arrhythmias after acute ST-elevation and non-ST-elevation myocardial infarction treated with percutaneous coronary intervention. Am J Cardiol. 2016;117(5):709–713. doi:10.1016/j.amjcard.2015.12.002
4. Shao C, Zhu J, Chen J, Xu W. Independent prognostic value of left atrial function by two-dimensional speckle tracking imaging in patients with non -ST-segment-elevation acute myocardial infarction. BMC Cardiovasc Disord. 2015;15:145. doi:10.1186/s12872-015-0135-9
5. Liszka J, Haberka M, Tabor Z, Finik M, Gasior Z. Two-dimensional speckle-tracking echocardiography assessment of left ventricular remodeling in patients after myocardial infarction and primary reperfusion. Arch Med Sci. 2014;10(6):1091–1100. doi:10.5114/aoms.2014.47821
6. Velasco O, Beckett MQ, James AW, et al. Real-time three-dimensional echocardiography: characterization of cardiac anatomy and function-current clinical applications and literature review update. Biores Open Access. 2017;6(1):15–18. doi:10.1089/biores.2016.0033
7. Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16(1):1–11. doi:10.1093/ehjci/jeu184
8. Bai C, Meng F, Feng P, et al. Application effect and evaluation of two-dimensional speckle tracking imaging on myocardial damage in patients with malignant lymphoma treated with anthracyclines. Evid Based Complement Alternat Med. 2021;2021:6355047. doi:10.1155/2021/6355047
9. Elshafey WEH, Al Khoufi EA, Elmelegy EK. Effects of sacubitril/valsartan treatment on left ventricular myocardial torsion mechanics in patients with heart failure reduced ejection fraction 2d speckle tracking echocardiography. J Cardiovasc Echogr. 2021;31(2):59–67. doi:10.4103/jcecho.jcecho_118_20
10. Li S, Dai X, Lv R, Li D. Analysis of the application of echocardiography technology in diagnosis of acute myocardial infection. J Infect Public Health. 2021;14(3):428–431. doi:10.1016/j.jiph.2019.08.005
11. Tamborini G, Piazzese C, Lang RM, et al. Feasibility and accuracy of automated software for transthoracic three-dimensional left ventricular volume and function analysis: comparisons with two-dimensional echocardiography, three-dimensional transthoracic manual method, and cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2017;30(11):1049–1058. doi:10.1016/j.echo.2017.06.026
12. Streiff C, Zhu M, Panosian J, Sahn DJ, Ashraf M. Comprehensive evaluation of cardiac function and detection of myocardial infarction based on a semi-automated analysis using full-volume real time three-dimensional echocardiography. Echocardiography. 2015;32(2):332–338. doi:10.1111/echo.12643
13. van den Borne SW, Isobe S, Verjans JW, et al. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J Am Coll Cardiol. 2008;52(24):2017–2028. doi:10.1016/j.jacc.2008.07.067
14. Fontanelli A, Bonanno C. Primary percutaneous coronary intervention in ‘early’ latecomers with ST-segment elevation acute myocardial infarction: the role of the infarct-related artery status. J Cardiovasc Med. 2011;12(1):13–18. doi:10.2459/JCM.0b013e32834038d8
15. Baroldi G, Bigi R, Cortigiani L. Ultrasound imaging versus morphopathology in cardiovascular diseases. Myocardial cell damage. Cardiovasc Ultrasound. 2005;3:32. doi:10.1186/1476-7120-3-32
16. Sheiban I, Fragasso G, Lu C, Tonni S, Trevi GP, Chierchia SL. Influence of treatment delay on long-term left ventricular function in patients with acute myocardial infarction successfully treated with primary angioplasty. Am Heart J. 2001;141(4):603–609. doi:10.1067/mhj.2001.113575
17. Tomoaia R, Beyer RS, Simu G, Serban AM, Pop D. Understanding the role of echocardiography in remodeling after acute myocardial infarction and development of heart failure with preserved ejection fraction. Med Ultrason. 2019;21(1):69–76. doi:10.11152/mu-1768
18. Orii M, Hirata K, Tanimoto T, et al. Two-dimensional speckle tracking echocardiography for the prediction of reversible myocardial dysfunction after acute myocardial infarction: comparison with magnetic resonance imaging. Echocardiography. 2015;32(5):768–778. doi:10.1111/echo.12726
19. Heusch G. Treatment of myocardial ischemia/reperfusion injury by ischemic and pharmacological postconditioning. Compr Physiol. 2015;5(3):1123–1145.
20. Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017;376(21):2053–2064. doi:10.1056/NEJMra1606915
21. Young Altahan R, Goldenberg I, Kimron L, Barsheshet A, Guetta V. Timing of coronary angiography and outcome in patients with non-ST elevation acute coronary syndromes and kidney disease: real-world data from the acute coronary syndromes Israeli survey. Cardiology. 2011;119(4):224–234. doi:10.1159/000332588
22. Huttin O, Marie PY, Benichou M, et al. Temporal deformation pattern in acute and late phases of ST-elevation myocardial infarction: incremental value of longitudinal post-systolic strain to assess myocardial viability. Clin Res Cardiol. 2016;105(10):815–826. doi:10.1007/s00392-016-0989-6
23. Kostic J, Djordjevic-Dikic A, Dobric M, et al. The effects of nicorandil on microvascular function in patients with ST segment elevation myocardial infarction undergoing primary PCI. Cardiovasc Ultrasound. 2015;13:26. doi:10.1186/s12947-015-0020-9
24. Curzen N, Gurbel PA, Myat A, Bhatt DL, Redwood SR. What is the optimum adjunctive reperfusion strategy for primary percutaneous coronary intervention? Lancet. 2013;382(9892):633–643. doi:10.1016/S0140-6736(13)61453-1
25. Huang J, Yan ZN, Fan L, Rui YF, Song XT. Left ventricular longitudinal function assessment in rabbits after acute occlusion of left anterior descending coronary artery by two-dimensional speckle tracking imaging. BMC Cardiovasc Disord. 2017;17(1):219. doi:10.1186/s12872-017-0655-6
26. Huttin O, Zhang L, Lemarie J, et al. Global and regional myocardial deformation mechanics of microvascular obstruction in acute myocardial infarction: a three dimensional speckle-tracking imaging study. Int J Cardiovasc Imaging. 2015;31(7):1337–1346. doi:10.1007/s10554-015-0690-2
27. Foster E, Lease KE. New untwist on diastole: what goes around comes back. Circulation. 2006;113(21):2477–2479. doi:10.1161/CIRCULATIONAHA.106.626697
28. Park SM, Hong SJ, Ahn CM, et al. Different impacts of acute myocardial infarction on left ventricular apical and basal rotation. Eur Heart J Cardiovasc Imaging. 2012;13(6):483–489. doi:10.1093/ejechocard/jer272
29. Bansal M, Leano RL, Marwick TH. Clinical assessment of left ventricular systolic torsion: effects of myocardial infarction and ischemia. J Am Soc Echocardiogr. 2008;21(8):887–894. doi:10.1016/j.echo.2008.01.011
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.