Back to Journals » International Journal of Women's Health » Volume 10
Completion of vaginal hysterectomy by electro surgery using anteroposterior approach in benign cases faced with obliterated posterior cul-de-sac
Authors Purohit R , Sharma J, Meher D, Rakh SR, Malik S
Received 18 April 2018
Accepted for publication 3 July 2018
Published 17 September 2018 Volume 2018:10 Pages 529—536
DOI https://doi.org/10.2147/IJWH.S171575
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Elie Al-Chaer
Ramkrishna Purohit, Jay Gopal Sharma, Devajani Meher, Sanjay Raosaheb Rakh, Surya Malik
Department of Obstetrics and Gynaecology, Purohit General Hospital, Bargarh, India
Background: Obliterated posterior cul-de-sac has been a real surgical challenge during vaginal hysterectomy. The present study demonstrates an anteroposterior approach to accomplish the vaginal hysterectomy in cases faced with an obliterated posterior cul-de-sac.
Methods: In a retrospective study in private setup, 51 consecutive cases with obliterated posterior cul-de-sac during vaginal hysterectomy due to severe benign pelvic adhesions were studied to know the feasibility of the anteroposterior approach. The upper limit of uterus size was that of 16 weeks of gestation.
Results: Vaginal hysterectomy was completed in 49 (96.08%) cases with obliterated posterior cul-de-sac due to severe benign pelvic adhesions. Two (3.92%) cases needed laparoscopic assistance to complete vaginal hysterectomy. Mean operation time was 109.92±40.13 (45–217) minutes due to the need for careful separation of adhesions from the uterus and indicated additional procedures. Mean weight of specimen uterus was 162±106.51 (40–460) grams. There was no major intra- or postoperative morbidity.
Conclusion: Completion of vaginal hysterectomy was feasible using the anteroposterior approach in most of the cases with obliterated posterior cul-de-sac due to severe benign pelvic adhesions.
Keywords: vaginal hysterectomy, obliterated posterior cul-de-sac, anteroposterior approach, difficult vaginal hysterectomy, severe pelvic adhesions, extraperitoneal uterosacral separation, sub-serosal morcellation, frozen pelvis
Introduction
Obliterated posterior cul-de-sac due to severe benign pelvic adhesions obstructs and challenges progress of vaginal hysterectomy. That situation arises occasionally in cases with chronic pelvic pain, severe dysmenorrhea, chronic pelvic inflammatory diseases, restricted uterine mobility, severe pelvic endometriosis, and history of previous pelvic operation. Challenges to find anatomical cleavage amidst severe adhesions by conventional methods increase the apprehension of ureteric injury, gut injury, failed vaginal hysterectomy, and need of laparoscopic or laparotomic conversion.1,2 Abdominal or laparoscopic hysterectomy in cases with obliterated posterior cul-de-sac by severe benign pelvic adhesions also is not without difficulty and surgical challenges.3–5 Many authors have previously tried to describe completion of the least invasive vaginal hysterectomy by conventional methods in cases with obliterated posterior cul-de-sac for benign adhesions.1,6 In the recent years, modalities like bipolar, ligasure, and harmonic have eased surgical dissection in a narrow space caused by adhesions between organs.
Experiences over the years since the description of Purohit technique of vaginal hysterectomy (PTVH) in 2003 using electrosurgery, and other procedures in this hospital,7–9 streamlined and developed a straight forward below described surgical approach to deal with obliterated posterior cul-de-sac to accomplish vaginal hysterectomy.
Methods
In a retrospective study in our hospital from March 2015 through February 2017, 51 consecutive cases with obliterated posterior cul-de-sac due to severe benign pelvic adhesions during vaginal hysterectomy were studied. The upper limit of the uterus size was 16 weeks of gestation. Anteroposterior approach as described below was used in all cases to accomplish vaginal hysterectomy. Written informed consent of each patient was obtained before the operation. Written informed consent was provided to review medical file for research purpose by each patient. Hospital authority permitted to review the records.
The institutional ethics committee of Purohit General Hospital approved the study. Data were analyzed to determine the feasibility of the anteroposterior approach to complete vaginal hysterectomy in benign cases with obliterated posterior cul-de-sac.
Anteroposterior approach
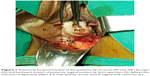
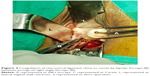
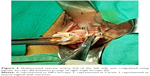
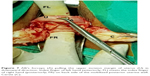
Vaginal hysterectomy was started and continued using procedures described in PTVH and its supplementary techniques.7,8 The posterior vaginal wall was dissected from the cervix extraperitoneally. After detection of the inability to open the posterior cul-de-sac due to obliterated posterior cul-de-sac, the posterior lip of cervix was pulled in anteriorly forward direction and the Sim’s speculum was enhanced upward through the extraperitoneal loose fascial plane between the cervix and rectum. That maneuver exposed the pillar of uterosacral ligament on both sides (Figure 1) running posterolaterally from the cervix. The blunt tip of a right angle forceps (PTVH forceps; Creative Surgicals, Mumbai, India) was inserted close to uterus through the loose tissue plane between uterosacral ligament and uterine wall (Figure 2), and the lower end of uterosacral ligament was hooked extraperitoneally by the bend of the forceps (Figure 2) following the similar technique used for cases without obliterated posterior cul-de-sac (Figure 3).7 The uterosacral ligament was then stretched and spread between the prongs of the right angle forceps, coagulated close to the uterine wall (Figure 4) by bipolar current (45–50 watts) through a bipolar forceps with 2 mm tip width, and separated from the uterus by scissors.7,8 The procedure was repeated on the contralateral side to release the uterosacral ligaments bilaterally. Extraperitoneal separation of scarred uterosacral ligaments was the cardinal step in this technique to increase descent of uterus towards the surgeon. Then, the uterine arteries were accessed by right angle forceps extraperitoneally and coagulated close to the uterus (Figure 5) using bipolar current of 45–50 watts and separated from uterus to reduce intraoperative bleeding.7 Then, the posterior dissection was stopped.
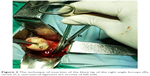  | Figure 3 The technique of insertion of the blunt tip of the right angle forceps (R), cervix (C), uterosacral ligament (U, arrow) of left side. |
Anteriorly, the vesicouterine loose fascial plane was dissected and anterior retractor was enhanced. Then, the cervix was split longitudinally in the midline, and the said incision was enhanced posteriorly up to the wall of adhesion that obstructed entry into the posterior cul-de-sac, and anteriorly, up to the uterovesical peritoneum if accessible. From the inner side of the midline cervico-uterine incision, sub-serosal morcellation of myometrium (Figure 6) by surgical knife was done repeatedly to excavate the inner core of the uterine musculatures leaving the serosa to decompress the bulk of the uterus.8 That decompression maneuver of the uterine wall automatically increased the downward descent of upper uterine incision, and the visibility of uterovesical pouch. Then, the anterior pouch was entered by scissors under vision.
Further, sub-serosal morcellation was done to decompress the upper wider bulk of body of uterus. Downward traction of the upper incision margin of the decompressed anterior wall of uterus at this stage increased visualization of the upper pedicles and adhesions. The round ligament, tube, and ovarian ligament of both sides were separated by scissors after bipolar coagulation (45–50 watts) using bipolar forceps to increase descend of fundus. Alli’s forceps held the round ligament stump on each side. Surgically freed portion of the fundus of uterus was excised under vision to reduce fundo-cervical length of the uterus, and increase visibility of posterior pelvic adhesions to the uterus. Then, the upper incised margin was pulled in downward direction by Alli’s forceps, and the tip of index finger was swept above it and the adhesions between the posterior wall of the uterus and posterior pelvic wall were gently broken by movement of the index finger to increase descend and mobility of the posterior wall of the uterus. Sub-serosal morcellation, if needed, was continued to thin out the remaining posterior uterine wall to further increase visibility of posterior pelvic adhesions to the uterus. The broad ligament adhesions at this stage were separated bilaterally after bipolar coagulation hemostasis close to the uterine wall between the prongs of right angle forceps.7 These maneuvers further mobilized down the wall of adhesion that initially obstructed entry into the posterior cul-de-sac.
Now, the tip of index finger of the right hand was guided from above along the back of the residual posterior uterine wall through the anterior opening, and the index finger of the left hand was guided from below along the posterior wall of cervix. Both fingers tip assessed thickness of wall of adhesion that obliterated posterior cul-de-sac (Figure 7) and then, a thin area along the “wall of adhesion” was gently penetrated by a finger to open a path, being the most interesting final step of the approach. Then, the tip of right angle forceps was guided by the posteriorly placed index finger to expose its tip anteriorly through the anterior opening, prongs of forceps were opened and the posterior uterine wall was divided in the midline longitudinally between the prongs of right angle forceps (Figure 8). Then, each half of the posterior wall was separated from the remaining lateral adhesions to complete the hysterectomy. In case of plastic adhesions of the rectum to a part of the uterine wall, a coat of serosa was spared with rectum to avoid rectal injury.
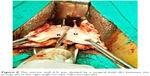  | Figure 8 The uterine wall (U) was divided by a surgical knife (K) between the prongs (P) of the right angle forceps. Alli’s forceps (A). |
After vaginal hysterectomy, adhered adnexa were mobilized from the lateral pelvic wall using transvaginal mobilization of adhered adnexa from the lateral pelvic wall using tactile feel.9
Adhesiolysis and adnexectomy were done vaginally under vision in indicated cases.9 Chocolate cystectomy if any, was done to preserve ovary in indicated cases.9
Endometriotic nodule if any, at the vaginal wall and uterosacrals was palpated by fingers and excised under vision using bipolar forceps hemostasis.
Deaver retractors and pelvic illuminator with fiber optic light source were used occasionally to improve exposure and visibility of the deeper field of operation procedure.8,9
Endometriotic nodule and puckered scars in the rectovaginal septum and uterosacrals were palpated by fingers and excised under vision using bipolar forceps hemostasis. Post-hysterectomy laparoscopy was done in cases with chronic pelvic pain and dysmenorrhea to find if any, a vaginally invisible condition in the upper part of pelvis that needed surgery.7 Before starting laparoscopy, the vault was temporarily closed by 2–3 Alli’s forceps horizontally to restore pneumoperitoneum.
Injection depot medroxyprogesterone acetate (DMPA) 150 mg was given every 3 months to patients with endometriosis for 2 years to prevent recurrence and patients were followed up every 6 months.
Results
Mean age of patients was 41.49±3.95 (35–50) years. The majority of patients had >1 vaginal birth. Eight (15.68%) cases were nulliparous. Four (7.84%) cases had a history of previous pelvic laparotomy for myomectomy. Two (3.92%) cases had a history of pelvic laparotomy for some other reasons. Two (3.92%) cases had a history of previous cesarean section. Thirteen (25.49%) cases had chronic pelvic pain, 31 (60.78) had dysmenorrhea, 2 (3.92%) had defecation pain during menstruation (dyschezia), and 21 (41.18%) had heavy menstrual bleeding. Restricted uterine mobility was found in all 51 (100%) cases, endometriotic nodule in fornices in 4 (7.84%), but none of the cases had infiltrating endometriosis into the lumen of rectum. Forty-six (90.2%) cases had uterus size below 12 weeks of gestation. Five (9.80%) cases had uterus size between 13–16 weeks of gestation. Twenty-four (47.06%) cases had associated adnexal cysts. Of them, 23 cases had a cyst located in the pelvis, and 1 had a big chocolate cyst of 15 cm located above the pelvis. Few cases had >1 indication of hysterectomy; fibroid uterus 14 (27.45%), grade IV endometriosis 36 (70.59%), chronic pelvic inflammatory disease 5 (9.80%), dysfunctional uterine bleeding 1 (1.96%), and adenomyosis uterus 5 (9.80%) cases. Preoperative evaluation was done in all cases using transabdominal and transvaginal ultrasonography, and MRI was used in few cases. Obliterated posterior cul-de-sac was confirmed or known by a prior diagnostic laparoscopy in 13 (25%) cases (Table 1).
  | Table 1 Patient characteristics |
Anterior cul-de-sac was not obliterated in all cases in spite of obliterated posterior cul-de-sac and was a clue to enter peritoneal cavity through anterior pouch. Vaginal hysterectomy was successfully completed in 49 (96.08%) of 51 cases. Two (3.92 %) cases needed laparoscopic assistance to complete vaginal hysterectomy. In 1 case, laparoscopic lysis (adhesions following previous myomectomy) of the posterior wall of fundus of uterus from the upper part of pelvis and in other, laparoscopic suction-aspiration decompression of the adhered large right ovarian chocolate cyst (15 cm) to the upper part of pelvis and uterus, allowed descent of uterus for a vaginal completion of hysterectomy.9 None of the cases needed laparotomic conversion to complete a hysterectomy. A coat of serosa of the uterus was spared with rectal wall in 3 (5.88%) cases to avoid rectal wall injury. Adnexectomy (uni- or bilateral) was indicated in 11 (21.57%) cases. It was successfully completed vaginally in 10 (19.60%) cases and failed in 1 (1.92%) case (Table 2).
  | Table 2 Intra- and postoperative outcomes |
That case needed laparoscopic adnexectomy after vaginal hysterectomy. Ovarian cystectomy was done successfully vaginally in all 12 (23.53%) indicated cases to preserve ovaries. Endometriotic nodules in the posterior vaginal fornix were excised in all 4 (7.84%) cases.
Post hysterectomy laparoscopy (PHL) was performed in 32 (62.75%) cases. The other 19 (59.38%) cases did not need PHL. However, only 10 (31.25%) of 32 cases had vaginally unreached adhesions and needed short laparoscopic procedures; 9 (28.12%) cases needed lysis of omental adhesions and 1 (3.12%) needed completion of adnexectomy of densely adhered adnexa following failed attempted vaginal adnexectomy. Other 22 (68.75%) of 32 cases had no other adhesions, did not need any laparoscopic procedure, indicating adequacy of vaginal hysterectomy, and additional procedures by the present method. The mean PHL time was 10.43±6.14 (5–28) minutes.
Outcomes of the present study were compared with a group of 51 cases with similar preoperative patients characteristics, that had no note of obliterated posterior cul-de-sac during vaginal hysterectomy by Purohit technique for benign indications,7,8 and found that the mean total operation time in study group was significantly (P<0.05) more than the control group (109.92±40.13 [45–217] vs 54.17±17.81 [25–90] minutes). The need of blood transfusion, mean specimen weight of uterus, post-operative complications, and mean hospital stay did not differ significantly in both groups. None of study and control cases had intraoperative injury to pelvic organs like rectum or ureter.
No recurrence of endometriosis was observed in 30 consecutive cases that completed 2 years follow-up.
Discussion
Inability to open posterior cul-de-sac due to dense benign adhesions, severe endometriosis during vaginal hysterectomy using conventional clamps and sutures, and inability to progress upward using a clear guideline to complete a vaginal hysterectomy have still been the possible reasons of conversion to abdominal hysterectomy.10 In spite of the benefits, bipolar hemostasis of lateral pedicles, modern equipment for increasing visibility and right angle forceps as an anatomy delineator have still been underutilized by gynecologic surgeons in improving feasibility of vaginal hysterectomy.7–9,11,12 A robot-assisted laparoscopic hysterectomy has been suggested by few studies as an alternative to conventional laparoscopic hysterectomy in such cases with severe pelvic adhesions to ease the operation and reduce morbidities.13,14
In the present study, vaginal hysterectomy was completed in majority of cases with obliterated posterior cul-de-sac due to severe benign pelvic adhesions. Parity did not affect completion of the least invasive vaginal hysterectomy; however, in nulliparous cases, a narrow speculum and retractors with bipolar forceps coagulation hemostasis eased operation.
Fixity of uterus to the anterior abdominal wall being a cause of failed vaginal hysterectomy had been excluded preoperatively in cases with previous cesarean section from the clinical and sonographic features.8,15
In cases with obliterated posterior cul-de-sac due to dense benign adhesions in the present study, rectal wall adhered to posterior surface of uterus–cervix, uterosacrals adhered to lateral wall of uterus, and raised the uterus–cervix to a high level and compromised uterine mobility. It also raised the level of “wall of adhesion” obstructing posterior cul-de-sac beyond reach. The uterosacral ligaments and uterine arteries, which were accessible vaginally extraperitoneally during a vaginal hysterectomy in spite of severe pelvic adhesions in the present method, were searched with difficulty from above through dense adhesions in cases of laparoscopic hysterectomy.4,16 The bipolar forceps coagulation using bipolar forceps close to uterus between prongs of a right angle forceps did not cause thermal injury to rectum or ureter in any case in this study. This study never used bi-clamp forceps for coagulation hemostasis of lateral pedicles due to space constraint in cases with severe pelvic adhesions.11,12 Adhesion of stretchable rectal wall to posterior wall of cervix did not obstruct downward descent of uterus. Sub-serosal morcellation was another very most important maneuver in this technique to remove obstruction caused by the bulk of uterus, and to increase visibility of anterior cul-de-sac, and of lateral adhesions with lateral pedicles. Sub-serosal morcellation and subsequent uterine decompression also allowed finger dissection of adhesions by tactile feel on the backside of uterus up to the posterior pouch. As was observed in this study, the “wall of adhesion” between cervix and rectum that obstructed access to posterior cul-de-sac was not very thick; rather, it was highly placed and unreached. After mobilization, it was easily reached and approached by tactile feel. Immobility of uterus due to benign dense adhesions being a relative contraindication to conventional vaginal hysterectomy, thus, did not affect progress of operation by the present method.10
Similar to our earlier observation, the endometriotic adhesions were easily broken by finger movement, post inflammatory adhesions were stronger than endometriotic adhesions and postoperative adhesions were very strong to break by finger in few cases.9
The approach of the present study had a special advantage of coagulation hemostasis in narrow lateral spaces using a bipolar forceps than that by conventional suture hemostasis.9 Thus, rate of failed vaginal hysterectomy due to inaccessible posterior cul-de-sac, and failed indicated adnexectomy due to severe pelvic adhesions had been minimized by the present study than that reported by earlier studies in similar situations.1,2,17 Previous history of myomectomy, similar to previous studies, may rarely cause a more difficult or a failed vaginal hysterectomy.18
Similar to our previous study, there occurred adequate exposure of operation field after vaginal hysterectomy up to the adnexa and infundibulopelvic ligament stump in indicated cases of adnexectomy in the present study.9 The pelvic illuminator with fiber optic light source adequately improved visibility when needed. Adhesions anterior to rectum, between folds of broad ligament, and between rectum and uterosacrals were accessible directly under vision, and adhesiolysis was done after a tactile feel. The adhesions in the upper part of pelvis, anterolateral pelvis, and anterior to bladder were not seen vaginally and, thus, are taken care by a short PHL in indicated cases.
Vaginal hysterectomy by the present method and a PHL in indicated cases may be enough to deal with such cases with severe pelvic adhesions due to endometriosis and other benign conditions. Symptoms of dysmenorrhea, dyspareunia, defecation pain during menstruation (dyschezia), etc, in cases with endometriosis were relieved by the present method of surgery without the need for a radical approach, as suggested by a study.19 Recurrence of endometriosis after surgery and DMPA therapy was not observed in cases that completed 2 years follow-up.
Long operation time was due to extensive adhesiolysis up to the adnexa and additional procedures. However, our total operation time of 109.92±40.13 minutes by vaginal hysterectomy was less than the operation time reported by Chalermchockchareonkit et al (185.1±48.7 and 139.9±52.4 minutes for laparoscopic hysterectomy and abdominal hysterectomy, respectively), and by few more previous studies for laparoscopic hysterectomy, in a similar condition of benign severe pelvic adhesions.3,4,20 It was nearly 2 times more than that of mean operation time for vaginal hysterectomy by electrosurgery in cases without severe endometriosis, and was nearly one and a half time more than that of mean operation time in cases with history of previous cesarean section.7,8 Compared with outcomes in our previous studies, however, the major intraoperative and postoperative complications like injury to bladder, ureter, and rectum did not increase due to vaginal hysterectomy and additional procedures in cases with severe benign pelvic adhesions by the present approach.7–9 Downward mobilization of the rectouterine adhesions to surgeon’s reach and dissection using tactile feel advantaged in this study over a laparoscopic surgery to avoid injury to rectum.19 In 13 (25 %) cases, obliterated posterior cul-de-sac was confirmed or known by a prior diagnostic laparoscopy and the hysterectomy and additional procedures were done in this study. So, it will not be unsafe to conduct a prospective study in the future to evaluate the validity of this study.
Conclusion
Completion of vaginal hysterectomy was feasible using the anteroposterior approach in most of the cases with obliterated posterior cul-de-sac due to severe benign pelvic adhesions.
Disclosure
The authors report no conflicts of interest in this work.
References
Sizzi O, Paparella P, Bonito C, Paparella R, Rossetti A. Laparoscopic assistance after vaginal hysterectomy and unsuccessful access to the ovaries or failed uterine mobilization: changing trends. JSLS. 2004;8(4):339–346. | ||
Furuhashi M, Suganuma N. A survey of vaginal hysterectomy ending in laparotomy. Arch Gynecol Obstet. 2002;267(2):57–59. | ||
Chalermchockchareonkit A, Tekasakul P, Chaisilwattana P, Sirimai K, Wahab N. Laparoscopic hysterectomy versus abdominal hysterectomy for severe pelvic endometriosis. Int J Gynaecol Obstet. 2012;116(2):109–111. | ||
Paul PG, Shabnam K, Bhosale SA, Kaur H, Talwar P, Thomas T. Laparoscopic hysterectomy in frozen pelvis – an alternative technique of retrograde adhesiolysis. Gynecol Surg. 2013;10(4):285–290. | ||
Song T, Kim TJ, Kang H, et al. Factors associated with complications and conversion to laparotomy in women undergoing laparoscopically assisted vaginal hysterectomy. Acta Obstet Gynecol Scand. 2012;91(5):620–624. | ||
Pelosi MA 3rd, Pelosi MA. Vaginal hysterectomy for benign uterine disease in the laparoscopically confirmed frozen pelvis. J Laparoendosc Adv Surg Tech A. 2009;7(6):345–351. | ||
Purohit RK. Purohit technique of vaginal hysterectomy: a new approach. BJOG. 2003;110(12):1115–1119. | ||
Purohit RK, Sharma JG, Singh S, Giri DK. Vaginal Hysterectomy by Electrosurgery for Benign Indications Associated with Previous Cesarean Section. J Gynecol Surg. 2013;29(1):7–12. | ||
Purohit RK, Joshi S, Sharma JG. Adnexectomy During Vaginal Hysterectomy for BenignIndications Using Bipolar Hemostasis of Lateral Pedicles and Transvaginal Adnexa Mobilization. J Gynecol Surg. 2015;31(2):86–91. | ||
Ray A, Pant L, Magon N. Deciding the Route for Hysterectomy: Indian Triage System. J Obstet Gynaecol India. 2015;65(1):39–44. | ||
Zubke W, Hornung R, Wässerer S, et al. Bipolar coagulation with the BiClamp forceps versus conventional suture ligation: a multicenter randomized controlled trial in 175 vaginal hysterectomy patients. Arch Gynecol Obstet. 2009;280(5):753–760. | ||
Samulak D, Wilczak M, Michalska MM, Pięta B. Vaginal hysterectomy with bipolar coagulation forceps (BiClamp) as an alternative to the conventional technique. Arch Gynecol Obstet. 2011;284(1):145–149. | ||
Chiu LH, Chen CH, Tu PC, Chang CW, Yen YK, Liu WM. Comparison of robotic surgery and laparoscopy to perform total hysterectomy with pelvic adhesions or large uterus. J Minim Access Surg. 2015;11(1):87–93. | ||
Bedaiwy MA, Rahman MY, Chapman M, et al. Robotic-assisted hysterectomy for the management of severe endometriosis: a retrospective review of short-term surgical outcomes. JSLS. 2013;17(1):95–99. | ||
Sheth SS, Shah NM, Varaiya D. A Sonographic and Clinical Sign to Detect Specific Adhesions Following Cesarean Section. J Gynecol Surg. 2008;24(1):27–36. | ||
Hsu WC, Chang WC, Huang SC, Sheu BC, Torng PL, Chang DY. Laparoscopic-assisted vaginal hysterectomy for patients with extensive pelvic adhesions: a strategy to minimise conversion to laparotomy. Aust N Z J Obstet Gynaecol. 2007;47(3):230–234. | ||
Sheth SS. Adnexectomy for benign pathology at vaginal hysterectomy without laparoscopic assistance. BJOG. 2002;109(12):1401–1405. | ||
Occhino JA, Gebhart JB. Difficult vaginal hysterectomy. Clin Obstet Gynecol. 2010;53(1):40–50. | ||
de La Hera-Lazaro CM, Muñoz-González JL, Perez RO, et al. Radical Surgery for Endometriosis: Analysis of Quality of Life and Surgical Procedure. Clin Med Insights Womens Health. 2016;9:7–11. | ||
Walid MS, Heaton RL. Total laparoscopic extirpation of a fixed uterus from benign gynecological disease. Gynecol Surg. 2011;8(2):157–159. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.