Back to Journals » Drug Design, Development and Therapy » Volume 14
Comparison of the Pharmacokinetics, Safety, and Tolerability of the Autoinjector (AI) and Pre-Filled Syringe (PFS) of SB4 in Healthy Subjects
Authors Shin D, Kim Y , Go A , Velinova M
Received 22 July 2019
Accepted for publication 5 December 2019
Published 8 January 2020 Volume 2020:14 Pages 43—50
DOI https://doi.org/10.2147/DDDT.S224103
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Donghoon Shin,1 Younsoo Kim,1 Ahra Go,1 Maria Velinova2
1Samsung Bioepis Co., Ltd., Incheon, Republic of Korea; 2PRA International, Groningen, the Netherlands
Correspondence: Donghoon Shin
Samsung Bioepis Co., Ltd, 107, Cheomdan-daero, Yeonsu-gu, Incheon 21987, Republic of Korea
Tel +82-31-8061-4534
Fax +82-31-8061-4393
Email [email protected]
Purpose: SB4 is an etanercept biosimilar, approved by the European Commission (EC) and the US Food and Drug Administration (FDA) following a demonstration of equivalent efficacy and safety and comparable quality data to the reference product. This study aimed to demonstrate equivalent pharmacokinetic (PK) profiles, safety, and tolerability between SB4 autoinjector (AI) and SB4 pre-filled syringe (PFS).
Patients and methods: This was an open-label, two-period, two-sequence, single-dose, cross-over study to evaluate bioequivalence of PK profiles, safety, and tolerability between SB4 AI and PFS in healthy adults. Treatment periods were separated by 14 days resulting in a 35-day washout between investigational product (IP) administration in Periods 1 and 2.
Results: A total of 50 subjects were randomized: 24 subjects in Sequence I and 26 in Sequence II, and 6 subjects discontinued from the study. The primary PK endpoints including area under the concentration–time curve from time zero to infinity (AUCinf) and to the last quantifiable concentration (AUClast), and maximum serum concentration (Cmax) were all within the equivalence margin of 80.00–125.00%. Safety and tolerability were comparable between the two treatments.
Conclusion: PK profiles showed that SB4 AI and PFS were bioequivalent in healthy subjects. Safety assessment was also comparable between the two treatments.
Keywords: biosimilar, etanercept, bioequivalence, autoinjector, pre-filled syringe
Introduction
Etanercept is a recombinant human tumor necrosis factor (TNFR) p75Fc fusion protein and was originally developed for the treatment of moderate-to-severe active RA.1 SB4 is the first biosimilar for etanercept reference product (ETN) developed by Samsung Bioepis Co., Ltd, and was approved by the European Commission (EC) and the US Food and Drug Administration (FDA) through a demonstration of similarity in structural characteristics, physicochemical properties, in vitro biological activities as well as its therapeutic equivalence to ETN.2–5
A Phase I study in healthy subjects showed that pharmacokinetic (PK) is equivalent between SB4 and reference etanercept (ETN) using the standard equivalence margin of 80–125%.6 In a Phase III, randomized, double-blind, and parallel-group study comparing SB4 with ETN, SB4 was shown to be equivalent with ETN in terms of the American College of Rheumatology 20% (ACR20) response rate at week 24 (78.1% for SB4 and 80.3% for ETN) in moderate to severe rheumatoid arthritis.7 In the Phase I and III studies, patients received 50mg of etanercept subcutaneously via a prefilled syringe (PFS).6,7 PFS is made of clear glass (type I) with a 27-gauge, stainless steel needle, rubber needle cover, and rubber plunger.8,9
Even though PFSs do not need to be reconstituted and offer greater patient convenience compared with vials, they can be difficult to use in patients with reduced manual dexterity.10 A systemic review showed that the median adherence rate for etanercept was 63% (range, 16–73%) in patients with rheumatoid arthritis.11 Low adherence to therapeutic regimen has negative consequences and can lead to substantial costs, increased disease flares, disease progression, increased disability, additional medical therapy, and sometimes surgery.12,13 Nonadherence can be intentional and nonintentional, and poor manual dexterity can impact the person’s ability and skill at medicine-taking leading to unintentional nonadherence.13 In addition, patients who experience difficulty when injecting may experience unnecessary pain and increased overall treatment costs.14,15
The autoinjector (AI) pen, which consists of type 1 glass with a stainless steel 27-gauge needle,8,9 was introduced to improve the injection experience in patients with reduced manual dexterity. This bridging study aimed to demonstrate equivalent PK profiles, safety, and tolerability between SB4 AI and PFS.
Materials and Methods
Subjects
Healthy male subjects aged 18–55 years with a body weight between 60.0 and 85.5 kg and a body mass index between 20.0 and 28.0 kg/m2 were included. In addition, subjects, who had clinical laboratory results within the normal range or outside the normal range but assessed as clinically non-significant by the investigator at screening were included. Patients were excluded if they had any of the following criteria: either active or latent tuberculosis (TB) as indicated by a positive test result for Mycobacterium tuberculosis, history of TB at screening, previous exposure to etanercept, biological agent or immunosuppressive agent within 120 days prior to the first investigational product (IP) administration in Periods 1 and 2.
Study Design
This is a Phase I, randomized, open-label, two-period, two-sequence, single-dose cross-over study in healthy male subjects performed at a single center in The Netherlands (EudraCT number: 2016-004993-16; ClinicalTrials.gov identifier: NCT03193203). Subjects were assigned 1:1 to either Treatment Sequence I in which they received SB4 PFS in Period 1 and SB4 AI in Period 2 or Treatment Sequence II in which they received SB4 AI in Period 1 and SB4 PFS in Period 2 with a washout period of 14 days between treatment periods (Figure 1). 50mg of SB4 AI or 50mg of SB4 PFS was administered subcutaneously (s.c.) in the abdomen. SB4’s active substance is etanercept and its excipients include sucrose, sodium chloride, sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate heptahydrate, and water.8 Blood samples for PK analysis were collected for analysis.
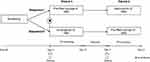 |
Figure 1 Study design. Abbreviation: PK, pharmacokinetics. |
Study Assessments
Pharmacokinetics (PK) Endpoints
The PK analysis set (PKS) included all subjects who were randomized and completed both Period 1 and Period 2 without any major protocol deviation. Reasons that may lead to exclusion from the PKS include incomplete administration of IP and other non-compliance with the protocol. Blood samples for PK analysis were collected at each time point at 0 h (pre-dose) and 6, 12, 24, 36, 48, 60, 72, 84, 96, 120, 144, 168, 216, 312 and 480 h post-dose. Serum concentration of etanercept was measured using an enzyme-linked immunosorbent assay (ELISA) test specific for the quantification of etanercept with the lower limit of quantification 20 ng/mL.6 The primary PK endpoints included area under the concentration–time curve from time zero to infinity (AUCinf) and to the last quantifiable concentration (AUClast), and maximum serum concentration (Cmax). The secondary PK endpoints were time to Cmax (Tmax), apparent volume of distribution during the terminal phase (Vz/F), terminal half-life (t1/2), and apparent total clearance (CL/F). To avoid a carryover effect, a subject with pre-dose concentrations greater than 5% of the Cmax value before IP administration in Periods 1 and 2 was excluded.
Safety
The safety set consists of all subjects who were randomized and received at least one IP. All adverse events (AEs) recorded from the study were coded using the Medical Dictionary for Regulatory Activities (MedDRA) Version 16.0. The assessment of safety was based 12-lead electrocardiogram (ECG), vital signs (including blood pressure and pulse rate measurements), physical examinations, and clinical laboratory data (hematology, biochemistry, and urinalysis). The incidence of treatment-emergent adverse events (TEAEs) by severity and causality and serious adverse events (SAEs) was summarized using the number of subjects and the frequency. Blood sampling for immunogenicity was performed at D1 prior to IP administration and at D21 in Period 2 at the end of the study.
Tolerability via Injection Site Reaction Evaluation
Subjects were instructed to report any pain or itching after IP administration. In addition to their reporting, subjects’ injection sites were assessed by the investigator or appropriate designees for 5 tolerability parameters: 1) Redness, 2) bruising, 3) swelling, 4) itching, and 5) pain immediately after IP administration at 0, 6 and 12 hrs after injection on Day 1 and then once daily from Day 2 to Day 8 of each period. Tolerability parameters followed a severity scale: None = 0, mild = 1, moderate = 2, and severe = 3. The Global Irritation Score (GIS), ranging from 0 to 15, was the sum of the individual grades. An injection site reaction with a score of 2 or greater was documented as an AE.
Statistical Analysis
Sample Size Calculation
With the equivalence limit of [80.00%, 125.00%], it was calculated that 19 subjects per treatment sequence were needed with the assumption of the true geometric mean ratio of 1.05 and intra-subject coefficient variation of 25% at 5% significance level, providing 90% power to reject the null hypothesis. Assuming a 15% drop-out rate, it was determined that a total of 46 subjects (23 per treatment sequence) were needed.
PK Data Analysis
Analysis of variance (ANOVA) was performed on the loge-transformed PK parameters of AUCinf, AUClast, and Cmax. The ANOVA model included treatment administration, sequence, and period as fixed effects and subject nested within the sequence as a random effect. Each ANOVA included calculation of least-square means (LSMeans) for the treatment administration. The assessment of bioequivalence was based upon the 90% CIs for the ratio of the geometric LSMeans (SB4 AI/SB4 PFS) for AUCinf, AUClast, and Cmax which had to be contained completely within the bioequivalence limits of 80.00–125.00%. Subsequently, ANOVA on the loge-transformed PK parameters was performed in subjects with body weight ≤75 kg and >75 kg, respectively. All PK parameters were calculated using PhoenixTM WinNonlin® Version 6.3.
Results
Subject Disposition
Among a total of 116 screened subjects, 50 were eligible for randomization. 24 subjects were randomized to Treatment Sequence I and 26 subjects to Treatment Sequence II. Baseline demographic characteristics of 50 subjects were well balanced between the two sequences (Table 1). Among 50 subjects, 44 completed the study, and 6 subjects discontinued from the study (Table 2).
 |
Table 1 Baseline Demographic Characteristics (Randomized Set) |
 |
Table 2 Disposition of Subjects (Randomized Set) |
PK
The six (6) discontinued subjects were excluded from the PKS. Among 44 subjects (23 in Sequence I and 21 in Sequence II), mean values of PK parameters of the PKS were similar between treatments (Table 3), and no unusual PK profile was detected. The maximum value for the pre-dose level was 0.057 µg/mL, which was less than 2% of mean of Cmax, and no subject was excluded from the PK analysis because of the pre-dose concentration greater than 5% of the Cmax. The mean serum concentration-time profiles in linear and semi-logarithmic scale were superimposable (Figure 2). The statistical comparison of primary PK parameters between SB4 AI and PFS showed that the geometric LSMean ratios (90% CI) for AUCinf, AUClast, and Cmax were 103.76% (98.36% to 109.46%), 103.97% (98.41% to 109.83%), and 104.40% (97.65% to 111.61%), respectively, with their corresponding 90% CIs within the pre-defined equivalence margin of 80.00% to 125.00% (Table 4). Of 44 subjects in the PKS, a total of 12 (27.3%) subjects were included in the ≤75 kg body weight subgroup and 32 (72.7%) subjects were included in the >75 kg body weight subgroup. The geometric LSMeans of AUCinf, AUClast and Cmax for the ≤75 kg body weight subgroup was numerically higher than the >75 kg body weight subgroup for both AI and PFS treatments (Table 4). The geometric LSMean ratios (90% confidence interval [CI]) between SB4 AI and SB4 PFS in both ≤75 kg and >75 kg body weight subgroups were within the pre-defined equivalence margin of 80.00% to 125.00% (Table 4).
 |
Table 3 Pharmacokinetic Parameters (PK Analysis Set) |
 |
Table 4 Statistical Comparison of Primary PK Between SB4 AI and PFS in Total Subjects and by Weight (PK Analysis Set) |
 |
Figure 2 Mean serum conc-time profile in linear and Semi-logarithmic scale. Abbreviations: AI, autoinjector; PFS, pre-filled syringe. |
Safety
A total of 50 subjects were included in the safety set. Among them, 50 subjects received SB4 AI, and 45 subjects received SB4 PFS. Overall, a single-dose administration of SB4 via AI was well tolerated, and the safety profile was comparable with PFS. The proportion of subjects who experienced TEAEs was similar between the AI and PFS groups (32, 64.0% and 21, 46.7% of subjects, respectively). The most frequent TEAEs across the 2 treatments were headache, oropharyngeal pain, pruritus, neutropenia, and rhinitis (Table 5). There were no deaths, serious adverse events (SAEs), or discontinuations of the study due to TEAEs. In addition, laboratory data, vital signs, physical findings, and ECG parameters did not show any relevant changes over time that might be related to the study drug.
 |
Table 5 Safety Profile (Safety Set) |
Tolerability via Injection Site Reaction Evaluation
No severe injection site reactions were observed, and there were two subjects whose injection site reactions were categorized as AEs. One subject experienced redness with a severity score of 2 (moderate) on injection on Day 6 of Period 1 (SB4 AI treatment), and it resolved the next day. The other subject experienced injection site reactions during SB4 PFS treatment. The first incidence was on Day 1 of Period 2 with GIS of 3 (1 for bruising, swelling, and pain), followed by GIS of 2 (1 for bruising and itching) on Day 2, then GIS of 2 (2 for bruising) on Day 3 to Day 6, and then GIS of 2 (2 for bruising) on Day 8. All injection site reactions were resolved by Day 18 of Period 2.
Most subjects, 84.0% (42/50) in SB4 AI and 82.2% (37/45) in SB4 PFS, had GIS of 0. 16.0% (8/50) of subjects who received SB4 AI and 17.8% (8/45) of subjects who received SB4 PFS had GIS ≥1. The most frequently reported injection site reaction for the SB4 AI and PFS treatment was mild pain on Day 1 immediately after IP administration (at 0.00 hrs) (3 subjects in each treatment).
Discussion
This study was a randomized, open-label, two-period, two-sequence, single-dose, cross-over study to demonstrate the bioequivalence of the PK profiles, safety, and tolerability between SB4 AI and SB4 PFS in healthy male subjects. This study demonstrates that the 90% CI of the geometric mean ratios between SB4 AI and PFS for AUCinf, AUClast, and Cmax were within the pre-defined range of 80.00–125.00% and thus considered to be bioequivalent.
It is known that body weight can be a significant covariate associated with pharmacokinetics of anti-TNFs.16,17 When the current study groups were divided into the ≤75 kg and >75 kg body weight subgroups, the higher body weight subgroup in both treatments showed 15% to 18% lower PK profiles compared to the lower body weight subgroup, and each body weight subgroup showed PK equivalence between SB4 AI and PFS. Therefore, it can be concluded that the impact of body weight on PK is the same between SB4 AI and PFS.
In terms of safety, no SAEs were observed, and the proportion of subjects who experienced TEAEs was similar between the SB4 AI and SB4 PFS treatment (64.0% and 46.7% of subjects, respectively).
Treatment with etanercept can lead to injection site reactions including bruising, erythema, itching, pain, and swelling.18 In this study, most subjects had no injection site reactions at any time point during the study and most of the reactions were mild in severity.
Previous studies have shown that AI is considered to be easier and more convenient to use compared to PFS in chronic conditions.10,19–21 Rheumatoid arthritis is a chronic, systemic disease leading to progressive joint destruction.22 It is estimated that 80–90% of patients with rheumatoid arthritis have their hands and wrists affected.23 Thus, offering a device that can accommodate self-injection for patients with hand and wrist involvement is important.
The SB4 AI has demonstrated that it offers convenience to patients. In a recent study, 220 patients with rheumatoid arthritis from Europe were surveyed through fact-to-face questionnaire-interview. Most patients (74%) reported that they prefer to use SB4 AI over reference etanercept AI due to the following factors: “Easy to operate” and “button-free autoinjector”.24 Similarly, when patients, nurses, and rheumatologists in Australia and Canada were shown how to use SB4 AI and reference etanercept AI and asked to fill out brief paper-and-pencil questionnaires, results showed that patients and health-care professionals were significantly more likely to indicate a preference for SB4 AI than reference etanercept AI for “ease of use”.25 Nurses and rheumatologists indicated that SB4 AI is simpler to learn and easier to use, thus it has the potential to reduce the time taken in training session.25 With the benefit of convenience, SB4 AI can potentially increase medication adherence in patients with rheumatologic conditions.
Conclusions
SB4 AI and PFS demonstrated equivalent PK parameters, tolerability, and safety in healthy subjects. Based on these results, it can be postulated that SB4 is well-tolerated, safe and effective when administered by either delivery method.
Ethics Approval and Informed Consent
This study was approved by the Independent Ethics Committee (IEC) of the foundation Beoordeling Ethiek Biomedisch Onderzoek of The Netherlands and conducted in accordance with the Declaration of Helsinki and was approved by an ethics committee. All subjects provided written informed consent.
Data Sharing Statement
Upon request, and subject to certain criteria, conditions and exceptions, Samsung Bioepis will provide access to individual de-identified participant data. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply. The proposals should be directed to the corresponding author. To gain access, data requestors must enter into a data access agreement with Samsung Bioepis.
Acknowledgments
Medical writing assistance was provided by Gihyun Myung, MD, an employee of Samsung Bioepis Co., Ltd.
Disclosure
Donghoon Shin, Younsoo Kim, and Ahra Go are employees of Samsung Bioepis Co., Ltd.
The authors report no other conflicts of interest in this work.
References
1. Garrison L, McDonnell ND. Etanercept: therapeutic use in patients with rheumatoid arthritis. Ann Rheum Dis. 1999;58(Suppl 1):I65–9.
2. Committee for Medicinal Products for Human Use (CHMP). Guideline on similar biological medicinal products containing monoclonal antibodies-non clinical and clinical issues. European Medicines Agency; 2012. Available from: http://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-monoclonal-antibodies-non-clinical_en.pdf.
3. Food and Drug Administration Center for Drug Evaluation and Research. Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. U.S. Department of Health and Human Services; 2015. Available from: https://www.fda.gov/media/82647/download.
4. Emery P, Vencovsky J, Sylwestrzak A, et al. 52-week results of the Phase 3 randomized study comparing SB4 with reference etanercept in patients with active rheumatoid arthritis. Rheumatology (Oxford). 2017;56(12):2093–2101. doi:10.1093/rheumatology/kex269
5. Emery P, Vencovsky J, Sylwestrzak A, et al. Long-term efficacy and safety in patients with rheumatoid arthritis continuing on SB4 or switching from reference etanercept to SB4. Ann Rheum Dis. 2017;76:1986–1991. doi:10.1136/annrheumdis-2017-211591
6. Lee YJ, Shin D, Kim Y, et al. A randomized phase I pharmacokinetic study comparing SB4 and etanercept reference product (Enbrel ®) in healthy subjects. Br J Clin Pharmacol. 2016;82(1):64–73. doi:10.1111/bcp.12929
7. Emery P, Vencovsky J, Sylwestrzak A, et al. A Phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in pts with active RA. Ann Rheum Dis. 2017;76(1):51–57. doi:10.1136/annrheumdis-2015-207588
8. European Medicines Agency. Benepali summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/benepali-epar-product-information_en.pdf.
9. U.S. Food & Drug Administration. Eticovo full prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761066s000lbl.pdf.
10. Borrás-Blasco J, Gracia-Pérez A, Rosique-Robles JD, Casterá MD, Abad FJ. Acceptability of switching adalimumab from a prefilled syringe to an autoinjection pen. Expert Opin Biol Ther. 2010;10(3):301–307. doi:10.1517/14712590903530633
11. Murage MJ, Tongbram V, Feldman SR, et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systemic literature review. Patient Prefer Adherence. 2018;12:1483–1503. doi:10.2147/PPA.S167508
12. Sharp LA. A medical anthropologist’s view on posttransplant compliance: the underground economy of medical survival. Transplant Proc. 1999;31(4A):31S–33S. doi:10.1016/S0041-1345(99)00121-9
13. Van Dem Bemt BJ, Zwikker HE, Van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. 2012;8(4):337–351. doi:10.1586/eci.12.23
14. DiMetteo MR. Variations in patient’s adherence to medical recommendation: a quantitative review of 50 years of research. Med Care. 2004;42:200–209. doi:10.1097/01.mlr.0000114908.90348.f9
15. Mohr DC, Cox D, Epstein L, Boudewyn A. Teaching patients to self-inject: pilot study of a treatment for injection anxiety and phobia in multiple sclerosis patients prescribed injectable medication. J Behav Ther Exp Psychiatry. 2002;33:39–47. doi:10.1016/S0005-7916(02)00011-3
16. Mostafa NM, Nader AM, Noertersheuer P, et al. Impact of immunogenicity on pharmacokinetics, efficacy and safety of adalimumab in adult patients with moderate to severe chronic plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31(3):490–497. doi:10.1111/jdv.13884
17. Wade JR, Parker G, Kosutic G, et al. Population pharmacokinetic analysis of certolizumab pegol in patients with Crohn’s disease. J Clin Pharmacol. 2015;55(8):866–874. doi:10.1002/jcph.v55.8
18. Enbrel® (etanercept). Full Prescribing Information. Thousand Oaks, CA: Immunex Corporation; 2016.
19. Kroon L. Overview of insulin delivery pen devices. J Am Pharm Assoc. 2003;49:e118–131. doi:10.1331/JAPhA.2009.08125
20. McCoy EK, Wright BM. A review of insulin pen devices. Postgrad Med. 2010;122:81–88. doi:10.3810/pgm.2010.05.2145
21. Paul C, Stalder JF, Thaçi D, et al. Patient satisfaction with injection devices: a randomized controlled study comparing two different etanercept delivery systems in moderate to severe psoriasis. J Eur Acad Dermatol Venereol. 2012;26(4):448–455. doi:10.1111/jdv.2012.26.issue-4
22. Eberhardt KB, Fex E. Functional impairment and disability in early rheumatoid arthritis-development over 5 years. J Rheumatol. 1995;22:1037–1042.
23. Durmus D, Uzuner B, Durmaz Y, et al. Michigan hand outcomes questionnaire in rheumatoid arthritis patients: relationship with disease activity, quality of life, and hand grip strength. J Back Musculoskelet Rehabil. 2013;26(4):467–473. doi:10.3233/BMR-130408
24. Thakur K, Biberger A, Handrich A, Rezk MF. Patient perceptions and preferences of two etanercept autoinjectors for rheumatoid arthritis: findings from a patient survey in Europe. Rheumatol Ther. 2016;3(2):245–256. doi:10.1007/s40744-016-0048-9
25. Egeth M, Soosar J, Nash P, et al. Patient and healthcare professional’s preference for Brenzys vs. Enbrel autoinjector for rheumatoid arthritis: a randomized crossover simulated-use study. Adv Ther. 2017;34(5):1157–1172. doi:10.1007/s12325-017-0523-x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
