Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Comparison of the Value of Neutrophil to High-Density Lipoprotein Cholesterol Ratio and Lymphocyte to High-Density Lipoprotein Cholesterol Ratio for Predicting Metabolic Syndrome Among a Population in the Southern Coast of China
Authors Chen T, Chen H, Xiao H, Tang H, Xiang Z, Wang X, Wang X, Zou H
Received 21 November 2019
Accepted for publication 15 February 2020
Published 28 February 2020 Volume 2020:13 Pages 597—605
DOI https://doi.org/10.2147/DMSO.S238990
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Tong Chen, Haishan Chen, Hua Xiao, Hongjuan Tang, Zhicong Xiang, Xin Wang, Xuan Wang, Hequn Zou
Department of Nephrology, The Third Affiliated Hospital, Southern Medical University, Guangzhou 510630, People’s Republic of China
Correspondence: Hequn Zou
Department of Nephrology, The Third Affiliated Hospital, Southern Medical University, Guangzhou 510630, People’s Republic of China
Email [email protected]
Background: This study aimed to determine the optimal cutoff values and evaluate the associations of neutrophil to high-density lipoprotein cholesterol ratio (NHR) and lymphocyte to high-density lipoprotein cholesterol ratio (LHR) with metabolic syndrome (MetS), stratified by sex.
Methods: A large-scale cross-sectional survey was conducted among 1401 adults from January to April 2018 in six communities in Wanzhai Town, Zhuhai City, on the southern coast of China. Receiver operating characteristics (ROC) analyses and logistic regression analysis were conducted to assess the optimal cutoff and value of NHR and LHR for predicting MetS.
Results: Hematological parameters showed the correlation with the occurrence of MetS (red blood cells, hemoglobin, and white blood cells and subtypes). Binomial logistic regression analysis found that LHR (OR: 3.671; 95% CI: 2.385– 5.651; p< 0.001) and NHR (OR: 1.728; 95% CI: 1.353– 2.207; p< 0.001) can predict MetS in females, independent of confounding factors. Although LHR (OR: 1.571; 95% CI: 1.001– 2.468; p=0.05) and NHR (OR: 1.163; 95% CI: 0.909– 1.48; p< 0.01) were independent risk factors for MetS in males after adjustment for age, current smoking, current alcohol use, physical activity, educational attainment, waist circumference, systolic pressure, diastolic pressure and hypersensitive C-reactive protein (hs-CRP), when further adjusted for fasting plasma glucose level, LHR and NHR, both lost their independence. ROC curves showed that LHR had the highest AUC for predicting MetS in females and NHR had the highest AUC in males. The cutoff points of LHR and NHR were 1.36 and 2.31 in females, and 1.96 and 3.38 in males.
Conclusion: LHR and NHR may become valuable makers and have strong predictive power for predicting MetS, especially in females.
Keywords: neutrophil to high-density lipoprotein cholesterol ratio, lymphocyte to high-density lipoprotein cholesterol ratio, metabolic syndrome
Introduction
Metabolic syndrome (MetS), affecting a growing number of people, comprises a group of serious global diseases that endanger human health and the main cause of mortality worldwide.1 MetS is a combination of metabolic disorders and involves complicated components, including central obesity, systemic hypertension, insulin resistance, dyslipidemia and elevated fasting glucose.2 The underlying pathophysiology of MetS is not clear; however, there are several widely accepted hypotheses to explain the process of MetS, one of which involves insulin resistance and chronic inflammation, which causes proinflammation and a prothrombotic state, and accelerates atherosclerosis.3
Several studies have found that hematological parameters, including white blood cell (WBC), red blood cell (RBC) and platelet counts, platelet to lymphocyte ratio, neutrophil to lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), monocyte to high-density lipoprotein cholesterol (HDL-C) ratio (MHR) and lymphocyte to high-density lipoprotein ratio (LHR), may be related to MetS and the atherosclerotic process, as potential indicators of prothrombotic and proinflammatory states.4–8 As known, HDL-C plays an important role in anti-inflammatory, antioxidant and antithrombotic progress, and can inhibit cytokine-induced expression of endothelial cell adhesion molecules.9,10 It was reported that the MHR was independently associated with coronary atherosclerosis in patients with stable coronary artery disease;11 however, there is inconsistency in changes in the lymphocyte count in MetS patients. Some studies reported that the number of lymphocytes was decreased in MetS patients, while other studies found that lymphocytes can take part in the pathogenesis of MetS.12,13 Our previous study showed that LHR may be a useful marker of inflammation to assess the presence and severity of MetS.14
The prevalence of MetS has been growing both in China and around the world, with a great number of people suffering from diabetes and cardiovascular diseases. Early diagnosis and prevention of MetS are essential. Hematological parameters can be easily measured from peripheral blood and hematological indicators, as discussed above, can be potential inflammatory makers for predicting MetS. Thus, the objective of this study is to investigate the relationship between NHR and LHR and MetS, to figure out which hematological parameters have higher value for predicting MetS, and to determine the optimal cutoff points, stratified by sex, among adults in southern China.
Methods
Study Population
This cross-sectional survey was conducted from January to April 2018 in six communities in Wanzhai Town, Zhuhai City, on the southern coast of China. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Third Affiliated Hospital of Southern Medical University. All subjects signed a written informed consent form. In total, 1862 residents were enrolled in the survey. Different criteria are used to define MetS. In our study, according to the definition of the International Diabetes Federation (IDF), MetS can be diagnosed when central obesity (waist circumference >90 cm for men or >80 cm for women) is accompanied by any two of the following four factors: (1) an elevated blood pressure (BP) of 130/85 mmHg or higher or receiving treatment for previously diagnosed hypertension; (2) elevated triglycerides (TG) of 1.7 mmol/L or higher or receiving drug treatment for elevated triglycerides; (3) an HDL-C level lower than 1.0 mmol/L for men or lower than 1.3 mmol/L for women or receiving drug treatment for reduced HDL-C; (4) a fasting blood glucose (FBG) of 5.6 mmol/L or higher or with previously diagnosed type 2 diabetes. Patients with diseases that can affect blood system were excluded, including active infection, hepatic, renal or hemolytic disorders, chronic inflammatory diseases, malignant tumor, heart disease, stroke history and current use of antiplatelet drugs. After exclusion, 1401 residents were enrolled in this study.
Data Collection
Questionnaires were used to collect data on age, gender, education, current smoking, current alcohol use, physical activity, and so on. After resting for at least 5 min, the blood pressure of each participant’s left arm was measured with a mercury sphygmomanometer three times, and the mean of the three readings was calculated. Waist circumference was measured at the midpoint between the lower rib edge at the midline and the top of the hip bone. All blood samples were collected after at least 10 h of fasting overnight. Blood samples collected in ethylenediaminetetraacetic acid (EDTA) anticoagulation tubes were used to analyze routine blood parameters, and blood samples collected in tubes containing inert separation gels were used to analyze the blood biochemistry. All blood samples were transported to the central laboratory of the Third Affiliated Hospital of Southern Medical University within 3 h for analysis. Serum creatinine, FBG, high-sensitivity C-reactive protein (hs-CRP), serum total cholesterol (TC), serum triglycerides TG) and serum HDL-C were measured by colorimetry. Whole blood cell counts were assessed using a Sysmex XN-1000 analyzer (Sysmex Corporation, Kobe, Japan). The lymphocyte to HDL-C ratio (LHR) and neutrophil to HDL-C ratio (NHR) were calculated manually. We also defined the severity of MetS based on the number of metabolic components.14
Statistical Analysis
Data were analyzed using Stata (version 15). Continuous variables were shown as mean ± SD if they had a normal distribution. Median and interquartile range were used to show skewed distributed continuous variables. The categorical variables were presented as absolute and relative (%) values or proportions. A two-tailed p value <0.05 was considered significant. The independent-sample t-test was used to compare the differences in the means between the groups, while the Wilcoxon test was used to compare the differences in the medians. The chi-squared (χ2) test was used for comparison of categorical variables. Correlation analysis was used to determine the association between MetS and variables (especially the hematological indicators). Binomial logistic regression analysis was used to identify the independent predictors of MetS, controlling for confounders. A receiver operating characteristics (ROC) curve analysis, which was quantified by the area under the ROC curve (AUC), was used to assess the value of the novel indicator NHR/LHR for predicting MetS. Youden’s index (sensitivity + specificity – 1) was used to determine the optimal cut-off point of each index. Two-tailed p values <0.05 were considered significant. Binomial logistic regression analysis was used to estimate the association between each SD increase of hematological indicators and MetS after adjusting for confounders by sex. The adjusted odds ratios (ORs) are presented with 95% confidence intervals (CIs).
Results
Baseline demographic characteristics and laboratory parameters of the population are shown in Table 1. HDL-C was significantly higher in the MetS(–) group. Lymphocyte, platelet and RBC counts, as well as LDL-C, serum creatinine, alcohol consumption, smoking, physical activity and educational level, did not differ between the two male MetS groups, but hypertension and diabetes history differed. Subjects in the MetS(+) group tended to be older and had larger waist circumference and higher levels of BP, fasting plasma glucose (FPG), TG, TC and hs-CRP. In the female population, physical activity, smoking, alcohol use, LDL-C and serum creatinine did not differ between the two female MetS groups, but educational level, hypertension and diabetes history, hemoglobin, and RBC, WBC, platelet, neutrophil and lymphocyte counts differed significantly. Individuals in the MetS(+) group also had significantly older age, larger waist circumference and higher levels of BP, FPG, TG, TC and hs-CRP. Females in the MetS(–) group had higher levels of HDL-C.
 |
Table 1 Basic Characteristics of the Study Subjects |
We defined the severity of MetS based on the number of metabolic components.14 In correlation analysis (Table 2), except for lymphocyte count in males, all parameters were significantly correlated with MetS severity. Among the correlations in the male population, MetS severity and NHR/LHR and hs-CRP presented the highest values, although smaller than in the female population. In the female population, there was a medium correlation between MetS severity and LHR (R=0.401; p<0.001), NHR (R=0.325; p<0.001) and hs-CRP (R=0.297; p<0.001).
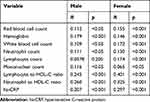 |
Table 2 Correlation Analysis Between Metabolic Syndrome Severity and LHR/NHR |
To determine independent variables for the presence of MetS, binomial logistic regression analysis was performed in females (Table 3) and males (Table 4). Males with higher NHR and LHR had a 1.36- and 1.45-fold increased risk of MetS (p=0.003 and p=0.025), respectively. After controlling for age, current smoking, current alcohol use, physical activity, educational attainment, waist circumference, systolic pressure, diastolic pressure and hs-CRP, the results also showed that NHR and LHR were independent predictors of MetS (Model 2). But NHR and LHR were no longer independent predictors when further adjustment was made for FPG level (Model 3).
 |
Table 3 Binomial Logistic Regression Analysis Showing Independent Predictors of Metabolic Syndrome in Females |
 |
Table 4 Binomial Logistic Regression Analysis Showing Independent Predictors of Metabolic Syndrome in Males |
Females also had increased risk, 3.87- and 1.37-fold higher (both p<0.001), respectively, for developing MetS than those with lower LHR and NHR. After adjustment for age, current smoking, current alcohol use, physical activity, educational attainment, waist circumference, systolic pressure, diastolic pressure and hs-CRP (Model 2) and FPG level (Model 3), the NHR (OR=1.72, p<0.001; OR=1.72, p<0.001, respectively) and LHR (OR=3.93, p<0.001; OR=3.67, p<0.001, respectively) of females were still associated with an increased risk of MetS.
The abilities of LHR and NHR to identify subjects with MetS were compared (Table 5). The accuracy of LHR and NHR, and their sensitivity and specificity in predicting MetS, were different between genders. The accuracy of LHR was higher for females than for males (AUC=0.752), and the optimal cutoff value of LHR was 1.36 (Figure 1). NHR with the higher AUC showed better accuracy in predicting MetS for males (AUC=0.665), followed by LHR (AUC=0.656), and the optimal cutoff value of NHR was 3.38 (Figure 2). Furthermore, for the risk factors assessed, the optimal cutoff value based on Youden's index in predicting MetS was higher for males than females.
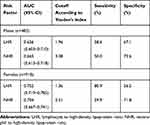 |
Table 5 Areas Under the ROC Curve (AUC), Sensitivity and Specificity by the Optimized Cutoff Points for LHR and NHR in Predicting Metabolic Syndrome |
 |
Figure 1 ROC curve analysis of the value of lymphocyte to high-density lipoprotein ratio (LHR), and neutrophil to high-density lipoprotein ratio (NHR) for predicting metabolic syndrome in females. |
 |
Figure 2 ROC curve analysis of the value of lymphocyte to high-density lipoprotein ratio (LHR), and neutrophil to high-density lipoprotein ratio (NHR) for predicting metabolic syndrome in males. |
Discussion
In this population-based cross-sectional study, we investigated the association between LHR/NHR and MetS in Wanzhai Town, Zhuhai City, on the southern coast of China. The main findings of this study were as follows. (1) LHR/NHR was significantly associated with the prevalence of MetS. (2) Patients with high LHR and NHR showed an increased risk of MetS, especially females, after adjustment for age, smoking, drinking, activity, waist circumference, systolic pressure, diastolic pressure and FPG. The association was more profound for females than for males. (3) The accuracy of LHR and NHR was different between genders. ROC curve analysis showed that LHR had stronger predictive value for MetS in females, while NHR had higher predictive value in males. To the best of our knowledge, this study was the first to analyze LHR and NHR as novel makers and their accuracy in predicting MetS stratified by gender. The results indicated that both LHR and NHR may be related to the chronic inflammation in the pathophysiology of MetS.
A previous study has shown that fat tissue is an endocrinologically and metabolically active organ, which can secrete cytokines and other messenger proteins called adipokines.15 Adipokines are a group of signal proteins which play an important role in inflammation, insulin resistance, lipid metabolism and atherosclerosis.16 MetS is a combination of metabolic factors, involving central obesity, hypertension, dyslipidemia, impaired glucose tolerance and insulin resistance. Subclinical chronic inflammation is independently associated with insulin resistance, and the role of chronic inflammation has been emphasized in previous studies. Subclinical chronic inflammation can be indirectly predicted by blood parameters, which means that the alteration of blood parameters has value for predicting chronic inflammation and progression of MetS.15–17
Several reports have indicated that hematological parameters are changed in patients with MetS.10,18-22 Some studies showed that this alteration can be related to insulin resistance and inflammation, because chronic inflammation can induce synthesis of several groups of cytokines and proteolytic enzymes, and decrease the formation of prostacyclin and nitric oxide, which can cause disruption of endothelial integrity and functional impairment, leading to an increase in WBCs and their subtypes, such as neutrophils. Elevated WBC counts, and counts of WBC subtypes, affected by chronic inflammatory risk factors, are more likely to be capable of vascular endothelium attachment and adherence, which can cause capillary leukocytosis and ultimately result in vasoconstriction and hypertension.23,24 Insulin and insulin growth factors can influence metabolism and proliferation of blood cells, when insulin resistance occurs through the development of MetS.25,26
HDL-C is recognized as an important protective factor in atherosclerosis and inflammation. HDL-C can counteract the migration of macrophages, remove oxidized LDL-C and promote the export of cholesterol from these cells. As an antiatherogenic lipoprotein, HDL-C can also inhibit the endothelial expression of adhesion molecules and prevent monocyte recruitment to the artery wall. So, HDL-C can have anti-inflammatory, antioxidant and antithrombotic effects.9,27-30
Previous studies have shown that hematological parameters have potential as surrogate and novel makers for predicting inflammation, MetS, diabetes and atherosclerosis.
Two studies found that patients with MetS had a higher NLR and the components of MetS can influence the NLR level.31,32 BMI and central obesity indicators such as waist circumference showed positive correlations with NLR;33–35 moreover, elevated NLR is considered valuable for predicting diabetes in obese subjects.36 But another study pointed out that NLR was not a better indicator of inflammation than CRP in obese individuals with MetS.37 In addition, the western dietary pattern was revealed to be significantly associated with increased odds ratios of high CRP and NLR in both genders.38 Monocytes play an important role in inflammation and atherosclerosis in MetS39 and the production of inflammation cytokines.40 The LMR has been recognized as a potentially predictive and prognostic maker for inflammation in distinct populations.7,41 The LMR was also found to be related to diabetic retinopathy and can be an independent risk factor in vascular pathologies such as coronary artery and peripheral vascular disease.42,43 Although the data on the MHR are limited, some studies revealed that MHR was also an important and valuable variable for cardiovascular events and MetS.44,45 In another study, it was found that MetS can involve changes in blood parameters, and the lymphocyte to HDL-C ratio may be a useful marker of inflammation to assess the presence and severity of MetS.14
In summary, in this study, two novel and independent hematological parameters were found. NHR and LHR can predict the progress and development of MetS, and they are easy to access and evaluate in patients.
There are a number of limitations of this study. First, since this is a retrospective single-center cross-sectional study, the analysis of causative effects of LHR and NHR on MetS is limited. Secondly, there was an absence of other inflammatory biomarkers and cytokines in this study. Thirdly, the sample size of this study was relatively small and the sample size of males was smaller than that of females. In addition, as the study involved Chinese people, the particular LHR and, NHR cut-off points cannot be used in other ethnic groups.
Funding
This study was supported by “Risk factors and prediction model of chronic kidney disease caused by metabolic syndrome: A multicentric prospective cohort study Clinical trial training project“ of Southern Medical University (LC2016PY047, 2016), Science and Technique Program of Guangzhou (201604020015, 2015), South Wisdom Valley Innovative Research Team Program (CXTD-004, 2014), and The National Natural Science Foundation of China (81873620).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28(4):629–636. doi:10.1161/ATVBAHA.107.151092
2. McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol. 2018;36(1):14–20. doi:10.1016/j.clindermatol.2017.09.004
3. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. doi:10.1007/s11906-018-0812-z
4. Balci KG, Balci MM, Sen F, et al. The role of baseline indirect inflammatory markers in prediction of response to cardiac resynchronisation therapy. Kardiol Pol. 2016;74(2):119–126. doi:10.5603/KP.a2015.0142
5. Lim HJ, Seo M-S, Shim J-Y, Kim KE, Shin YH, Lee YJ. The association between platelet count and metabolic syndrome in children and adolescents. Platelets. 2015;26(8):758–763. doi:10.3109/09537104.2014.995613
6. Yang H, Fu Y-Q, Yang B, et al. Positive association between the metabolic syndrome and white blood cell counts in Chinese. Asia Pac J Clin Nutr. 2017;26(1):141–147. doi:10.6133/apjcn.102015.13
7. Ertem AG, Yayla C, Acar B, et al. Relation between lymphocyte to monocyte ratio and short-term mortality in patients with acute pulmonary embolism. Clin Respir J. 2018;12(2):580–586. doi:10.1111/crj.2018.12.issue-2
8. Akboga MK, Canpolat U, Sahinarslan A, et al. Association of serum total bilirubin level with severity of coronary atherosclerosis is linked to systemic inflammation. Atherosclerosis. 2015;240(1):110–114. doi:10.1016/j.atherosclerosis.2015.02.051
9. Cockerill GW, Rye K-A, Gamble JRVadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15(11):1987–1994. doi:10.1161/01.ATV.15.11.1987
10. Wu S, Lin H, Zhang C, et al. Association between erythrocyte parameters and metabolic syndrome in urban Han Chinese: a longitudinal cohort study. BMC Public Health. 2013;13:989. doi:10.1186/1471-2458-13-989
11. Akboga MK, Balci KG, Maden O, et al. Usefulness of monocyte to HDL-cholesterol ratio to predict high SYNTAX score in patients with stable coronary artery disease. Biomark Med. 2016;10(4):375–383. doi:10.2217/bmm-2015-0050
12. Berhane M, Melku M, Amsalu A, Enawgaw B, Getaneh Z, Asrie F. The role of neutrophil to lymphocyte count ratio in the differential diagnosis of pulmonary tuberculosis and bacterial community-acquired pneumonia: a Cross-Sectional Study at Ayder and Mekelle Hospitals, Ethiopia. Clin Lab. 2019;65(4). doi:10.7754/Clin.Lab.2018.180833
13. Stentz FB, Kitabchi AE. Transcriptome and proteome expressions involved in insulin resistance in muscle and activated T-lymphocytes of patients with type 2 diabetes. Genomics Proteomics Bioinformatics. 2007;5(3–4):216–235. doi:10.1016/S1672-0229(08)60009-1
14. Chen H, Xiong C, Shao X, et al. Lymphocyte to high-density lipoprotein ratio as a new indicator of inflammation and metabolic syndrome. Diabetes Metab Syndr Obes. 2019;12:2117–2123. doi:10.2147/DMSO
15. Nikolopoulou A, Kadoglou NP. Obesity and metabolic syndrome as related to cardiovascular disease. Expert Rev Cardiovasc Ther. 2012;10(7):933–939. doi:10.1586/erc.12.74
16. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi:10.1016/j.jacc.2010.05.034
17. Akboga MK, Yalcin R, Sahinarslan A, Demirtas CY, Abaci A. Effect of serum YKL-40 on coronary collateral development and SYNTAX score in stable coronary artery disease. Int J Cardiol. 2016;224:323–327. doi:10.1016/j.ijcard.2016.09.042
18. Wang YY, Lin S-Y, Liu P-H, et al. Association between hematological parameters and metabolic syndrome components in a Chinese population. J Diabetes Complications. 2004;18(6):322–327. doi:10.1016/S1056-8727(04)00003-0
19. Lao XQ, Neil Thomas G, Jiang C, et al. White blood cell count and the metabolic syndrome in older Chinese: the Guangzhou Biobank Cohort Study. Atherosclerosis. 2008;201(2):418–424. doi:10.1016/j.atherosclerosis.2007.12.053
20. Tanigawa T, Iso H, Yamagishi K, et al. Association of lymphocyte sub-populations with clustered features of metabolic syndrome in middle-aged Japanese men. Atherosclerosis. 2004;173(2):295–300. doi:10.1016/j.atherosclerosis.2003.12.019
21. Kim JA, CHOI YS, HONG JI, et al. Association of metabolic syndrome with white blood cell subtype and red blood cells. Endocr J. 2006;53(1):133–139. doi:10.1507/endocrj.53.133
22. Tamariz LJ, Young JH, Pankow JS, et al. Blood viscosity and hematocrit as risk factors for type 2 diabetes mellitus: the atherosclerosis risk in communities (ARIC) study. Am J Epidemiol. 2008;168(10):1153–1160. doi:10.1093/aje/kwn243
23. Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45(10):1638–1643. doi:10.1016/j.jacc.2005.02.054
24. Shankar A, Klein BE, Klein R. Relationship between white blood cell count and incident hypertension. Am J Hypertens. 2004;17(3):233–239. doi:10.1016/j.amjhyper.2003.11.005
25. Pasini E, Flati V, Paiardi S, et al. Intracellular molecular effects of insulin resistance in patients with metabolic syndrome. Cardiovasc Diabetol. 2010;9:46. doi:10.1186/1475-2840-9-46
26. Bersch N, Groopman JE, Golde DW. Natural and biosynthetic insulin stimulates the growth of human erythroid progenitors in vitro. J Clin Endocrinol Metab. 1982;55(6):1209–1211. doi:10.1210/jcem-55-6-1209
27. Ghattas A, Griffiths HR, Devitt A, et al. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62(17):1541–1551. doi:10.1016/j.jacc.2013.07.043
28. Murphy AJ, Chin-Dusting JP, Sviridov D, Woollard KJ. The anti inflammatory effects of high density lipoproteins. Curr Med Chem. 2009;16(6):667–675. doi:10.2174/092986709787458425
29. Murphy AJ, Woollard KJ, Hoang A, et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. 2008;28(11):2071–2077. doi:10.1161/ATVBAHA.108.168690
30. Rohatgi A. High-density lipoprotein function measurement in human studies: focus on cholesterol efflux capacity. Prog Cardiovasc Dis. 2015;58(1):32–40. doi:10.1016/j.pcad.2015.05.004
31. Sur G, Floca E, Kudor-Szabadi L, et al. The relevance of inflammatory markers in metabolic syndrome. Maedica (Buchar). 2014;9(1):15–18.
32. Surendar J, Indulekha K, Mohan V, Pradeepa R. Association of neutrophil-lymphocyte ratio with metabolic syndrome and its components in Asian Indians (CURES-143). J Diabetes Complications. 2016;30(8):1525–1529. doi:10.1016/j.jdiacomp.2016.08.006
33. Furuncuoglu Y, Tulgar S, Dogan AN, Cakar S, Tulgar YK, Cakiroglu B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci. 2016;20(7):1300–1306.
34. Kitahara CM, Trabert B, Katki HA, et al. Body mass index, physical activity, and serum markers of inflammation, immunity, and insulin resistance. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2840–2849. doi:10.1158/1055-9965.EPI-14-0699-T
35. Vuong J, Qiu Y, La M, Clarke G, Swinkels DW, Cembrowski G. Reference intervals of complete blood count constituents are highly correlated to waist circumference: should obese patients have their own “normal values?” Am J Hematol. 2014;89(7):671–677. doi:10.1002/ajh.23713
36. Yilmaz H, Ucan B, Sayki M, et al. Usefulness of the neutrophil-to-lymphocyte ratio to prediction of type 2 diabetes mellitus in morbid obesity. Diabetes Metab Syndr. 2015;9(4):299–304. doi:10.1016/j.dsx.2014.04.009
37. Bahadir A, Baltaci D, Turker Y, et al. Is the neutrophil-to-lymphocyte ratio indicative of inflammatory state in patients with obesity and metabolic syndrome? Anatol J Cardiol. 2015;15(10):816–822. doi:10.5152/AnatolJCardiol.
38. Syauqy A, Hsu C-Y, Rau H-H, et al. Association of dietary patterns, anthropometric measurements, and metabolic parameters with C-reactive protein and neutrophil-to-lymphocyte ratio in middle-aged and older adults with metabolic syndrome in Taiwan: a cross-sectional study. Nutr J. 2018;17(1):106. doi:10.1186/s12937-018-0417-z
39. Khan IM, Pokharel Y, Dadu RT, et al. Postprandial monocyte activation in individuals with metabolic syndrome. J Clin Endocrinol Metab. 2016;101(11):4195–4204. doi:10.1210/jc.2016-2732
40. Johnsen SH, Fosse E, Joakimsen O, et al. Monocyte count is a predictor of novel plaque formation: a 7-year follow-up study of 2610 persons without carotid plaque at baseline the Tromso Study. Stroke. 2005;36(4):715–719. doi:10.1161/01.STR.0000158909.07634.83
41. Yayla C, Akboğa MK, Gayretli Yayla K, et al. A novel marker of inflammation in patients with slow coronary flow: lymphocyte-to-monocyte ratio. Biomark Med. 2016;10(5):485–493. doi:10.2217/bmm-2016-0022
42. Yue S, Zhang J, Wu J, Teng W, Liu L, Chen L. Use of the monocyte-to-lymphocyte ratio to predict diabetic retinopathy. Int J Environ Res Public Health. 2015;12(8):10009–10019. doi:10.3390/ijerph120810009
43. Gary T, Pichler M, Belaj K, et al. Lymphocyte-to-monocyte ratio: a novel marker for critical limb ischemia in PAOD patients. Int J Clin Pract. 2014;68(12):1483–1487. doi:10.1111/ijcp.2014.68.issue-12
44. Vahit D, Mehmet KA, Samet Y, Hüseyin E. Assessment of monocyte to high density lipoprotein cholesterol ratio and lymphocyte-to-monocyte ratio in patients with metabolic syndrome. Biomark Med. 2017;11(7):535–540. doi:10.2217/bmm-2016-0380
45. Acikgoz SK, Açıkgöz E, Şensoy B, Topal S, Aydoğdu S. Monocyte to high-density lipoprotein cholesterol ratio is predictive of in-hospital and five-year mortality in ST-segment elevation myocardial infarction. Cardiol J. 2016;23(5):505–512. doi:10.5603/CJ.a2016.0026
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
