Back to Journals » Cancer Management and Research » Volume 12
Comparisons of Therapeutic and Aesthetic Effects of One-Stage Implant-Based Breast Reconstruction with and without Biological Matrix
Authors Gao P, Wang Z, Kong X, Wang X, Fang Y , Wang J
Received 19 September 2020
Accepted for publication 2 December 2020
Published 29 December 2020 Volume 2020:12 Pages 13381—13392
DOI https://doi.org/10.2147/CMAR.S282442
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Peng Gao, Zhongzhao Wang, Xiangyi Kong, Xiangyu Wang, Yi Fang, Jing Wang
Department of Breast Surgical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China
Correspondence: Yi Fang; Jing Wang
Department of Breast Surgical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China
Email [email protected]; [email protected]
Background: Biological matrix can provide coverage of compromised muscle and augment the subpectoral pocket in the one-stage reconstruction. However, few studies compared one stage implant-based breast reconstruction with and without biological matrix. The primary endpoint of our study was to assess the patient-reported outcomes (PROs) based on BREAST-Q version 2.0 and analyze complications between SIS matrix-assisted implant-based breast reconstruction (IBBR) and no-matrix-assisted IBBR.
Methods: This retrospective single-center study was conducted from May 2015 to April 2019, and we analyzed 155 patients who underwent one-stage IBBR with at least 1 year of follow-up. Seventy-nine patients underwent one-stage IBBR with SIS matrix group and 76 patients underwent one-stage IBBR without SIS matrix group were evaluated of PROs with BREAST-Q version 2.0 (from 3 different domains) and compared with complications. Complications occurred in patients were divided into major complications and minor complications.
Results: In the satisfaction domain, the mean score for satisfaction with breasts was 60.27 (17.71) in the SIS matrix group and 54.49 (14.76) in the no-matrix group, p=0.045. The multivariate logistic regression for postoperative complications in the whole series pointed out a statistical significance for age> 40 years old (odds ratio 3.314, 95% CI 1.012– 10.854, p=0.048) and patients with endocrine therapy (odds ratio 0.260, 95% CI 0.092– 0.736, p=0.011).
Conclusion: Patients who underwent SIS matrix-assisted one-stage IBBR yield better results in PROs of satisfaction with breasts. Other domains and complications between the two groups had no significant difference.
Keywords: breast cancer, one-stage implant-based breast reconstruction, biological matrix, BREAST-Q version 2.0
Introduction
Implant-based reconstruction becomes more popular in the therapeutic course following skin- and nipple-sparing mastectomy (SSM/NSM).1 The advent of biological matrix can provide coverage of compromised muscle and fascia to increase the structural strength and augment the subpectoral pocket in the direct-to-implant reconstruction (one-stage).2 Direct-to-implant reconstruction with biological matrix can avoid the need for an additional operation in tissue expander/implant reconstruction (two-stage). However, whether biological matrix can really improve aesthetic outcome has always been a debate during the past decade.
Biological tissue-derived matrices are composed of human or animal tissues such as dermis, pericardium, or small intestine submucosa, and acellular dermal matrices (ADMs) are commonly used in the implant-based breast reconstruction (IBBR).3 The clinical use of biological matrix has changed the technique of implant-based breast reconstruction. However, outcomes of studies of IBBR with biological matrices vary widely. Some studies had demonstrated the benefits of ADMs and other biological matrices. As the extension of the elevated pectoralis major muscle, the biological matrix can directly support the implant so that the skin can avoid the negative consequence of internal pressure of the implant. It is also thought to achieve a good-looking breast in the cosmetic outcome. And biological matrix can possibly reduce the incidence of capsular contraction.4–7 However, some conclusions did not yield superior results in surgery with biological matrix. As a non-vascularised material in a setting with poorly circulated mastectomy flaps, the use of biological matrix in breast reconstruction might increase the risk of complications.8,9 And a previous prospective randomized trial showed that implant removal and other surgical complications were significantly higher in one-stage IBBR with ADM.10
With a general shift in health care towards patient-centeredness and with the improvement of surgical techniques, surgeons not only value the basic outcomes of surgical results but pay more attention to understand the patient-reported outcomes (PROs) following breast reconstruction.11 BREAST-Q, which was introduced since 2009, has been increasingly used and has become the gold standard to assess PROs after breast reconstruction in many countries. And the BREAST-Q version 2.0 has changed some scales in detail to better assess health-related quality of life (QOL) and patient satisfaction.12,13 However, outcomes of patient satisfaction after biological matrix-assisted breast reconstruction are scarce.
Currently, a wide variety of biological matrices have been increasingly used in IBBR. SIS matrix (Biodesign Surgisis, Cook Biotech), which is derived from porcine small intestine submucosa, is used in IBBR to offload the weight of the implant from the lower pole of the tissue envelope.14 Many researches have studies different biological matrices’ value between one-stage and two-stage IBBR.15–17 To our knowledge, seldom studies have analyzed the surgical outcomes and PROs only based on one-stage IBBR and evaluating PROs with BREAST-Q version 2.0.
The primary endpoint of this study was to assess the PROs based on BREAST-Q version 2.0 between SIS matrix-assisted one-stage IBBR and no-matrix-assisted one-stage IBBR. Besides, the second endpoint is to analyze the complications between the two groups.
Methods
Study Population
This retrospective study is to evaluate PROs in post-mastectomy breast reconstruction which is approved by the Good Clinical Practice Center of Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College, and all participants had written the informed consent before the study. Patients in this study undergoing direct-to-implant reconstruction from May 2015 to April 2019 were eligible for inclusion in our single cancer center. Two breast oncology surgeons were included in our study to perform the one-stage implant-based breast reconstruction. Patients undergoing one-stage IBBR with SIS matrix or without matrix were diagnosed with breast cancer by pathology at least one side, and only patients with a minimum follow-up of 1year from reconstruction were included. Exclusion criteria included patients <18 years of age, contraindications with porcine, and patients who previously underwent breast plastic surgery.
Questionnaire Collection
PROs were measured with BREAST-Q version 2.0, which include 1) the health-related QOL domain (psychosocial well-being, physical well-being: chest, and sexual wellbeing), 2) the satisfaction domain (satisfaction with breasts, satisfaction with implants) and 3) the experience domain (satisfaction with surgeon). Values for BREAST-Q 2.0 subscales were converted to the equivalent Rasch transformed scores that range from 0 to 100, by using the scoring table. And higher scores reflect a superior patient satisfaction or better QOL.18,19 Surveys were completed online or by mobile-phone with questionnaires.
Patient Variables
Baseline data collected during the initial consultation included age, body mass index (BMI), SIS matrix or not, implant volume, laterality (unilateral versus bilateral procedures), Histology, lymph node management (sentinel lymph node or axillary lymph node dissection), Surgical type (NSM/SSM), time to drain removal, whether wearing a good-fitting sports bra, neoadjuvant/adjuvant chemotherapy, radiation therapy, hormone therapy, targeted therapy, diabetes, smoking status (current or former), and the length of follow-up.
Complications
Postoperative complications were assessed, including seroma, infections, implant loss, tumor recurrence/metastasis, dehiscence, capsular contracture, skin flap necrosis, nipple-areola complex necrosis, hematoma, implant displacement, and chronic pain. Complications in this study were divided into major and minor complications.19,20 Major complications were designated as those requiring reoperation. And minor complications were these which can be treated in dressing rooms.
Surgical Techniques
The surgical techniques used were similar in both SIS matrix-assisted and no-matrix-assisted IBBR. During surgery, all rules for hygienic prosthetic surgery were followed meticulously to reduce the likelihood of bacterial contamination. Patients with no cefazolin allergy received one prophylactic dose of an antibiotic 30 min before surgical incision and two doses in the 24 h after surgery. All patients received mentor implants (Mentor, CPG331 Gel Breast Implant, Cohesive III, Leiden, The Netherlands). Before handling the implant, gloves of surgeons on the operating table were changed. The implant and the SIS matrix were infiltrated with antibiotic solution at least 10 minutes (100 mL normal saline solution containing 40 mg gentamicin and 0.5 mg adrenaline) before submuscular IBBR.
The technique for one-stage IBBR with SIS matrix was to release the inferior origin of the pectoralis major muscle and create a subpectoral pocket. And the SIS matrix was fixed to the chest wall to cover and support the lower and lateral areas of the implant. The SIS matrix sized 5 cm×30 cm was tailored accordingly to the individual case, and it was used to completely close the pocket according to the insufficiency of pectoralis major muscle. The decision on whether to perform SIS matrix-assisted or no-matrix-assisted IBBR was made mainly according to the sufficiency of the pectoralis major muscle during the operation and also based on preoperatively risk factors (such as patients who had contraindications with porcine and patients who request to use the biological matrix to assist their breast reconstruction). If pectoralis major muscle was deemed sufficient to completely cover the implant, the surgeon could do one-stage IBBR without SIS matrix (supplement with serratus anterior if necessary). The technique for one-stage IBBR without SIS matrix was similar before covering the pocket. If the pectoralis major muscle was not enough, the surgeon needed to appropriately separate the serratus anterior to fix to the lower and lateral edge of the pectoralis major muscle to completely cover the implant. Before stitching the skin, the wound bed was rinsed with a large quantity of 0.9% saline\povidone-iodine solution. Two drains were placed at the exit of the inframammary fold, one between the implant and the axilla, and one in the lateral breast gutter. The drains remained in place for at least 7 days until the output was less than 20 mL in 24 h. After surgery, all patients were instructed to wear a good-fitting sports bra for at least 1 month to help create a better postoperative shape. And the good-fitting sports bra could also help to avoid implant malposition during the early postoperative stage.
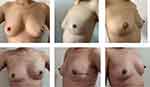
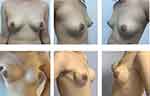
Patients were provided an informed consent for clinical photographs to be used for academic purposes. And Figures 1 and 2 show the comparative photographs before and after one-stage IBBR.
Statistical Analysis
Descriptive statistics were used for patients’ demographics, disease-related variables, treatment-related variables, and clinical outcomes. Baseline demographics and complications were analyzed with Student’s tests for continuous variables, Pearson chi-square tests, and Wilcoxon-Mann–Whitney test for categorical variables. To further explore the effects of procedure type on outcomes, the main patient- and surgery-related variables were entered into logistic regression models for the occurrence of postoperative major/minor complications. And results are expressed as odds ratios with 95% CIs.
Mean [standard deviation (SD)] BREAST-Q Version 2.0 scores of post-operation were reported for the overall cohort, and we assessed differences between groups using Student’s tests.
We used IBM SPSS (version 25) for statistical analyses and the level of significance used for all analyses was two tailed and set at p < 0.05.
Results
Among 155 one-stage IBBR patients captured in the institutional database, one-stage IBBR with SIS matrix was done in 79 (51.0%) patients and one-stage IBBR without SIS matrix was done in 76 (49%) patients. In total, 131 (84.5%) patients of 155 completed BREAST-Q Version 2.0 at postoperative year 1 or greater.
Sociodemographic Results and Medical History
Patients’ baseline characteristics were similar in the two groups (Table 1). The mean age of all included women was 40.3 (7.16 Y) years and the overall mean BMI was 21.82 (SD 2.20) kg/m.2 In both groups, 5(3.32%) patients had a history of smoking, and they all stop smoking after reconstruction. There were no patients who had a history of diabetes in the study. All of the 155 operations were therapeutic with cancer pathology results and there was no prophylactic surgery. The mean follow-up after placement of the definite implant at the time of completing the BREAST-Q version 2.0 was 17.55 months (SD 4.83) in the one-stage group and 33.37 months (SD 16.65) in the two-stage group.
 |
Table 1 Baseline Characteristics of the Included Patients Who Received Breast Reconstruction Surgeries |
Surgical and Cancer-Related Characteristics
Unilateral treatment was predominant with the patients of 154 (99.4%), and the bilateral treatment of patients was only 1(0.6%). Among all patients, NSM was more common in 120 (77.4%) patients than SSM in 35 (22.6%) patients. The mean implant size was 234.6 (54.76) cc in the two groups. In total, 24 (15.5%) patients underwent IBBR for ductal carcinoma in situ (DCIS), 45 (29.0%) patients had invasive carcinoma, 77 (49.7%) patients were diagnosed of carcinoma with DCIS, and 9(5.8%) patients had other kinds of malignant tumors. There were 110 (71.0%) patients with sentinel lymph node management and 53 (34.2%) patients with axillary lymph node dissection. A total of 13 (8.4%) patients underwent neoadjuvant chemotherapy, and no patients underwent adjuvant radiotherapy. After reconstruction, treatment with adjuvant chemotherapy was in 94 (60.6) patients, and patients who received postoperative radiation therapy were 28 (18.1%). Endocrine therapy was given to 96 (61.9) patients and 37 (23.9) patients received targeted therapy.
Two drains were used in all cases, and the last drain was removed at the day of 18.8 (SD 8.91). In total, 113 (72.9%) patients wore the postoperative good-fitting sports bra the next day after surgery, and 42 (27.1%) patients did not follow the doctor’s advice without wearing a good-fitting sports bra. (Table 1)
BREAST-Q Version 2.0 Scores
Of the two cohorts, the postoperative BREAST-Q Version 2.0 was completed by 68 (86.1%) of 131 patients in the SIS matrix-assisted one-stage IBBR group, and 63 (82.9%) of 131 patients in the no-matrix-assisted one-stage IBBR group (Table 2) In the satisfaction domain, the mean score for satisfaction with breasts was 60.27 (17.71) in the SIS matrix group and 54.49 (14.76) in the no-matrix group, p=0.045. In the health-related QOL and the experience domains, the mean score for the SIS matrix group and no-matrix group were no statistically significant differences.
 |
Table 2 Postoperative Scales of BREAST-Q Version 2.0 |
In total, 12 (15.2%) of 79 patients in the SIS matrix-assisted one-stage IBBR group and 13 (17.1%) of 76 patients can not complete the questionnaire. For the reason of implant removal, 3 (3.80%) patients refused to complete the questionnaire in the SIS matrix-assisted IBBR group and 2 (2.63%) patients in the no-matrix-assisted IBBR group. Because of the cosmetically unsatisfactory results, there were 7 (8.86%) patients who refused to complete the questionnaire in the SIS matrix-assisted IBBR group and 7 (9.21%) patients in the no-matrix-assisted IBBR group. In the two cohorts, 5 (3.23%) people were lost of follow-up during the questionnaire survey, with 2 (2.53%) in the SIS matrix-assisted IBBR group and 3(3.95%) in the no-matrix-assisted IBBR group. Due to the major complication of metastasis to the lung, 1(1.32%) patient in the no-matrix-assisted IBBR group refused to complete the questionnaire.
Complications
Major complications (reoperations) occurred in 5 (6.33%) patients in the SIS matrix-assisted one-stage IBBR group, compared with 3 (3.95%) in the no-matrix-assisted one-stage IBBR group. In the no-matrix-assisted one-stage IBBR group, there was 1 (1.32%) case of lung metastasis which was treated with surgery and chemotherapy (Table 3) Cases of minor complications in the SIS matrix group were 9 (11.4%), and there were 5 (6.6%) minor complications occurred in the no-matrix group. The most common minor complications were seroma [n = 8 (5.17%)] in the two groups.
 |
Table 3 Univariate Analysis Results of the Complications Between SIS Matrix-Assisted One-Stage IBBR Group and No-Matrix-Assisted One-Stage IBBR Group |
For implant removal and reoperations, there were 5 (6.33%) patients in the SIS matrix-assisted one-stage IBBR group and 2 (2.63%) patients in the no-matrix-assisted one-stage IBBR group who had reoperations to remove implants. Among the 7 (4.52%) in 155 patients, 6 (3.87%) removal surgeries were done because of wound healing problems (necrosis, wound dehiscence, and wound infection). The other 1 (0.65%) removal was due to the uncomfortability of implant and she finally chose to replace it with autologous reconstruction.
The multivariate logistic regression for postoperative complications (both major and minor complications) in the whole series pointed out a statistical significance for age>40 years old (odds ratio 3.314, 95% CI 1.012–10.854, p=0.048), and patients with endocrine therapy (odds ratio 0.260, 95% CI 0.092–0.736, p=0.011). Other variables show no significant influence on the complications in our study.
During the survey, the overall survival rate was 100%, and no patients underwent locoregional recurrence, except 1 (0.65%) patient with lung metastasis.
Discussion
Acellular dermal matrices, as biological matrices, were first described in IBBR in 2005.21,22 Since then, this technique has increasingly been used and been overwhelmingly promising. Besides, different kinds of biological and synthetic matrices have been introduced to support implant-based breast reconstruction.23–25 These matrices can allow for increasing the volume of the submuscular pouch, potentially increasing the number of one-stage breast reconstructions. However, there is still a lack of high-quality evidence to demonstrate the impact of biologic matrix use on the outcomes of IBBR. Most studies were based on one-stage IBBR and two-stage IBBR, and some studies compared outcomes between two different matrices based on one-stage IBBR or two-stage IBBR. We review and summarize some large sample studies regarding biological matrices or synthetic meshes based on one-stage IBBR or two-stage IBBR (Table 5).26–28 To our knowledge, few studies have compared one-stage IBBR with and without ADM. Our retrospective study tried to investigate these possible benefits of SIS matrix-assisted one-stage IBBR relative to no-matrix-assisted one-stage IBBR.
For patient-reported QOL, scores of BREAST-Q version 2.0 showed that patients who underwent matrix-assisted IBBR felt better than no-matrixed patients in the domain of satisfaction with breast. This result was measured after surgery at least one year, and it demonstrated the benefits of biological matrix to make women feel more satisfied with their breasts postoperatively. From the patients’ view, biological matrix and ADMs might create a more natural-looking breast. In the domain of the health-related QOL and satisfaction with surgeon, we found that there was no significant difference between the two groups.
With the longer survivorship of breast cancer, the patient’s perspective of care becomes more and more important in outcome discussions and assessment.29,30 The BREAST-Q is used to measure response to treatment by clinicians in real-world practice, investigators in clinical trials, and by institutions for quality improvement initiatives.31 BREAST-Q version 2.0, which was updated from 2017, could better reflect the health-related quality of life of patients from more comprehensive views. The whole completion rate of postoperative questionnaire was 84.5%, and only 5(3.23%) patients who did not have major complication refusing the questionnaire. The completion rates were no more than 80% in some previous researches.16,32,33 The higher completion rate in our study demonstrated the patients’ satisfaction with reconstructions. And in the patients’ experience domain, the mean score of satisfaction with surgeon was 85.49 (21.38) in SIS matrix-assisted one-stage IBBR group and 81.81 (23.07) in another group. These reflected the experienced surgical techniques of our surgeons, and we could also find this point from the lower mean scores of physical well-being: chest (the lower scores reflected a better outcome in this scale). In the health-related QOL domain, the mean scores of psychosocial well-being were 73.52 (19.96) and 72.22 (18.8) in the two groups, and it shows that the reconstruction can give patients good health and self-esteem. However, the limitation is that we lack the preoperative research to compare the expectations of patients. For the sexual well-being domain, the completion rates dropped to 50.6% in SIS matrix-assisted one-stage IBBR and 52.6% in no-matrix-assisted one-stage IBBR. Some patients chose to skip the sexual questions which made them feel uncomfortable, and it indicated the conservative of women in China to some extent.
Comparing complications was the second endpoint in our study. We found no significant difference between the two groups, no matter the major complications or minor complications. Although some previous studies discovered that one-stage IBBR with ADM was associated with higher odds ratios for surgical complications, reoperation, and removal of the implant.10,34 In our study, we also found a lower whole complication rate than previous researches, and the rate aligned with other previous retrospective studies.6,35,36 From baseline characteristics, we noticed that there only 5 former smokers and no diabetics in the two groups, and maybe this was the reason for the lower complication rate. In our institution, we usually did not suggest patients with diabetes to have reconstruction with implants, because diabetics can result in many disadvantages for wound healing and other complications from the general surgery’s view. And the 5 patients who smoked all gave up smoking after reconstruction with our recommendations.
However, patients who underwent SIS matrix-assisted one-stage IBBR had longer days [21.08 (9.53)] to remove drains comparing with no matrix group [16.52 (7.61)], P=0.001. And Hadar Israeli Ben-noon et al37 also reported that immediate prosthetic breast reconstruction with ADM could increase time to drain removal. Although, we did not find higher odds ratios of drain removal factor from multivariate logistic analysis for whole complications.
Due to the lower complication rate and not so many patients enrolled, we decided to analyze major and minor complications together using multivariate logistic analysis in the whole series. Age older than 40 years old and body mass index greater than 25 kg/m2 were assigned as categorical variables and risk factors in the multivariate logistic analysis (Table 4) From the outcomes, age older than 40 years old had an increased comparative risk with patients who were under 40 years old (odds ratio 3.314, 95% CI 1.012–10.854, p=0.048). And treatment with endocrine therapy was another patient-related characteristic, which was a significant risk factor for major or minor complications (odds ratio 0.260, 95% CI 0.092–0.736, p=0.011). We did not find the risk factor of endocrine therapy that had a significant influence in other previous studies. Patients who were given treatment with endocrine therapy had positive hormone receptors, which might give them positive psychological expectations than patients who had negative hormone receptors. Radiotherapy, an established risk factor for postoperative complications in implant-based breast reconstruction, was also not identified as a risk factor, most likely because of the low number of patients receiving radiotherapy in our study. All patients who underwent radiotherapy were after reconstruction, and we did suggest patients who received radiotherapy before surgery to have reconstruction. Whether having complications with radiotherapy was usually related to the experience of the radiologist, and our patients benefited to the advanced comprehensive treatment in our cancer center.
 |
Table 4 Multivariate Logistic Analysis of Patient and Surgery-Related Characteristics as Risk Factors for Postoperative Complications (Major + Minor) in the Whole Series |
 |
Table 5 Studies Regarding Biological Matrices or Synthetic Meshes Based on One-Stage IBBR or Two-Stage IBBR |
In retrospect, one-stage IBBR without matrix did not have significantly lower PROs and complications compared with patients who underwent one-stage IBBR with matrix. However, we still did not recommend IBBR without matrix. On the one hand, reconstruction without matrix was based on the experienced surgical skills, and surgeons needed to determine whether there is sufficient subcutaneous tissue. On the other hand, patients who underwent SIS matrix one-stage IBBR had better satisfaction with breast in our study, and previous analysis had also reported the lower complications and appreciable benefits in reconstruction with matrix.7,38
There are some limitations to our study. Firstly, our study lacked the preoperative BREAST-Q version 2.0 questionnaire. However, prospective randomized clinical studies comparing one stage implant-based breast reconstruction with and without biological matrix did not appear feasible, because their application depended on the implant-covering skin situation.24 And this led to the longer mean duration of follow-up of the no-matrix-assisted one-stage IBBR group[33.37 (16.65)]. Secondly, due to the small patient sample size, our study did not further investigate the effects of baseline characteristic factors (such as good-fitting sports bra condition) that influenced the BREAST-Q scores between SIS matrix-assisted one-stage IBBR and no-matrix-assisted one-stage IBBR. In our study, these limitations might result in the tendency that one-stage IBBR with biological matrix is better. However, we hoped that larger sample size researches can make outcomes more scientific and rigorous.
Women who opt for breast reconstruction often do so to “regain femininity” or to “feel whole again.”39 And we want to give patients a better health-related quality of life after reconstruction through this study. Overall, SIS matrix-assisted one-stage IBBR in our study can make patients attain a high score of satisfaction with breast than no-matrix-assisted one-stage IBBR, and risks for complications have no significant difference between the two groups. However, investigations are still needed to adequately assess the association between biological matrix procedure choice and complication rates.
Ethics
This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 81872160), the Beijing Municipal Natural Science Foundation (Key Project) (No. 19G10077), the Beijing Municipal Natural Science Foundation (No. 7204293), the Special Research Fund for Central Universities, Peking Union Medical College (No. 3332019053), the Beijing Hope Run Special Fund of Cancer Foundation of China (No. LC2019B03), the Beijing Hope Run Special Fund of Cancer Foundation of China (No.LC2019L07).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131(1):15–23. doi:10.1097/PRS.0b013e3182729cde
2. Ibrahim AM, Koolen PG, Ganor O, et al. Does acellular dermal matrix really improve aesthetic outcome in tissue expander/implant-based breast reconstruction? Aesthetic Plast Surg. 2015;39(3):359–368. doi:10.1007/s00266-015-0484-x
3. Dieterich M, Faridi A. Biological matrices and synthetic meshes used in implant-based breast reconstruction - a review of products available in Germany. Geburtshilfe Frauenheilkd. 2013;73(11):1100–1106. doi:10.1055/s-0033-1350930
4. Maisel Lotan A, Ben Yehuda D, Allweis TM, Scheflan M. Comparative study of meshed and nonmeshed acellular dermal matrix in immediate breast reconstruction. Plast Reconstr Surg. 2019;144(5):1045–1053. doi:10.1097/PRS.0000000000006116
5. Salzberg CA, Dunavant C, Nocera N. Immediate breast reconstruction using porcine acellular dermal matrix (Strattice): long-term outcomes and complications. J Plast Reconstr Aesthet Surg. 2013;66(3):323–328. doi:10.1016/j.bjps.2012.10.015
6. Scheflan M, Grinberg-Rashi H, Hod K. Bovine acellular dermal matrix in immediate breast reconstruction: a retrospective, observational study with SurgiMend. Plast Reconstr Surg. 2018;141(1):1e–10e. doi:10.1097/PRS.0000000000003982
7. Zhao X, Wu X, Dong J, Liu Y, Zheng L, Zhang LA. Meta-analysis of postoperative complications of tissue expander/implant breast reconstruction using acellular dermal matrix. Aesthetic Plast Surg. 2015;39(6):892–901. doi:10.1007/s00266-015-0555-z
8. Barber MD, Williams L, Anderson ED, et al. Outcome of the use of acellular-dermal matrix to assist implant-based breast reconstruction in a single centre. (1532-2157 (Electronic))
9. Hallberg H, Rafnsdottir S, Selvaggi G, et al. Benefits and risks with acellular dermal matrix (ADM) and mesh support in immediate breast reconstruction: a systematic review and meta-analysis. J Plast Surg Hand Surg. 2018;52(3):130–147. doi:10.1080/2000656X.2017.1419141
10. Dikmans RE, Negenborn VL, Bouman MB, et al. Two-stage implant-based breast reconstruction compared with immediate one-stage implant-based breast reconstruction augmented with an acellular dermal matrix: an open-label, Phase 4, multicentre, randomised, controlled trial. Lancet Oncol. 2017;18(2):251–258. doi:10.1016/S1470-2045(16)30668-4
11. Porter ME What is value in health care? (1533-4406 (Electronic))
12. Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353. doi:10.1097/PRS.0b013e3181aee807
13. Cano SJ, Klassen AF, Fau - Scott AM, et al. The BREAST-Q: further validation in independent clinical samples. (1529-4242 (Electronic))
14. Centeno RF. Surgisis acellular collagen matrix in aesthetic and reconstructive plastic surgery soft tissue applications. Clin Plast Surg. 2009;36(2):229–40, vii. doi:10.1016/j.cps.2008.12.004
15. Ho G, Nguyen TJ, Shahabi A, Hwang BH, Chan LS, Wong AK. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg. 2012;68(4):346–356. doi:10.1097/SAP.0b013e31823f3cd9
16. Headon H, Kasem A, Manson A, Choy C, Carmichael AR, Mokbel K Clinical outcome and patient satisfaction with the use of bovine-derived acellular dermal matrix (SurgiMend™) in implant based immediate reconstruction following skin sparing mastectomy: A prospective observational study in a single centre. (1879-3320 (Electronic))
17. Zhong T, Temple-Oberle C, Hofer SO, et al. The Multi Centre Canadian Acellular Dermal Matrix Trial (MCCAT): study protocol for a randomized controlled trial in implant-based breast reconstruction. Trials. 2013;14:356. doi:10.1186/1745-6215-14-356
18. Pusic A, Klassen A, Cano S. BREAST-Q Recon V2.0 English. (Copyright ©2017, Memorial Sloan Kettering Cancer Center and the University of British Columbia).
19. Nelson JA, Allen RJ
20. Baldelli I, Fau Cardoni G, Franchelli S et al. Implant-based breast reconstruction using a polyester mesh (Surgimesh-PET): a retrospective single-center study. (1529-4242
21. Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005;55(3):232–239. doi:10.1097/01.sap.0000168527.52472.3c
22. Kahan BC, Morris TP. Reporting and analysis of trials using stratified randomisation in leading medical journals: review and reanalysis. BMJ. 2012;345:e5840. doi:10.1136/bmj.e5840
23. Dieterich M, Reimer T, Dieterich H, Stubert J, Gerber B. A short-term follow-up of implant based breast reconstruction using a titanium-coated polypropylene mesh (TiLoop((R)) Bra). Eur J Surg Oncol. 2012;38(12):1225–1230. doi:10.1016/j.ejso.2012.08.026
24. Dieterich M, Paepke S, Zwiefel K, et al. Implant-based breast reconstruction using a titanium-coated polypropylene mesh (TiLOOP Bra): a multicenter study of 231 cases. Plast Reconstr Surg. 2013;132(1):8e–19e. doi:10.1097/PRS.0b013e318290f8a0
25. Gschwantler-Kaulich D, Schrenk P, Bjelic-Radisic V, et al. Mesh versus acellular dermal matrix in immediate implant-based breast reconstruction - a prospective randomized trial. Eur J Surg Oncol. 2016;42(5):665–671. doi:10.1016/j.ejso.2016.02.007
26. Farhangkhoee H, Matros E, Disa J. Trends and concepts in post-mastectomy breast reconstruction. J Surg Oncol. 2016;113(8):891–894. doi:10.1002/jso.24201
27. Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150(1):9–16. doi:10.1001/jamasurg.2014.2895
28. Negenborn VL, Young-Afat DA, Dikmans REG, et al. Quality of life and patient satisfaction after one-stage implant-based breast reconstruction with an acellular dermal matrix versus two-stage breast reconstruction (BRIOS): primary outcome of a randomised, controlled trial. Lancet Oncol. 2018;19(9):1205–1214. doi:10.1016/S1470-2045(18)30378-4
29. Srinivasa DR, Garvey PB, Qi J, et al. Direct-to-implant versus Two-Stage Tissue Expander/Implant Reconstruction: 2-year risks and patient-reported outcomes from a prospective, multicenter study. Plast Reconstr Surg. 2017;140(5):869–877. doi:10.1097/PRS.0000000000003748
30. Woerdeman LA, Hage JJ, Hofland MM, Rutgers EJ. A prospective assessment of surgical risk factors in 400 cases of skin-sparing mastectomy and immediate breast reconstruction with implants to establish selection criteria. Plast Reconstr Surg. 2007;119(2):455–463. doi:10.1097/01.prs.0000246379.99318.74
31. Voineskos SH, Klassen AF, Cano SJ, Pusic AL, Gibbons CJ. Giving meaning to differences in BREAST-Q scores: minimal important difference for breast reconstruction patients. Plast Reconstr Surg. 2020;145(1):11e–20e. doi:10.1097/PRS.0000000000006317
32. Eichler C, Vogt N, Brunnert K, et al. Comparison between SurgiMend and epiflex in 127 breast reconstructions. Plast Reconstr Surg Glob Open. 2015;3(6):e439. doi:10.1097/GOX.0000000000000409
33. Ohkuma R, Buretta KJ, Fau - Mohan R, et al. Initial experience with the use of foetal/neonatal bovine acellular dermal collagen matrix (SurgiMend™) for tissue-expander breast reconstruction. (1878-0539 (Electronic))
34. Israeli Ben-Noon H, Farber N, Weissman O, et al. The effect of acellular dermal matrix on drain secretions after immediate prosthetic breast reconstruction. J Plast Surg Hand Surg. 2013;47(4):308–312. doi:10.3109/2000656X.2013.766202
35. Lee KT, Mun GH. Updated evidence of acellular dermal matrix use for implant-based breast reconstruction: a meta-analysis. Ann Surg Oncol. 2016;23(2):600–610. doi:10.1245/s10434-015-4873-9
36. Reaby LL. Reasons why women who have mastectomy decide to have or not to have breast reconstruction. Plast Reconstr Surg. 1998;101(7):1810–1818. doi:10.1097/00006534-199806000-00006
37. Sorkin M, Qi J, Kim HM, et al. Acellular dermal matrix in immediate expander/implant breast reconstruction: a multicenter assessment of risks and benefits. Plast Reconstr Surg. 2017;140(6):1091–1100. doi:10.1097/PRS.0000000000003842
38. McCarthy CM, Lee CN, Halvorson EG, et al. The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg. 2012;130(5Suppl 2):57S–66S. doi:10.1097/PRS.0b013e31825f05b4
39. Salzberg CA, Ashikari AY, Koch RM, Chabner-Thompson E. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg. 2011;127(2):514–524. doi:10.1097/PRS.0b013e318200a961
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.