Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Comparison of UVA Protection Factor Measurement Protocols
Authors Hedayat K , Ahmad Nasrollahi S , Firooz A , Rastegar H , Dadgarnejad M
Received 6 January 2020
Accepted for publication 8 April 2020
Published 8 May 2020 Volume 2020:13 Pages 351—358
DOI https://doi.org/10.2147/CCID.S244898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Kamand Hedayat,1 Saman Ahmad Nasrollahi,1,2 Alireza Firooz,1,2 Hossein Rastegar,2 Manouchehr Dadgarnejad3
1Center for Research and Training in Skin Diseases and Leprosy (CRTSDL), Tehran University of Medical Sciences (TUMS), Tehran, Iran; 2Cosmetic Products Research Center, Iranian Food and Drug Administration, Ministry of Health and Medical Education, Tehran, Iran; 3Iranian Food and Drug Administration, Ministry of Health and Medical Education, Tehran, Iran
Correspondence: Saman Ahmad Nasrollahi
Center for Research and Training in Skin Diseases and Leprosy (CRTSDL), Tehran, University of Medical Sciences (TUMS), No. 415 Taleqani Avenue, Tehran 1416613675, Iran
Tel/Fax +982188963804
Email [email protected]
Background: In the past, it was taught that UVA wavelengths (320- 400nm) only plays a major role in skin aging but recently the scientific researches also show that UVA cause cancerous keratinocyte cells in deep layer of the epidermis. Therefore, the protective ability of the product against UVA is important in addition to protection against UVB rays. The UVA protective factor (UVA-PF) is used to evaluate the effectiveness of sunscreen products against UVA rays. This study aims to review and compare all outstanding protocols in the field of UVA-PF measurement and finally the introduction of the best method of measuring UVA-PF based on the further benefits.
Materials and Methods: Four standards including ISO 24443 (AS/NZS 2604: 2012 recommended approach), CEN 2006, FDA 2007 and FDA 2011 are selected.
Results: In order to measure UVA-PF with in vivo method, two standards of CEN 2006 and FDA 2007 recommended persistent pigment darkening (PPD) method. Although the general principle of both is similar, there are some differences in detail. For in vitro measurement of UVA-PF, CEN and FDA 2011 standards use critical wavelengths. FDA 2007 introduces the modified Diffey fraction, and ISO 24443 standard meets the UVA-PF measurement in a manner that is consistent with PPD.
Conclusion: Finally, this review discussed the comparison of all in vitro and in vivo UVA-PF measurement standards and provided information in the form of texts and tables to move towards the creation of an integrated standard. Since in vitro methods of UVA-PF measurement are not reproducible due to differences in test conditions, it may be concluded that the in vivo PPD method is a more suitable option.
Keywords: sunscreen, ultraviolet, UVA protection factor, in vivo, in vitro
Introduction
Part of the sunlight reaching the ground involves the ultraviolet spectrum consisting of three sections of UVC (200–290nm), UVB (290–320nm) and UVA (320–400nm). UVC is completely absorbed by the earth’s atmosphere. The amount of UVA reaching the ground is more than 20 times the UVB.1 UVA plays a major role in premature aging and photocarcinogenesis. UVA increases the release of inflammatory factors, including IL10 from keratinocyte cells. Additionally, it increases the expression of inflammatory proteins such as TNFα, IL1α, IL6, and IL8.2,3 UVA plays a considerable role in the creation of mutations in mitochondrial DNA in human skin with the help of ROS (reactive oxygen spices). It also causes premature skin aging by creating a mutation in fibroblast cells in the human skin.4 The role of increased expression of the p53 protein in causing UVA-induced apoptosis is undeniable.5 8-hydroxy-2′-deoxyguanine (8-oxoG) is a valuable indicator to track the DNA oxidative damage caused by UVA, and it can lead to G → T transversions.6
Therefore, protection against UVA along with UVB is highly important.
The UVA protective factor (UVA-PF) is used to evaluate the effectiveness of sunscreen products against UVA rays.
Materials and Methods
In this study, global standards in the field of UVA-PF determination were reviewed and compared. Four standards, namely ISO 24443 (provided by European union, at May 2013), CEN2006 (provided by European Union, South Africa, Japan in Brussels, at July 12, 2006), FDA 2007 (provided by United States, at August 27, 2007) and FDA 2011 (provided by United States, at June 17, 2011) have set up different protocols in the field of UVA protection factor. Some are dealt with using in vivo methods whereas others are dealt with using in vitro methods.7–11
Results
Due to the role of UVA in the occurrence of skin damages, it is important to use sunscreens which protect the skin from both UVA and UVB. In vitro methods of UVA-PF measurement are not reliable and reproducible due to differences in test conditions, such as distinction of substrates, variant amount of applied product and UV dose of irradiation.12 Among the FDA 2007 and CEN standards introducing the in vivo PPD approach, both have advantages and disadvantages so that using a better approach in each case can yield better results.
Discussion
In vivo UVA-PF Determination Test Assessment
First, we will go through in vivo UVA-PF determination methods conducted using Immediate Pigment Darkening (IPD), Persistent Pigment Darkening (PPD) and Protection Factor A (PFA). However, in CEN and FDA 2007 standards, the PPD method is accepted only in the in vivo UVA-PF determination method (Table 1).
 |
Table 1 In vivo UVA-PF Determination Protocol Comparison |
In the PPD method, like the SPF (Sun Protection Factor) measurement method, people meeting the criteria for the test are selected with a specified number for participation. In the SPF test, the endpoint is an erythemal response versus the PPD test in which the endpoint is persistence pigment darkening of the test subsites. Test sites and subsites are designed in specific dimensions blindly on the participant bodies. The test and reference sunscreen products are weighed in the defined amount and are applied onto the participant’s skin. Then, with a specific method, the product is spread over the skin followed by a waiting period for product to be dried. Individuals, based on a predetermined manner, are exposed to UVA radiation while sitting or lying position on the abdomen. After a certain amount of time from the last UV exposure, the level of permanent darkness of the pigment is evaluated as the final point of the test, and UVA-PF is calculated by a set of formulas (which will be discussed in “Statistics and Calculations” section ).
Selection Criteria for Participants
Despite the criteria mentioned in Table 1 (FDA 2007 and CEN common criteria), CEN standards further their precautions for entering the test.
The criteria for a healthy male and female to be considered include:
- Skin type II, III and IV13
- Not being involved in any sun tests within the last two months (no remaining marks on the back).
- Not having sun exposure on the back area for at least 2 months prior to the study.
- Absence of scars, or active dermal lesions on the areas of the backtested.
- Test area must be uniform in color, without nevi, blemishes or solar lentigo without excessive hairs.
The CEN also set a series of exclusion test criteria;
- Subjects not fitting the previous inclusion criteria.
- Pregnant or lactating women,
- Past history of allergy, photoallergic, phototoxic, or other abnormal responses to sunlight or sensitivity to cosmetic products, toiletries, sunscreens, latex and/or topical drugs.
- Subjects with dermatological problems on the test area.
- Subjects having used self-tanning products on the back in the previous month.
Consequently, the CEN appears more suitable for individuals selecting criterion owing to greater consistency.
Number of Participants
The FDA 2007 and CEN use 20 to 25 people and 10 to 20 people, respectively. Like other clinical studies, the higher number of the participants, brings higher test accuracy. On the other hand, the large sample size lead to some problems such as handling the protocol, providing enough samples and volunteer’s dropout.
Reference Sunscreen Formulations
It is recommended that a standard sample be used to verify the results of each test sample.
The test is valid if the average UVA-PF obtained for the reference sunscreen product is within the specified range. According to Table 1, both reference sunscreens are good choices, since their UVA-PF results are close to each other, and their active ingredients are approved.
Test Sites and Subsites Size
What is important in choosing the size of the sites and sub-sites is the possibility of using multi-source solar simulator. It means, choosing smaller sizes for the sites and sub-sites can result in multiple sub-sites being exposed to UV at the same time. Therefore, the CEN standard features (for site size 30 cm2 and for subsites circles 8mm in diameter) are more appropriate in this field.
For distances between the two sub-sites, FDA 2007 suggests an interval of 1 cm. CEN does not specify any interval between subsites, making the FDA 2007 feature considerable for subsites distances.
Loading Dose Amount
The loading quantity of the test and reference product is determined in almost the same amount. CEN suggests the range 2mg/cm2±2.5%, and FDA 2007 proposes 2mg/cm2, which should be uniformly loaded and spread by a finger cot. The product should be placed in small droplets throughout the test area and spread with special movements.
The spreading time should take 20 to 50 s. Product loading areas should be selected randomly.
Waiting Period Between Sunscreen Application and UV Exposure
The waiting time suggested between the product loading and the UV exposure is at least 15 min and 15 to 30 min by both FDA 2007 and CEN, respectively.
Radiation Device and the Periodical Inspection of Its Lamp
Table 1 presents the list of the radiation device specifications for each of the two standards. Regarding the periodic inspection of the device, FDA 2007 and the CEN standard suggest that the inspection be conducted every 6 and 12 months, respectively.
According to the FDA 2011, calibration data of solar simulators and their UV lamps are stable for periods longer than 1 year; Thus, the periodic assessment of the device is better to consider once a year.
Exposure Trend
As shown for the exposure trend in Table 1, the concept of both methods is the same.
Time and Condition of MPD Assessment
MPD is the smallest amount of UVA radiation producing visible tangible pigmentation with visible borders. When the pigment darkness is fixed, the MPD is evaluated visually. For the MPD evaluation, CEN and FDA 2007 suggest 2 to 4 and 3 to 24 h after the last site exposure, respectively. Eye evaluation should be blindly by an experienced observer under adequate and uniform conditions of light. FDA notes that it appears the pigment darkening is most stable about 3 h or more after post-irradiation, and is thus proposing that this observation occur at 3 to 24-h post-irradiation. This time range provides increased flexibility in the test method without sacrificing accuracy. Therefore, FDA 2007 criteria appear to be superior.
In vitro UVA-PF Determination Test Assessment
All in vitro methods are based on the evaluation of UV passing through a thin layer of sunscreen spreading over a rough plate so that the UV level is calculated before and after exposure to the controlled dose from the radiation source. However, at the stage of UVA protection level calculation, each standard sets a different benchmark (Table 2).
 |
Table 2 In vitro UVA-PF Determination Protocol Comparison |
The FDA 2007 standard uses the modified Diffey fraction where the area under the UVA1 absorption curve (340nm to 400nm) is divided into the total absorption region of UVA and UVB (290nm to 400nm).14,15 Nevertheless, FDA 2011 abolished the modified Diffey fraction for the following reasons:
- This fraction provides considerable attention to the UVA1 region, while this area does not play a major role in producing harmful skin effects.
- If one or more ingredients of the product produce, a high absorption in the short wavelength UVA2 region and low or no adsorption in the UVA1 region a large ratio is resulted, while this product does not produce a wide range of absorption.
- This proposed ratio does not show a reliable degree of protection against UVA and does not apply anywhere else in the world.
- One way to increase this fraction is to reduce high-absorption compounds in the UVA2 and UVB regions, but this will reduce the level of protection in these areas of the spectrum (in other words, for increasing the Diffey fraction, SPF should decrease).
The ISO 24443 standard calculates the UVA protective factor in line with the PPD test results. The two standards CEN and FDA 2011 consider the critical wavelength method to be appropriate.
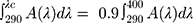
A (λ) is the mean absorption at each wavelength, and d (λ) is the wavelength interval between each calculation.
The critical wavelength is the wavelength in which the region below the absorption curve comprises 90% of the total area under the UV absorption curve.
The FDA concluded that the accompanying critical wavelength test (to measure the extent of UVB and UVA protection) and the SPF test (to measure the magnitude of UVB and UVA protection) provided a complete measurement of the wide spectrum capability. For a product to be labeled with a broad-spectrum statement, it should generate a critical wavelength equal to or greater than 370 nm.
Test Plates
Plates, as the loading place, play a key role in the test. Among the plates of quartz, including PMMA (polymethylmethacrylate), hydrated collagen, epidermal skin and transpore tape, the PMMA plates are chosen for its newness. The following reasons are determining factors of preference to the other plate types:
- It is cheaper than quartz.
- It is ready to use and it does not need to be cleaned or re-roughened.
- It is used for more than a decade.
As for the size of plates, when plates with dimensions of 16 cm2 yield acceptable results, it is not necessary to use plates with larger dimensions (10.2cm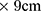 ). Regarding the degree of roughness of these plates, the standard FDA 2011 proposes pages with a roughness of 2 to 7 µg, and the ISO 24443 offers 6-micron roughness. The choice of a constant roughness of 6 µm that is identical to all these tests seems reasonable.
). Regarding the degree of roughness of these plates, the standard FDA 2011 proposes pages with a roughness of 2 to 7 µg, and the ISO 24443 offers 6-micron roughness. The choice of a constant roughness of 6 µm that is identical to all these tests seems reasonable.
Reference Sunscreen Formulation
According to Table 2, only ISO 24443 has a reference sunscreen product. The reference sunscreen S2 brings a UVA-PF of 12.7.
Radiation Source
Before measuring the radiation passage through the plates covered with the product, an irradiated phase for these products is performed to consider optical stability. FDA 2011 believes that the solar simulator (FDA 2007 proposed device) radiation is filtered such a way that its energy at wavelengths below 300nm is less than the spectrophotometer sensitivity even when no sunscreen is loaded on to the plate (i.e. the passage rate is 100%).
Therefore, FDA 2011 proposes a radiation source producing continuous spectral distribution of UV radiation from 290 to 400 nanometers. Standard CEN proposes the solar simulator equipped with filters.
Wavelength Intervals Between Measurement Operations
To measure the passage of radiated UV at each wavelength, the radiation intervals must be intermittent. These intervals are set in the intervals 1nm and 5nm according to Table 2. It was mentioned in FDA 2011: The submissions stated that specifying a smaller interval would produce more accurate results and noted that current spectrometers are capable of making measurements at 1 nm intervals.
The Dynamic Range of the Spectrometer
Dynamic range is one of the indicators to calibrate the UV spectrophotometer. The dynamic range of spectrometer must be sufficient to accurately measure the passage of high-absorption sunscreens at all UV wavelengths (290nm to 400nm).
Product Loading Amount
The amount and process of loading the product on the rough section of the plate is specified in each standard. To ensure accuracy in the amount of product loading, we can use the pipette weight measurement method before and after loading or with the aid of density factor. The product is loaded in droplets of equal volume. The FDA 2007, CEN and ISO 24443 use a value of 2 and 2 mg/cm2 and 1.3 mg/cm2 respectively. The FDA believes that UV passage from a thick layer of 2mg/cm2 is less, and leads to inconsistent responses. Therefore, the FDA set the loading amount at 0.75 mg/cm2 to ensure that the UV passing level is within the dynamic range of UV detectors.
Product Distributing Manner
The smooth spreading of the sunscreen product on the plate considerably affects the accuracy of the test. As Table 2 shows, the spreading process can be either one-phase or two-phase with use or non-use of a finger cot. The FDA 2011 considers the two-phase spreading a more effective way to achieve a solid and thick layer. This contributes to a better distribution for a wide range of drug forms than the 10-s soft distribution technique, and is also similar to the actual use of consumers. Therefore, the FDA 2011 and ISO 24443 opinion in a product-spreading manner seems the best options.
Pre-Irradiation Dose
This operation is performed to check the reduction of optical stability. The sunscreen is loaded onto the plate and then exposed to a source of radiation. According to FDA 2011, since at one time and place on the earth’s surface, the radiation of the sun is equal for products with high or low SPF and UVA-PF, then the pre-irradiation dose in the form of an SPF or UVA-PF fraction is not logical. Thus, FDA 2011 suggests that the pre-radiation dose is a constant value. The FDA received information, which showed that products containing Avobenzone, were completely destroyed in the face of exposure to a dose equivalent to 2–3 MED (Minimal Erythemal Dose).
This is the worst scenario for optical degradation, since Avobenzone is the most unstable sunscreen against light in sunscreens monographs. 1MED in skin type 2 is equivalent to 250 J/m2. Thus, in FDA 2011, the pre-irradiation dosage is set 4MED that is equivalent to 800J/m2.
Number of Radiation Passing Measurement
After exposure of products to pre-radiation dose, the plates covered with the product are placed in the spectrophotometer to measure the passage. According to FDA 2011 opinion (5 times measurement from 3 pages), when a satisfactory result is obtained with fewer computations, then no more time and cost are needed.
Statistics and Calculations
According to CEN, the critical wavelength is obtained from the following formula:
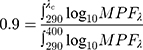
where λC is the critical wavelength, and MPF is the monochromatic protection factor, which is the inverse of the transmittance at a given wavelength.
According to ISO 24443, initially, the individual UVA protection factor (UVA-PFI) on each base (at least 4 bases) is calculated according to the following formula:
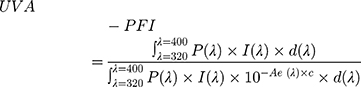
where P(λ) is the PPD action spectrum.
I(λ) is the spectral irradiance received from the UVA source.
Ae(λ) is the mean monochromatic absorbance of the test product layer after UV exposure.
d(λ) is the wavelength step (1 nm).
C is the coefficient of adjustment. The initial absorbance curve values are multiplied by a scalar value “C” until the in vitro calculated SPF values are equal to the in vivo measured SPF. This is accomplished in an iterative calculation process. The initial absorbance values multiplied by this “C” value become the adjusted sunscreen absorbance curve that is used for determination of the initial UVA-PF0 value, and the exposure dose. The “C” value typically lies between 0.8 and 1.6 for valid interpretation. If it is outside this range, new samples should be prepared to validate the original observations.
Thus, according to the formula below, the average UVA-PF calculation is computed:
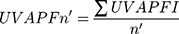
If this condition Confident interval (CI) n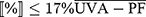 is met, the test is valid; otherwise, the number of bases should be increased as long as the condition is fulfilled.
is met, the test is valid; otherwise, the number of bases should be increased as long as the condition is fulfilled.
If the mentioned condition is not met after using 10 bases, the test is declared invalid.
According to FDA 2011 and FDA 2007 standards, the UV passing level is calculated from the following formula:
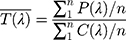
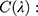 Measurments of spectral irradiance transmitted for each wavelength through control PMMA plates coated with 15 µl of glycerin.
Measurments of spectral irradiance transmitted for each wavelength through control PMMA plates coated with 15 µl of glycerin.
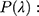 Measurements of spectral irradiance transmitted for each wavelength λ through the PMMA plate covered with the sunscreen product. The average absorption in each wavelength (
Measurements of spectral irradiance transmitted for each wavelength λ through the PMMA plate covered with the sunscreen product. The average absorption in each wavelength (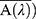 is calculated from the negative logarithm of the mean UV passing as follows:
is calculated from the negative logarithm of the mean UV passing as follows:
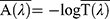
According to FDA 2011, the critical wavelength is calculated from this formula;
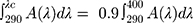
According to FDA 2007, the Diffey fraction ratio is calculated as follows:
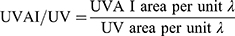
UVA I area per unit λ is given as:
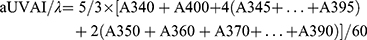
UV area per unit λ is given as:
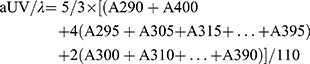
Conclusion
Due to the role of UVA in the occurrence of skin damages, the use of sunscreen products which protects the skin against UVA and UVB, seems indispensable. To assess UVA-PF, the in vivo method is preferred owing to its precision and repeatability, as well as simulation of the actual human use conditions. Among FDA 2007 and CEN standards introducing the in vivo PPD approach, both have advantages and disadvantages so that using a better approach in each case can yield better results.
Disclosure
The authors declare no conflict of interests.
References
1. Yadav N, Banerjee M. Molecular and genetic response of human skin under ultraviolet radiation. Photocarcinogenesis Photoprotection. 2018;15–27. doi:10.1007/978-981-10-5493-8_3
2. Levandovski R, Pfaffenseller B, Carissimi A, et al. The effect of sunlight exposure on interleukin-6 levels in depressive and non-depressive subjects. Biomed Central Psychiatry. 2013;13:75. doi:10.1186/1471-244X-13-75
3. Schneider LA, Raizner K, Wlaschek M, et al. UVA-1 exposure in vivo leads to an IL-6 surge within the skin. Exp Dermatol. 2017;26(9):830–832. doi:10.1111/exd.13286
4. Brem R, Macpherson P, Guven M, et al. Oxidative stress induced by UVA photoactivation of the tryptophan UVB photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) inhibits nucleotide excision repair in human cells. Sci Rep. 2017;7(1). doi:10.1038/s41598-017-04614-8
5. Hiramoto K, Yamate Y, Yokoyama S. Ultraviolet A eye irradiation ameliorates atopic dermatitis via p53 and Clock Gene Proteins in NC/Nga Mice. Photochem Photobiol. 2018;94(2):378–383. doi:10.1111/php.12853
6. Brash DE. UV signature mutations. Photochem Photobiol. 2015;91(1):15–26. doi:10.1111/php.12377
7. Food and Drug Administration proposed rule: sunscreen drug products for over-the-counter human use; proposed amendment of final monograph 07-4131. C2007. [cited August 27, 2007]. Available from: https://www.federalregister.gov/d/07-4131/p-1.
8. Food and Drug Administration proposed rule: labeling and effectiveness testing; sunscreen drug products for over-the-counter human use 2011-14766. C2011. [cited June 17, 2011]. Available from: https://www.federalregister.gov/d/2011-14766/p-2.
9. Australian/New Zealand standard: sunscreen products – evaluation and classification. C2012. [
10. European Commission: standardisation mandate assigned to cen concerning methods for testing efficacy of sunscreen products. C2006. [
11. International Organization for Standardization Determination of sunscreen UVA photoprotection in vitro C2012 [
12. Moyal D. UVA protection labeling and in vitro testing methods. Photochem Photobiol Sci. 2010;9:516–523. doi:10.1039/b9pp00139e
13. Fitzpatrick TB. The validity and practicability of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi:10.1001/archderm.1988.01670060015008
14. Diffey BL. A method for broad-spectrum classification of sunscreens. Int J Cosmet Sci. 1994;16:47–52. doi:10.1111/j.1467-2494.1994.tb00082.x
15. Diffey BL, Matts PJ, Tanner PJ. In vitro assessment of the broad-spectrum ultraviolet protection of sunscreen products. J Am Acad Dermatol. 2000;43:1024–1035. doi:10.1067/mjd.2000.109291
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
