Back to Journals » Infection and Drug Resistance » Volume 15
Compassionate Use of Contezolid for the Treatment of Tuberculous Pleurisy in a Patient with a Leadless Pacemaker
Authors Kang Y, Ge C, Zhang H, Liu S, Guo H, Cui J
Received 14 May 2022
Accepted for publication 1 August 2022
Published 12 August 2022 Volume 2022:15 Pages 4467—4470
DOI https://doi.org/10.2147/IDR.S373082
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yixin Kang,1,* Cheng Ge,2,* Huan Zhang,3 Saizhe Liu,4 Hongyang Guo,4 Junchang Cui1
1Department of Respiratory Diseases, the First Medical Center, Chinese People’s Liberation Army General Hospital, Beijing, 100853, People’s Republic of China; 2Department of Cardiology, the First Medical Center, Chinese People’s Liberation Army General Hospital, Beijing, 100853, People’s Republic of China; 3Center of Medicine Clinical Research, the First Medical Center, Chinese People’s Liberation Army General Hospital, Beijing, 100853, People’s Republic of China; 4Department of Cardiology, the Sixth Medical Center, Chinese People’s Liberation Army General Hospital, Beijing, 100853, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongyang Guo; Junchang Cui, The First Medical Center, Chinese People’s Liberation Army General Hospital, No. 28 Fuxing Road, Haidian District, Beijing, 100853, People’s Republic of China, Email [email protected]; [email protected]
Abstract: We report the case of an 87-year-old woman with tuberculous pleurisy. She developed adverse effects in the form of thrombocytopenia and gastrointestinal hemorrhage with isoniazid, and thrombocytopenia with linezolid. Her treatment was switched to contezolid plus cycloserine for a 4-week antibiotic duration, with a favorable outcome.
Keywords: contezolid, tuberculous pleurisy
Introduction
Contezolid is a novel oxazolidinone antibiotic that was approved by the National Medical Products Administration of China (NMPAC) on 1 June 2021 mainly for the treatment of complicated skin and soft tissue infections (cSSTI).1 Contezolid has a broad antibacterial spectrum against staphylococci, streptococci as well as enterococci. Besides, contezolid can also be used to treat clinically relevant drug-resistant pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus epidermidis (MRSE), penicillin-resistant Streptococcus pneumoniae (PRSP), and vancomycin-resistant Enterococcus (VRE).1 Compared to linezolid, contezolid has a good safety profile with a significantly reduced potential for myelosuppression and monoamine oxidase inhibition (MAOI).2 Here, we present a case of contezolid for the treatment of tuberculous pleurisy. Reviewing the literature, this case was reported for the first time worldwide.
Case Presentation
An 87-year-old female with R-R long intervals due to atrial fibrillation admitted to our hospital. On Day +15, she underwent leadless pacemaker implantation (“on Day +X” means that “on Day X after admission”). She received regular infection prevention treatment both preoperatively and postoperatively. On Day +16, she developed an unexplained low-grade fever without cough or sputum. She also had night sweats. Blood counts indicated slightly elevated neutrophil, C-reactive protein (CRP), and interleukin-6 (IL-6) levels. The T-SPOT test repeatedly suggested elevated levels of antigen A and antigen B. Additionally, her blood sedimentation level was significantly elevated, fluctuating between 60 mm/h and 70 mm/h.
On Day +23, she received regular anti-infective therapy with a specific regimen of cefoperazone-sulbactam 3 g/8 h. On Day +42, she underwent chest ultrasonography, which suggested that the amount of pleural fluid had increased compared to the previous amount. At this point, we considered regular anti-infection treatment ineffective. On Day +46, she underwent thoracentesis and drainage. We sent her pleural fluid for testing, and the results suggested that her pleural fluid/serum protein was >0.5. Therefore, we considered the pleural effusion to be exudate. Considering that the patient was an elderly woman, the patient’s family refused to undergo pleural biopsy.
On Day +57, she received diagnostic anti-tuberculosis treatment. The specific regimen was isoniazid 0.3 g/d, rifapentine 0.9 g/week, pyrazinamide 0.25 g/8 h, levofloxacin 0.5 g/d, and methylprednisolone 40 mg/d. Additionally, she underwent a computer tomography (CT) scan of the chest, as shown in Figure 1A. We observed a moderate amount of liquid density shadow in the chest cavity bilaterally, mainly on the right side, with a CT value of 5 HU. Chest CT also suggested that adjacent lung tissue was compressed. After diagnostic anti-tuberculosis treatment, her low-grade fever resolved, and pleural fluid decreased. Besides, she has a 20-year history of exposure to tuberculosis (TB) patients. Therefore, we made a clinical diagnosis of tuberculous pleurisy for this patient.
On Day +72, she underwent ultrasonography of the chest, which showed no fluid in the chest cavity bilaterally. On Day +74, her platelet dropped from 189*109/L to 43*109/L. We considered thrombocytopenia in relation to the use of isoniazid. Therefore, we temporarily stopped the anti-tuberculosis treatment. Her platelet levels returned to normal after stopping anti-tuberculosis treatment.
On Day +92, we added linezolid 600 mg/12 h and moxifloxacin 0.4g/d. After using linezolid and moxifloxacin, her symptoms were better than before. On Day +110, she reappeared with thrombocytopenia, and she received platelet transfusion therapy. We believed that the cause of this thrombocytopenia was related to the use of linezolid. Therefore, we adjusted the anti-tuberculosis regimen to cycloserine 0.25 g/d and contezolid 400 mg/d. During the follow-up treatment, her platelet count increased from 68*109/L to 324*109/L. She did not have any further adverse reactions. Therefore, we increased the drug doses of cycloserine and contezolid. On Day +121, we modified the drug dose to cycloserine 0.25 g/12 h and contezolid 400 mg/12 h. On Day +131, she underwent another chest CT examination, as shown in Figure 1B. This result indicated a significant reduction in bilateral pleural effusion compared with the image in Figure 1A. Her body temperature fluctuated within the normal range. Therefore, we considered the anti-tuberculosis treatment effective.
For the treatment of heart diseases, rivaroxaban 10 mg/d was used for the treatment of atrial fibrillation. She was bedridden for a long time. Her pacemaker function was normal prior to death. Therefore, heart diseases have little relationship with the death of this patient. We considered that she died of a pulmonary embolism on Day +136. Besides, Figure 2 shows the treatment of this patient.
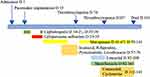 |
Figure 2 Antimicrobial regimens used in this patient and correlated with side effects, and interventions during inpatient days. D, patient day(s). |
Discussion
Contezolid is a new class of oxazolidinones with a significant safety profile. It demonstrated good in vivo and in vitro activity against M. tuberculosis.3 Shoen et al collected 22 M. tuberculosis isolates and conducted in vivo and in vitro tests. In vitro susceptibility tests showed that contezolid was comparable to linezolid against both drug-susceptible and drug-resistant M. tuberculosis.3 In addition, the activity of contezolid in a murine tuberculosis model was also similar to that of linezolid.
Linezolid was the first oxazolidinone antibiotic used in humans. Linezolid has promising in vitro and in vivo activities against M. tuberculosis.4,5 For patients with extensively-drug resistance M. tuberculosis pulmonary infections, linezolid was effective for achieving culture conversion.6 Therefore, linezolid is a useful drug in the treatment of human drug-resistant tuberculosis. However, the ribosomal target site of linezolid action is homologous to the mitochondrial protein synthesis pathway. The clinical application of linezolid has been limited due to its adverse effects. Most of the adverse effects of linezolid were associated with mitochondrial toxicity.7 The common adverse effects of linezolid included myelosuppression (ie: thrombocytopenia, leukopenia, pancytopenia, and anemia). The myelosuppressive effects of linezolid are generally reversible. In our previously described case, the patient developed severe thrombocytopenia after the use of linezolid. Her platelet levels rose to normal after stopping linezolid. Besides, she did not experience any adverse effects during the application of contezolid. And contezolid demonstrated good clinical efficacy for tuberculous pleurisy. However, the optic neuropathy caused by linezolid often results in irreversible visual impairment.8
Contezolid has a specific structure. This can improve its binding to bacterial targets and reduce the adverse effects on mitochondria. Therefore, contezolid can more efficiently kill bacteria and reduces the risk of drug resistance. Moreover, contezolid does not affect the function of mitochondria and may reduce myelosuppressive toxicity.9 In regard to drug metabolism, flavin mono plus oxidase 5 (FMO5) is the main metabolic pathway of contezolid. Drugs metabolized by FMOs can reduce the adverse effects of drug‒drug interactions.
In conclusion, in the case we describe, contezolid demonstrated a good safety and efficacy profile in the treatment of M. tuberculosis pulmonary infections. Contezolid can be considered as an option for the treatment of tuberculous pleurisy. However, the current NMPAC-approved indication for contezolid is for cSSTI only. Therefore, the efficacy and safety of contezolid in the treatment of M. tuberculosis pulmonary infections still needs to be verified by large-scale randomized controlled trials. In addition, it is meaningful to determine whether the use of the less toxic oxazolidinone contezolid for the treatment of M. tuberculosis pulmonary infections would improve efficacy and result in a shorter, simpler course.
Ethical Approval Statement
The patient has provided written informed consent to publish their case details and any accompanying images. Besides, Chinese People’s Liberation Army General Hospital has approved this case report for publication.
Funding
This work was supported by the Military biosafety research fund (No. 19SWAQ06) and the “13th Five-Year” Military Key Discipline Construction Project of the PLA Medical College. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors have no conflicts of interest to declare. All coauthors have seen and agree with the contents of the manuscript and there is no financial interest to report.
References
1. Hoy SM. Contezolid: first Approval. Drugs. 2021;81:1587–1591. doi:10.1007/s40265-021-01576-0
2. Gordeev MF, Yuan ZY. New potent antibacterial oxazolidinone (MRX-I) with an improved class safety profile. J Med Chem. 2014;57:4487–4497. doi:10.1021/jm401931e
3. Shoen C, DeStefano M, Hafkin B, et al. In Vitro and In Vivo Activities of Contezolid (MRX-I) against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2018;1:62.
4. Erturan Z, Uzun M. In vitro activity of linezolid against multidrug-resistant Mycobacterium tuberculosis isolates. Int J Antimicrob Agents. 2005;26:78–80. doi:10.1016/j.ijantimicag.2005.03.006
5. Maltempe FG, Caleffi-Ferracioli KR, Do Amaral RCR, et al. Activity of rifampicin and linezolid combination in Mycobacterium tuberculosis. Tuberculosis. 2017;104:24–29. doi:10.1016/j.tube.2017.02.004
6. Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012;367:1508–1518. doi:10.1056/NEJMoa1201964
7. Vinha DC, Rubinstein E. Linezolid: a review of safety and tolerability. Journal of Infection. 2009;59:S59–S74. doi:10.1016/S0163-4453(09)60009-8
8. Junping L, Ramesh CT, Tripathi BJ. Drug-Induced Ocular Disorders. Drug Safety. 2008;31:127–141. doi:10.2165/00002018-200831020-00003
9. Wright A, Deane-Alder K, Marschall E, et al. Characterization of the Core Ribosomal Binding Region for the Oxazolidone Family of Antibiotics Using Cryo-EM. ACS Pharmacol Transl Sci. 2020;3:425–432. doi:10.1021/acsptsci.0c00041
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

