Back to Journals » Clinical Ophthalmology » Volume 10
Comparison study of intraocular pressure reduction efficacy and safety between latanoprost and tafluprost in Japanese with normal-tension glaucoma
Authors Ikeda Y, Mori K, Tada K, Ueno M, Kinoshita S, Sotozono C
Received 11 March 2016
Accepted for publication 25 May 2016
Published 24 August 2016 Volume 2016:10 Pages 1633—1637
DOI https://doi.org/10.2147/OPTH.S108213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yoko Ikeda,1,2,* Kazuhiko Mori,1,* Kaori Tada,3 Morio Ueno,1 Shigeru Kinoshita,4 Chie Sotozono1
1Department of Ophthalmology, Kyoto Prefectural University of Medicine, 2Oike-Ganka Ikeda Clinic, 3Department of Ophthalmology, Japanese Red Cross Society Kyoto Daini Hospital, 4Department of Frontier Medical Science and Technology for Ophthalmology, Kyoto Prefectural University of Medicine, Kyoto, Japan
*These authors contributed equally to this work
Purpose: To evaluate and compare the intraocular pressure (IOP) reduction efficacy and safety between the ophthalmic solutions 0.005% latanoprost (Lat) and 0.0015% tafluprost (Taf) in Japanese patients with normal-tension glaucoma (NTG).
Methods: In this randomized nonmasked study, we prospectively enrolled 30 Japanese NTG patients who had used Lat monotherapy for more than 4 weeks, and randomly divided them into the following two groups: 1) Lat-to-Taf group (LT group) and 2) Taf-to-Lat group (TL group). At the beginning of the study, both groups were switched from initial Lat to Lat or Taf for 12 weeks, and then switched over to the other drug (crossover) for 12 additional weeks. At 0, 4, 12, 16, and 24 weeks, we evaluated each patient’s IOP, conjunctival injection, and corneal epitheliopathy score, and at 0, 12, and 24 weeks, we evaluated their eyelash changes and pigmentation of the eyelids and irises.
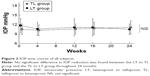
Results: The mean IOP of the LT group (15 eyes) was 10.5, 10.6, and 11.1 mmHg, at 0, 12, and 24 weeks, respectively, whereas that of the TL group (15 eyes) was 11.7, 11.1, and 10.5 mmHg at 0, 12, and 24 weeks, respectively. No significant differences were found between the two groups and in the intragroup comparisons. Moreover, no significant differences were found between Lat and Taf in regard to the conjunctival injection score and corneal epitheliopathy score. Eyelash changes and eyelid and iris pigmentation were similar in both groups.
Conclusion: The findings of this study show that Lat and Taf have equivalent efficacy and safety in Japanese patients with NTG.
Keywords: latanoprost, tafluprost, normal-tension glaucoma, crossover
Introduction
Glaucoma is one of the major leading causes of blindness worldwide.1 In Japan, glaucoma has become the primary cause of blindness since 2004.2 Normal-tension glaucoma (NTG) is the most common type of glaucoma in Japan,3 with a prevalence rate of 3.6% in people over 40 years of age. The ophthalmic solutions 0.005% latanoprost (Lat)4,5 and 0.0015% tafluprost (Taf)6,7 are topical prostaglandin analogs. Both solutions greatly reduce intraocular pressure (IOP) by increasing the uveoscleral outflow as a prostaglandin F receptor agonist.8 Lat was first approved for clinical use in 1996 and has been used globally since then as a first-line drug for the treatment of glaucoma. On the other hand, the IOP reduction efficacy and safety of Taf, first launched in 2008, is reportedly equivalent to that of Lat9 for patients with primary open-angle glaucoma or ocular hypertension. Although there have been reports on the efficacy of Taf for NTG patients,10,11 to the best of our knowledge, no comparison crossover studies have been conducted to investigate the efficacy and safety between Lat and Taf for NTG patients.
In this prospective crossover study involving Japanese NTG patients, the IOP reduction efficacy and safety of Taf were compared with those of Lat.
Subjects and methods
All study procedures were approved by the Institutional Review Board of Kyoto Prefectural University of Medicine, Kyoto, Japan, and were performed in accordance with the tenets set forth in the Declaration of Helsinki. This study was a randomized nonmasked study. All study participants were recruited between March 2009 and March 2011. We selected and enrolled 30 Japanese NTG patients who had used Lat monotherapy at least 4 weeks at the Glaucoma Special Clinic of Kyoto Prefectural University of Medicine. Written informed consent was obtained from all patients after receiving a detailed explanation of the nature and possible consequences of their participation in the study.
Each of the 30 enrolled patients were randomly divided into one of the following two groups: 1) Lat-to-Taf group (LT group: 15 patients, 7 male eyes and 8 female eyes; mean age: 63.5±11.5 years) and 2) Taf-to-Lat group (TL group: 15 patients, 8 male eyes and 7 female eyes; mean age: 69.1±8.8 years). At the beginning of the study, both groups were switched from initial Lat to Lat or Taf for 12 weeks, and then switched over to the other drug (crossover) to use for 12 additional weeks (Figure 1). At 0, 4, 12, 16, and 24 weeks, we evaluated their IOP, conjunctival injection score (Grades 0–3),12 and corneal epitheliopathy score (area density [AD] score).13 Then, at 0, 12, and 24 weeks, we evaluated adverse events of eyelash changes and pigmentation of the eyelids and irises via a slit-lamp photograph taken at each visit. The incidence of eyelash and pigmentation was judged via slit-lamp photographs, with a comparison between the current and baseline photographs. In all patients, IOP was measured by using an applanation tonometer (H03 R 90030515; Haag-Streit, Bern, Switzerland) by the same glaucoma specialist throughout the protocol periods, and those IOP measurements were obtained at approximately the same time of day for each patient. If data were available from both eyes, then the right-eye data were used. We evaluated the mean deviation of the Humphrey 30-2 threshold of static visual field program at baseline or within 3 months. Statistical analysis was performed using the Student’s t-test and Fisher’s exact test.
  | Figure 1 Study design. |
For each patient, Lat and Taf eye drops were prescribed at the Glaucoma Special Clinic. As this was not a masked study, both the patient and the doctor knew which eye drop was being used. A calculated sample size of 15 enrolled subjects per arm providing the power of 80% was based on a noninferiority limit of 1.5 mmHg, a standard deviation of 1.5 mmHg for change in IOP.
Results
There were no dropout cases, and all patients completed the study. Of the total 30 subjects, 28 were newly prescribed Lat, yet 1 subject in both the LT and TL groups used Lat for more than 4 weeks. The backgrounds of the patients are detailed in Table 1. There were no significant differences in age, sex ratio, or mean deviation obtained from Humphrey perimetry sita standard 30-2 between the LT and TL groups.
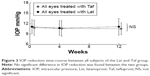
As for the IOP time course of all subjects, at 0, 12, and 24 weeks, respectively, the mean IOP of the LT group (15 eyes) was 10.5, 10.6, and 11.1 mmHg, whereas that of the TL group (15 eyes) was 11.7, 11.1, and 10.5 mmHg, and no significant differences were found between the two groups (Student’s t-test) (Figure 2). At 0, 4, and 12 weeks, respectively, the mean IOP of the Lat intragroup (30 eyes) was 10.8, 10.6, and 10.5 mmHg, whereas that of the Taf intragroup (30 eyes) was 10.8, 10.7, and 11.1 mmHg, and no significant differences were found between the two intragroups (Student’s t-test) (Figure 3).
The mean conjunctival hyperemia score at 0, 4, 12, 16, and 24 weeks, respectively, was 1.0±0.4, 1.0±0.5, 0.9±0.3, 0.9±0.4, and 0.9±0.4 in the LT group and 1.0±0.4, 1.1±0.5, 1.1±0.6, 1.0±0.5, and 1.1±0.5 in the TL group, and no significant differences were found between two groups (Student’s t-test) (Figure 4). The respective mean AD score at 0, 4, 12, 16, and 24 weeks was 0.5±0.9, 1.0±1.4, 0.7±1.0, 0.9±1.4, and 0.7±1.0 in the LT group and 1.0±1.3, 0.7±1.0, 0.7±1.0, 0.5±1.0, and 0.8±1.3 in the TL group, and no significant differences were found between the two groups (Student’s t-test) (Figure 5).
The rates of the adverse events of eyelash change and eyelid and iris pigmentation induced by Lat or Taf are shown in Table 2. The incidence of eyes showing increased eyelash length was 30.0% by Lat and 26.7% by Taf, and the incidence of eyes showing increased eyelash amount was 16.7% by Lat and 30.0% by Taf. The incidence of eyes showing increased eyelid pigmentation was 3.3% by Lat and 20.0% by Taf, and the incidence of eyes showing increased iris pigmentation was 6.7% by Lat and 3.3% by Taf. There was no significant difference in the incidence of eyelash change (length and amount) as well as eyelid and iris pigmentation between the two groups (Fisher’s exact test).
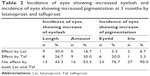  | Table 2 Incidence of eyes showing increased eyelash and incidence of eyes showing increased pigmentation at 3 months by latanoprost and tafluprost |
Discussion
Although there have been reports on the efficacy of Taf for NTG patients, to the best of our knowledge, this present study is the first crossover prospective report to investigate and compare IOP reduction efficacy and safety between Lat and Taf in NTG patients. In this study, no significant differences were found between Lat and Taf in regard to IOP reduction effects, conjunctival hyperemia score, and corneal epitheliopathy score, as well as eyelash change and eyelid and iris pigmentation. It has been reported that conjunctival hyperemia becomes stronger when switching from Lat to travoprost or bimatoprost,14,15 and the same tendency was found to be true in this present study when switching from Lat to Taf. Although there were no statistically significant differences, elongation of eyelash length was more frequently found in Lat, whereas increased eyelash amount was more frequently found in Taf. Since the patients involved in this study were already users of Lat for over 1 month, it was difficult to make a simple comparison between the two drugs in regard to the adverse events of newly appearing eyelid and iris pigmentation or eyelash elongation. In this study, there was a tendency of more eyelid pigmentation with Taf than with Lat. According to past reports for eyelash length, eyelash amount, eyelid pigmentation, and iris pigmentation, the appearance rate was 0–25.8/0.38%, 26/46%, 1.5–6.0/1.08%–4%, and 0–12.3/4.0% with Lat/Taf,7,13,14,16–21 respectively. Our results were nearly the same as those of the previous reports, except for iris and eyelid pigmentation. Our findings showed that eyelid pigmentation was greater with Taf than with Lat, yet with no significant difference. We must investigate further eyelid pigmentation induced by Taf.
It should be noted that this study did include some limitations. The first limitation was that this was not a masked study, as both the subjects and the attending doctor knew which eye drop was being used. The second limitation was that since the baseline IOP of all subjects using Lat was low, it was difficult to find the switching efficacy of the eye drop.
Conclusion
The findings of this study show that Lat and Taf have equivalent safety and efficacy in Japanese NTG patients with low IOP.
Acknowledgments
The authors wish to thank Mr John Bush for reviewing this manuscript. The abstract of this paper was submitted at the ARVO Annual Meeting, May 1–5, 2011 as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Investigative Ophthalmology and Visual Science Vol 52, Issue 14. The actual paper, however, has never been published. Clinical trial number Umin-CTR, UMIN000002017.
Disclosure
The authors report no conflicts of interest in this work.
References
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. | ||
Nakae K, Masuda K. Recent causes of visual disturbances in Japan. Comparison with causes fifteen years ago. J Clin Exp Med. 2008;225(8):691–693. | ||
Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111(9):1641–1648. | ||
Digiuni M, Fogagnolo P, Rossetti L. A review of the use of latanoprost for glaucoma since its launch. Expert Opin Pharmacother. 2012;13(5):723–745. | ||
Arias A, Schargel K, Ussa F, Canut MI, Robles AY, Sanchez BM. Patient persistence with first-line antiglaucomatous monotherapy. Clin Ophthalmol. 2010;4:261–267. | ||
Aihara M. Clinical appraisal of tafluprost in the reduction of elevated intraocular pressure (IOP) in open-angle glaucoma and ocular hypertension. Clin Ophthalmol. 2010;4:163–170. | ||
Parrish RK, Palmberg P, Sheu WP; XLT Study Group. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003;135(5):688–703. | ||
Takagi Y, Nakajima T, Shimazaki A, et al. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Exp Eye Res. 2004;78(4):767–776. | ||
Konstas AG, Quaranta L, Katsanos A, et al. Twenty-four hour efficacy with preservative free tafluprost compared with latanoprost in patients with primary open angle glaucoma or ocular hypertension. Br J Ophthalmol. 2013;97(12):1510–1515. | ||
Nakano T, Yoshikawa K, Kimura T, Suzumura H, Nanno M, Noro T. Efficacy and safety of tafluprost in normal-tension glaucoma with intraocular pressure of 16 mmHg or less. Jpn J Ophthalmol. 2011;55(6):605–613. | ||
Mizoguchi T, Ozaki M, Unoki K, Dake Y, Eto T, Arai M. A randomized crossover study comparing tafluprost 0.0015% with travoprost 0.004% in patients with normal-tension glaucoma [corrected]. Clin Ophthalmol. 2012;6:1579–1584. | ||
Japanese Ocular Allergology Society. Guidelines for the clinical management of allergic conjunctival disease (2nd edition). Nippon Ganka Gakkai Zasshi. 2010;114:831–870. | ||
Chiba T, Kashiwagi K, Chiba N, et al. Comparison of iridial pigmentation between latanoprost and isopropyl unoprostone: a long term prospective comparative study. Br J Ophthalmol. 2003;87(8):956–959. | ||
Aihara M, Oshima H, Araie M; EXTraKT study group. Effects of SofZia-preserved travoprost and benzalkonium chloride-preserved latanoprost on the ocular surface – a multicentre randomized single-masked study. Acta Ophthalmol. 2013;91(1):e7–e14. | ||
Maruyama Y, Ikeda Y, Mori K, et al. Comparison between bimatoprost and latanoprost-timolol fixed combination for efficacy and safety after switching patients from latanoprost. Clin Ophthalmol. 2015;9: 1429–11436. | ||
Kuwayama Y, Nomura A. Prospective observational post-marketing study of tafluprost for glaucoma and ocular hypertension: short-term efficacy and safety. Adv Ther. 2014;31(4):461–471. | ||
Inoue K, Shiokawa M, Higa R, et al. Adverse periocular reactions to five types of prostaglandin analogs. Eye. 2012;26(11):1465–1472. | ||
Noecker RS, Dirks MS, Choplin NT, et al. A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol. 2003;135(1):55–63. | ||
Netland PA, Landry T, Sullivan EK, et al. Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2001;132(4):472–484. | ||
Manni G, Centofanti M, Parravano M, Oddone F, Bucci MG. A 6-month randomized clinical trial of bimatoprost 0.03% versus the association of timolol 0.5% and latanoprost 0.005% in glaucomatous patients. Graefes Arch Clin Exp Ophthalmol. 2004;242(9):767–770. | ||
Gandolfi S, Simmons ST, Sturm R, Chen K, VanDenburgh AM; Bimatoprost Study Group 3. Three-month comparison of bimatoprost and latanoprost in patients with glaucoma and ocular hypertension. Adv Ther. 2001;18(3):110–121. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.