Back to Journals » Clinical Ophthalmology » Volume 14
Comparison of the Intraocular Pressure-Lowering Efficacy and Safety of the Brinzolamide/Brimonidine Fixed-Dose Combination versus Concomitant Use of Brinzolamide and Brimonidine for Management of Open-Angle Glaucoma or Ocular Hypertension
Authors Wang N, Lu DW, Pan Y, Astakhov Y, Iureva T, Adewale A, Walker TM
Received 17 September 2019
Accepted for publication 31 December 2019
Published 23 January 2020 Volume 2020:14 Pages 221—230
DOI https://doi.org/10.2147/OPTH.S231402
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ningli Wang,1 Da-Wen Lu,2 Yingzi Pan,3 Yury Astakhov,4 Tatyana Iureva,5 Adeniyi Adewale,6 Thomas M Walker6
1Beijing Tongren Eye Center, Beijing, People’s Republic of China; 2Tri-Service General Hospital, Taipei, Taiwan; 3Peking University First Hospital, Beijing, People’s Republic of China; 4Pavlov First Saint Petersburg State Medical University, Saint Petersburg, Russia; 5Irkutsk Branch of the Academician S.N. Fyodorov Eye Microsurgery Federal State Institution, Irkutsk, Russia; 6Novartis Pharmaceuticals Corporation, Fort Worth, TX, USA
Correspondence: Ningli Wang
Beijing Tongren Eye Center, No. 1 Dongjiaomin Str., Dongcheng District, Beijing 100041, People’s Republic of China
Tel +861058265922
Email [email protected]
Objective: To demonstrate that the intraocular pressure (IOP)-lowering efficacy of a twice-daily brinzolamide 10 mg/mL (BRINZ)/brimonidine 2 mg/mL (BRIM) fixed-dose combination (BBFC) was non-inferior to its individual components (BRINZ+BRIM) dosed concomitantly in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT). Safety was also evaluated.
Methods and Analysis: This was a Phase III, multicenter, observer-masked study in patients from China, Russia and Taiwan. Patients aged ≥ 18 years with a mean IOP ≥ 21 mmHg and ≤ 36 mmHg in the same eye after washout of other IOP-lowering medications were included. Eligible patients were randomized (1:1) to receive BBFC or BRIZ+BRIM eye drops twice daily for 3 months. The primary endpoint was the mean change in diurnal IOP (averaged over 09:00, +2 h, and +7 h) from baseline to Month 3. Adverse events (AEs) were recorded throughout the study.
Results: The per-protocol set included 349 patients (BBFC, n=172; BRINZ+BRIM, n=177). The mean±standard deviation diurnal IOP at baseline was 24.6± 2.66 mmHg in both groups. At Month 3, the least square mean±standard error change in diurnal IOP from baseline was − 7.2± 0.34 mmHg and − 7.3± 0.34 mmHg with BBFC and BRINZ+BRIM, respectively (between-group difference: 0.1 mmHg [95% CI − 0.5, 0.7]). In the BBFC and BRINZ+BRIM groups, 53.3% and 55.0% of patients achieved a diurnal IOP < 18 mmHg, and 43.2% and 37.4% of patients, respectively, achieved a mean diurnal IOP reduction > 30% from baseline at Month 3. Ocular AEs were reported in 28.7% (BBFC) and 22.5% (BRINZ+BRIM) of patients; conjunctival hyperemia was the most frequent ocular AE (BBFC, 6.4%; BRINZ+BRIM, 6.8%). Non-ocular AEs were reported in 32.4% (BBFC) and 30.4% (BRINZ+BRIM) of patients.
Conclusion: The study findings demonstrate that the efficacy of twice-daily BBFC was non-inferior to BRINZ+BRIM in patients with OAG/OHT. The safety profile of BBFC was similar to that of BRINZ+BRIM.
Keywords: brinzolamide/brimonidine fixed-dose combination, intraocular pressure reduction, ocular hypertension, open-angle glaucoma
Introduction
Open-angle glaucoma (OAG) is a progressive optic neuropathy and a common cause of irreversible blindness worldwide.1 Ocular hypertension (OHT) refers to raised intraocular pressure (IOP) in patients without detectable glaucomatous damage on standard clinical tests.2,3 Elevated IOP is a major risk factor for glaucoma; IOP reduction is the only proven and effective medical approach for slowing progression of glaucoma and reducing the associated risk of vision loss.4–6 The Asia-Pacific Glaucoma Guidelines recommend monotherapy with topical IOP-lowering agents as the first-line therapy for OAG and OHT.7 In patients for whom monotherapy is insufficient, combination therapy with two or more IOP-lowering agents is recommended to achieve and maintain the target IOP.8
However, an increase in the number of medications is associated with a decrease in treatment adherence and patient persistence to these medications,9–11 which may reduce the effectiveness of multidrug regimens. Fixed-dose combinations (FDCs) of IOP-lowering agents offer greater convenience and improved treatment adherence than concomitant use of two or more medications.11,12 Simbrinza® (Novartis Pharma AG, Basel, Switzerland) is a FDC of brinzolamide 10 mg/mL and brimonidine 2 mg/mL (BBFC). Brinzolamide is a carbonic anhydrase inhibitor that decreases aqueous humor secretion. Brimonidine has a dual mechanism of action of reducing aqueous humor production and increasing uveoscleral outflow. BBFC is approved in the European Union and many other countries as a twice-daily regimen for the treatment of OAG or OHT when monotherapy is insufficient for IOP reduction.13 In the United States, BBFC is approved as a thrice-daily regimen for the treatment of OAG or OHT.14
Here, we report on a study conducted to assess the efficacy and safety of BBFC versus concomitant administration of brinzolamide 10 mg/mL (AZOPT®, Novartis Pharma AG, Basel, Switzerland; BRINZ) and brimonidine 2 mg/mL (Brimonidine, Novartis Pharma AG, Basel, Switzerland, BRIM) in patients with OAG or OHT from China, Russia and Taiwan.
Materials and Methods
Study Design
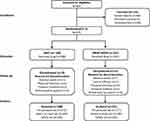
This was a 3-month, prospective, Phase III, randomized, observer-masked, active-controlled study conducted from April 2015 to November 2016 across 26 centers from the three aforementioned countries (ClinicalTrials.gov, NCT02339584). The study consisted of 2 sequential phases (a screening/eligibility phase and a treatment/follow-up phase) involving six visits (Figure 1). The screening phase included a washout period of 5–28 days during which patients who met the initial inclusion and exclusion criteria discontinued their prior IOP-lowering agents. Following the washout period, two eligibility visits, E1 and E2, were scheduled 3–8 days apart. During the treatment period, eligible patients were randomized 1:1 to either BBFC or to brinzolamide and brimonidine given concomitantly (BRINZ+BRIM), dosed twice daily (at approximately 09:00 and 21:00) in both eyes for 3 months. Efficacy and safety was evaluated at Weeks 2 and 6 (09:00 and +2 h [following dosing]) and Month 3 (09:00, +2 h and +7 h [following dosing]). If only one of a patient’s eyes was dosed, the dosed eye was selected as the study eye. If both eyes were dosed, the eye with the higher IOP at 09:00 averaged across the two eligibility visits was selected as the study eye. If both eyes were equal, the right eye was selected as the study eye. Randomization was stratified by country and region; the treatment group was assigned to each patient using an interactive response system. Investigators, the study sponsor, investigational center staff, and clinical monitors were masked to treatment assignments to avoid any bias.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with the International Council on Harmonization of Technical Requirements for Pharmaceuticals for Human Use E6 Good Clinical Practice Consolidated Guideline and other country-specific regulatory guidelines, as applicable. The trial protocol, protocol amendments and informed consent forms were approved by an Independent Ethics Committee/Institutional Review Board (Supplementary Material Appendix 1). All sites received ethical approval prior to recruitment. All patients provided written informed consent before study initiation.
Patients
Inclusion Criteria
Male or female patients, aged 18–95 years, diagnosed with OAG or OHT who were insufficiently controlled on monotherapy or were currently on multiple IOP-lowering medications were included. Patients were required to have a mean IOP between ≥21 mmHg and ≤36 mmHg in at least one eye (the same eye) at the 09:00 and the +2 h time points of both eligibility visits, and a mean IOP ≤36 mmHg at any time point.
Key Exclusion Criteria
Key exclusion criteria were Schaffer angle Grade <2 (measured by gonioscopy); cup/disc ratio >0.80; severe central visual field loss, defined as a sensitivity of ≤10 decibels in at least two of the four visual field test points closest to the point of fixation; best-corrected visual acuity (BCVA) score worse than 55 Early Treatment Diabetic Retinopathy Study (ETDRS) letters; or chronic, recurrent, or severe inflammatory eye disease, ocular trauma, ocular infection, or intraocular surgery (a full list of criteria is provided in Supplementary Material Appendix 2).
Assessments
Efficacy and Safety assessments
IOP was measured using a Goldmann applanation tonometer at screening, at both eligibility visits, at Weeks 2 and 6 at 09:00 and +2 h, and at Month 3 at 09:00, +2 h, and +7 h. The 09:00 IOP assessment was performed prior to the study medication dosing. All IOP measurements for any individual patient were preferably performed by the same operator using the same tonometer.
Overall ocular safety was monitored through anatomic and functional assessments including BCVA, slit-lamp and fundus ophthalmoscopy performed at baseline and study visits. BCVA was assessed using a standardized ETDRS chart. Ocular signs were evaluated through slit-lamp biomicroscopy examination of the cornea, iris/anterior chamber, lens, and eyelids/conjunctiva. Both BCVA and slit-lamp examinations were performed on study visit days before measurement of IOP.
Automated perimetry assessments of visual field function were performed with the Humphrey Field Analyzer using standard full threshold, FASTPAC, SITA-Standard, or SITA-FAST testing algorithms or the Octopus Perimeter using Program G1 or G1X and/or dG2 at screening and at the Month 3 visit.
Dilated fundus examination of the vitreous, retina, macula, choroid, and optic nerve was performed on both eyes after measurement of IOP at screening and at the Month 3 visit. Adverse events (AEs) were recorded throughout the study.
Study Endpoints
The primary endpoint was a diurnal IOP change (averaged over 09:00, +2 h and +7 h) from baseline at Month 3. The supportive efficacy endpoints were: diurnal IOP change from baseline at each visit (Weeks 2 and 6 and Month 3); IOP at Week 2 (09:00 and +2 h), Week 6 (09:00 and +2 h), and Month 3 (09:00, +2 h, and +7 h); IOP mean change and mean percentage change from baseline by visit and time point (at Week 2 [09:00 and +2 h], Week 6 [09:00 and +2 h], and Month 3 [09:00, +2 h, and +7 h]); the percentage of patients with a diurnal IOP <18, <16, and <14 mmHg at Month 3; and the percentage of patients with >20%, >25%, and >30% diurnal IOP reduction from baseline at Month 3. Safety endpoints included cardiovascular parameters (pulse and blood pressure), fundus, BCVA and slit-lamp examination, visual field loss, and AEs.
Statistics
Sample Size Calculation
At a one-sided significance level of 0.025 with a non-inferiority margin of 1.5 mmHg, and assuming a common standard deviation of 3.5 mmHg and an inferiority of BBFC to BRINZ+BRIM of 0.1 mmHg with respect to the mean diurnal IOP change from baseline, 320 evaluable patients would yield 95% power to demonstrate non-inferiority of BBFC versus BRINZ+BRIM. Assuming a non-evaluable rate of 15%, a sample size of 376 patients was estimated so as to have at least 320 evaluable patients.
Statistical Method
All statistical analyses were performed using Statistical Analysis Software (SAS Institute, Cary, NC, USA). The primary endpoint was analyzed using an analysis of covariance (ANCOVA) model that had change in diurnal IOP from baseline as a response variable, treatment and region as fixed effects, site as a random effect, and baseline IOP as a covariate. Non-inferiority was assessed on the primary endpoint based on the upper limit of the 95% confidence interval (CI) for the between-group treatment difference in the least square mean (LSM) diurnal IOP change from baseline at 3 months of <1.5 mmHg. Supportive efficacy endpoints and safety were analyzed descriptively.
The primary efficacy analysis set was the per-protocol (PP) set, which included all patients who received study medication, met the pre-randomization inclusion/exclusion criteria, and who had at least one scheduled on-therapy study visit. Safety was analyzed using the safety analysis set, which included all patients who received at least one dose of study medication.
Results
Of the 493 patients enrolled, 379 were randomized to receive treatment (BBFC, n=188; BRINZ+BRIM, n=191), and 349 completed the study (BBFC, n=173; BRINZ+BRIM, n=176; Figure 2). AEs were the most common reason for discontinuation from the study in both groups (Figure 2). The PP set included 349 patients (BBFC, n=172; BRINZ+BRIM, n=177) and the safety set included 379 patients (BBFC, n=188; BRINZ+BRIM, n=191).
Baseline and Demographic Characteristics
The age (mean ± standard deviation [SD]) of the study population was 52.4 ± 16.25 years, and 55.9% of patients were female. At baseline, approximately 68% of patients had a diagnosis of OAG (with or without a pseudoexfoliative or pigment dispersion component) and 32% of patients had a diagnosis of OHT. Overall, the baseline and demographic characteristics were similar between the BBFC and BRINZ+BRIM groups (Table 1).
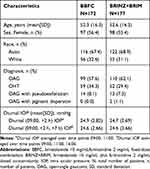 |
Table 1 Demographic and Baseline Characteristics by Treatment Groups (Per-Protocol Set) |
Primary Efficacy Outcome
At baseline, the mean ± SD diurnal IOP was 24.6 ± 2.66 mmHg in both groups. At Month 3, the IOP was 17.9 ± 3.31 mmHg with BBFC and 17.8 ± 3.44 mmHg with BRINZ+BRIM. The LSM ± standard error (SE) change in diurnal IOP from baseline at Month 3 was −7.2 ± 0.34 mmHg (95% CI −7.9, −6.5) with BBFC and −7.3 ± 0.34 mmHg (95% CI −7.9, −6.6) with BRINZ+BRIM (between-group difference: 0.1 mmHg [95% CI −0.5, 0.7]). The upper limit of the 95% CI was <1.5 mmHg, demonstrating the non-inferiority of BBFC to BRINZ+BRIM (Table 2).
 |
Table 2 Change in Diurnal IOP from Baseline at Month 3 (Per-Protocol Set) |
Supportive Efficacy Outcomes
The diurnal IOP decreased from baseline in both groups at Weeks 2 and 6 and at Month 3 (Table 3). The mean IOP and the change in mean IOP from baseline was comparable between the two treatment groups at each visit for all time points; the greatest mean reductions in IOP were observed at the +2 h time point at each visit (Figures 3 and 4A). The percentage reduction in IOP ranged from 20.5%–33.3% with BBFC and 20.7–33.9% with BRINZ+BRIM, across all visits (Figure 4B). In all, 53.3% and 55.0% of patients on BBFC and BRINZ+BRIM, respectively, achieved a mean diurnal IOP <18 mmHg at Month 3 (Supplementary Information Figure 1A). The percentages of patients with a >20%, >25%, and >30% diurnal IOP reduction from baseline at Month 3 were numerically greater with BBFC than BRINZ+BRIM (Supplementary Information Figure 1B).
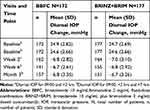 |
Table 3 Mean Diurnal IOP Change in BBFC and BRINZ+BRIM Treatment Groups from Baseline by Study Visits (Per-Protocol Set) |
Safety Outcomes
The mean duration of exposure was similar between the two treatment groups (BBFC, 85 days; BRINZ+BRIM, 86 days). Overall, 50.0% and 46.6% of patients in the BBFC and BRINZ+BRIM groups, respectively, experienced at least 1 AE; of these, the AEs were considered to be treatment-related in 21.3% and 20.4% of patients, respectively (Table 4). Ocular AEs were reported in 28.7% of patients receiving BBFC and in 22.5% of patients receiving BRINZ+BRIM; of these, 14.9% (BBFC) and 12.6% (BRINZ+BRIM) were related to the treatment. The ocular AE with the highest incidence was conjunctival hyperemia in both groups (6.4% in BBFC; 6.8% in BRINZ+BRIM). No deaths or serious ocular AEs were reported during the study. Four non-ocular serious AEs, which were not fatal, were reported in each group (BBFC: deafness neurosensory, pancreatitis, ligament injury, and renal colic; BRINZ+BRIM: uterine leiomyoma, diabetic neuropathy, vocal cord leukoplakia, and gastritis). In all, 18 patients (11 in BBFC and 7 in BRINZ+BRIM) discontinued the study due to an AE. In 17 patients, these AEs were considered related to the study drug (Table 4 and Supplementary Table 1). The BCVA, slit-lamp examination and fundus examination results, cup/disc ratio, corneal thickness, visual field assessment, blood pressure and pulse rate were comparable between both groups (Supplementary Tables 2–6).
 |
Table 4 Adverse Events Reported in BBFC and BRINZ+BRIM Groups (Safety Set) |
Discussion
This study was the first to evaluate the efficacy and safety of BBFC in patients with OAG or OHT from China, Russia and Taiwan. The results of this study demonstrated that BBFC administered twice daily was non-inferior to BRINZ+BRIM in terms of IOP-lowering efficacy in these patients. A clinically relevant IOP-lowering effect of BBFC was observed as early as Week 2 and was maintained throughout the study period. Overall, these results are consistent with a previous global multicenter study that demonstrated the non-inferiority of twice-daily BBFC versus individual drugs administered concomitantly in patients with OAG or OHT.15
The Early Manifest Glaucoma Trial demonstrated that each 1 mmHg decrease in mean IOP reduces the risk of glaucoma progression by 10%.16 Thus, a 7.2 mmHg reduction with twice-daily BBFC treatment observed in this study is clinically significant with the potential to reduce glaucoma progression. The Advanced Glaucoma Intervention Study showed that an IOP <18 mmHg is associated with reduced progression of visual field deterioration.17 In the present study, a mean diurnal IOP of <18 mmHg at Month 3 was observed in as many as 50% of patients. These findings are especially relevant considering that the patients included in this study did not achieve their target IOP with monotherapy or with other multiple IOP-lowering agents.
The greatest reduction in IOP with BBFC was observed at the 11.00 time point (+2 h following dosing), which corresponds to the peak effect for both of the individual components of the product. The 12 hr trough effect of BBFC was observed at the 09:00 time point. The peak and trough efficacy of BBFC in this study was consistent with that observed in other studies that compared the efficacy of twice-daily BBFC with its components administered either separately or concomitantly in patients with OAG/OHT. At Month 3, the peak effect was 16.4 mmHg and trough effect was 19.3 mmHg in the study by Aung et al; in the study by Gandolfi et al, the corresponding values were 16.0 mmHg and 19.2 mmHg).15,18 Assessment of IOP later in the day can give a better understanding of the diurnal IOP control. In this study, at the Month 3 visit, clinically relevant IOP reductions were achieved with BBFC at each of the three time points (09:00: −5.2 mmHg; +2 h: −8.3 mmHg; +7 h: −6.8 mmHg). These results demonstrate that good diurnal IOP control can be achieved with BBFC treatment. Similar findings have been reported for a Phase III trial that assessed the IOP-lowering effect with twice-daily BBFC in patients with OAG or OHT (mean IOP change from baseline at Month 3: 09:00, −7.7 mmHg; +2 h: −9.7 mmHg).18
Gandolfi et al demonstrated the similarity in IOP-lowering efficacy of twice-daily BBFC and concomitant administration of BRINZ and BRIM (Month 3 mean IOP change: –8.5 mmHg versus –8.4 mmHg).15 The findings of the present study add to the existing knowledge that BBFC is non-inferior to BRINZ+BRIM.
The safety profile of BBFC was similar to concomitant administration of the individual components (BRINZ+BRIM). The AEs reported for BBFC in this study during the 3-month period were comparable with those reported in other studies; in the present study, the proportion of serious AEs was 2.1% (vs 2.6% in Aung et al and 2.4% in Gandolfi et al) while ocular hyperemia was reported in 2.1% (vs 5.7% and 3.5%).15,18 Thus, the safety profile of BBFC observed in this study was consistent with its known safety profile and no new safety signals were reported.13
The use of FDCs has many advantages over concomitant administration of two or more drugs. FDCs confer greater convenience to the patient, provide multiple mechanisms of action, and minimize or eliminate potential washout effects that arise with multidrug regimens.12,19 In addition, FDCs reduce exposure to preservatives that may be associated with ocular surface disease symptoms.19 BBFC contains considerably less of the preservative benzalkonium chloride (0.03 mg/mL) compared with the levels in the individual components used concomitantly (BRINZ, 0.15 mg/mL; BRIM, 0.05 mg/mL) and may therefore reduce the risk of ocular surface damage, intolerability, and associated non-compliance.15 Similar to other FDCs, BBFC may have the potential to improve adherence and persistence to treatment relative to therapy with separate drugs.11,20
An added advantage of BBFC in patients with OAG or OHT is that it is the only available FDC without a β-blocker. BBFC is therefore a suitable option for patients with OAG or OHT who have comorbid conditions where β-blockers are contraindicated or use of systemic therapeutic regimens (eg systemic β-blockers)21 make them susceptible to adverse drug reactions (eg depression of systemic cardiovascular function observed with timolol).22,23
IOP is subject to fluctuations during the 24 hr cycle and some studies have observed that peak diurnal IOP occurs early in the morning. Hence, a limitation of the study is that IOP was assessed at a limited number of time points during the day in the study, and not at early morning, evening, or nocturnal time points.
Conclusions
BBFC administered twice daily is non-inferior to BRINZ+BRIM in terms of IOP-lowering efficacy in patients with OAG or OHT from China, Russia and Taiwan. The safety profile of BBFC is comparable to that of the individual components, BRINZ and BRIM. For patients with OAG or OHT requiring additional IOP-lowering therapy, BBFC is an efficacious option.
Availability of Data
The data used to support the conclusion of this study are included within the article and in the Supplementary Material.
Acknowledgment
The authors thank Swati Bhandari, PhD (Novartis Healthcare Pvt. Ltd., India) for medical writing support and editorial assistance during the development of the manuscript.
Funding
This study was sponsored by Alcon Research Ltd., Fort Worth, TX, USA.
Disclosure
Meeting presentation: Data included in this manuscript were previously presented at the following congresses: 13th European glaucoma society (EGS) 2018, Florence, Italy; 36th World Ophthalmology Congress (WOC) 2018, Barcelona, Spain. Ningli Wang has received honoraria and personal fees from Novartis. Adeniyi Adewale and Thomas M. Walker are employees of Novartis. Adeniyi Adewale is also a marketer of brinzolamide/brimonidine fixed-dose combination. The authors report no other conflicts of interest in this work.
References
1. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. doi:10.1016/S0140-6736(04)16257-0
2. Gordon MO, Kass MA. What we have learned from the ocular hypertension treatment study. Am J Ophthalmol. 2018;189:xxiv–xxvii. doi:10.1016/j.ajo.2018.02.016
3. Leibowitz HM, Krueger DE, Maunder LR, et al. The framingham eye study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24(Suppl):335–610.
4. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi:10.1001/archopht.120.10.1268
5. Blumberg D, Skaat A, Liebmann JM. Emerging risk factors for glaucoma onset and progression. Prog Brain Res. 2015;221:81–101.
6. Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385(9975):1295–1304. doi:10.1016/S0140-6736(14)62111-5
7. Asian Pacific Glaucoma Society. Asia Pacific glaucoma guidelines. 3 Edition. 2016. Available from: https://www.apglaucomasociety.org/Public/Public/Resources/APGG.aspx.
8. Noecker RJ. The management of glaucoma and intraocular hypertension: current approaches and recent advances. Ther Clin Risk Manag. 2006;2(2):193–206. doi:10.2147/tcrm.2006.2.issue-2
9. Fechtner RD, Realini T. Fixed combinations of topical glaucoma medications. Curr Opin Ophthalmol. 2004;15(2):132–135. doi:10.1097/00055735-200404000-00013
10. Sleath B, Robin AL, Covert D, Byrd JE, Tudor G, Svarstad B. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113(3):431–436. doi:10.1016/j.ophtha.2005.10.034
11. Higginbotham EJ. Considerations in glaucoma therapy: fixed combinations versus their component medications. Clin Ophthalmol. 2010;4:1–9.
12. Nguyen QH. Combination of brinzolamide and brimonidine for glaucoma and ocular hypertension: critical appraisal and patient focus. Patient Prefer Adherence. 2014;8:853–864. doi:10.2147/PPA
13. European Medicines Agency. Simbrinza® Summary of Product Characteristics. Switzerland: Novartis Pharma AG. Available from:: https://www.ema.europa.eu/documents/product-information/simbrinza-epar-product-information_en.pdf.
14. Food and Drug Administration. Simbrinza® prescribing information. Available from https://www.drugs.com/pro/simbrinza.html.
15. Gandolfi SA, Lim J, Sanseau AC, Parra Restrepo JC, Hamacher T. Randomized trial of brinzolamide/brimonidine versus brinzolamide plus brimonidine for open-angle glaucoma or ocular hypertension. Adv Ther. 2014;31(12):1213–1227. doi:10.1007/s12325-014-0168-y
16. Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48–56. doi:10.1001/archopht.121.1.48
17. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS investigators. Am J Ophthalmol. 2000;130(4):429–440. doi:10.1016/S0002-9394(00)00538-9
18. Aung T, Laganovska G, Hernandez Paredes TJ, Branch JD, Tsorbatzoglou A, Goldberg I. Twice-daily brinzolamide/brimonidine fixed combination versus brinzolamide or brimonidine in open-angle glaucoma or ocular hypertension. Ophthalmology. 2014;121(12):2348–2355. doi:10.1016/j.ophtha.2014.06.022
19. Hollo G, Topouzis F, Fechtner RD. Fixed-combination intraocular pressure-lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expert Opin Pharmacother. 2014;15(12):1737–1747. doi:10.1517/14656566.2014.936850
20. Robin AL, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy? Ophthalmology. 2005;112(5):863–868. doi:10.1016/j.ophtha.2004.12.026
21. Schuman JS. Effects of systemic β-blocker therapy on the efficacy and safety of topical brimonidine and timolol. Ophthalmology. 2000;107(6):1171–1177. doi:10.1016/S0161-6420(00)00081-6
22. Waldock A, Snape J, Graham CM. Effects of glaucoma medications on the cardiorespiratory and intraocular pressure status of newly diagnosed glaucoma patients. Br J Ophthalmol. 2000;84(7):710–713. doi:10.1136/bjo.84.7.710
23. Javitt JC, Schiffman RM. Clinical success and quality of life with brimonidine 0.2% or timolol 0.5% used twice daily in glaucoma or ocular t hypertension: a randomized clinical trial. Brimonidine outcomes study group I. J Glaucoma. 2000;9(3):224–234. doi:10.1097/00061198-200006000-00005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.