Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Comparison of the Efficacy and Safety of Transarterial Chemoembolization with or without Lenvatinib for Unresectable Hepatocellular Carcinoma: A Retrospective Propensity Score–Matched Analysis
Authors Chen YX , Zhang JX, Zhou CG, Liu J , Liu S, Shi HB, Zu QQ
Received 3 May 2022
Accepted for publication 23 July 2022
Published 1 August 2022 Volume 2022:9 Pages 685—694
DOI https://doi.org/10.2147/JHC.S373250
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Manal Hassan
Yu-Xing Chen,1,* Jin-Xing Zhang,1,* Chun-Gao Zhou,1 Jin Liu,2 Sheng Liu,1 Hai-Bin Shi,1 Qing-Quan Zu1
1Department of Interventional Radiology, The First Affiliated Hospital with Nanjing Medical University, Nanjing, 210029, People’s Republic of China; 2Department of Clinical Medicine Research Institution, The First Affiliated Hospital with Nanjing Medical University, Nanjing, 210029, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qing-Quan Zu, Department of Interventional Radiology, The First Affiliated Hospital with Nanjing Medical University, No. 300 Guangzhou Road, Nanjing, 210029, People’s Republic of China, Tel +86-25-68306316 ; +86-13913807470, Fax +86-25-83724440, Email [email protected]
Background: Combination of angiogenesis inhibitor may achieve better therapeutic synergistic efficacy, considering of tumor hypoxia and promoted angiogenesis after transarterial chemoembolization (TACE). This study aimed to compare the safety and efficacy of TACE plus lenvatinib (TACE-lenvatinib) with TACE alone for patients with unresectable hepatocellular carcinoma (HCC).
Methods: Between June 2019 and September 2021, a total of 215 patients diagnosed with unresectable HCC were retrospectively reviewed, including 53 patients who received TACE-lenvatinib and 162 patients who received TACE alone. The patient selection bias between the TACE-lenvatinib group and the TACE group was balanced by propensity score matching analysis at a 1:2 ratio. Progression-free survival (PFS), overall survival (OS) and tumor response were evaluated in the two groups.
Results: After propensity score matching analysis, 34 patients receiving TACE-lenvatinib and 68 patients receiving TACE alone were enrolled. The median PFS and OS times in the TACE-lenvatinib group were significantly greater than those in the TACE group (PFS: 8.3 months vs 4.6 months, P = 0.008; OS: 27.7 months vs 18.4 months, P = 0.043). The objective response rate (ORR) in the TACE-lenvatinib group was higher than that in the TACE alone group (64.1% vs 36.5%, P = 0.002). Univariate and multivariate analyses revealed that TACE-lenvatinib treatment was an independent favorable prognostic factor for both PFS and OS.
Conclusion: For unresectable HCC patients, the TACE-lenvatinib appeared superior to TACE alone regarding tumor control, PFS, and OS. However, considering the limitations of this study, these results should be interpreted as preliminary and warrant further confirmation.
Keywords: carcinoma, hepatocellular, chemoembolization, therapeutic, lenvatinib, survival analysis, propensity score
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer in the world and the fourth leading cause of cancer-related mortality, with nearly 78,000 deaths per year.1 The majority of patients are diagnosed at an intermediate or advanced stage, which implies that they missed the opportunity to undergo radical surgery.2
Transarterial chemoembolization (TACE) is recommended as the primary treatment for patients with HCC at an intermediate or a locally advanced stage.3,4 However, a considerable number of patients are either not sensitive to TACE alone or develop TACE resistance, resulting in a poor prognosis.5,6 The potential mechanisms of TACE ineffectiveness or resistance may be due to the upregulation of vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) after the procedure,7,8 thus leading to an exploration of synergistic and complementary treatment modalities with TACE. Three randomized controlled trials comparing the efficacy of TACE plus sorafenib with TACE alone showed unexpected negative results for this combination therapy.9–11 Compared with these trials, the recent TACTICS trial prolonged the duration of sorafenib administration. The results demonstrated that TACE plus sorafenib yielded longer progression-free survival (PFS) in unresectable HCC patients; however, it did not bring an overall survival (OS) benefit compared with TACE alone.12,13
Lenvatinib is a novel molecule tyrosine kinase inhibitor that has been approved in several countries as a first-line treatment for HCC. Compared with sorafenib, lenvatinib has higher selective inhibition of VEGF receptors.14 Moreover, lenvatinib inhibits FGF receptors and rearranged during transfection (RET), thereby suppressing tumor angiogenesis and tumor cell proliferation.15 These effects could further convert the immunosuppressive status of the tumor microenvironment and boost the antitumor immune response.16–18 Therefore, in the face of tumor hypoxia and increased levels of VEGF and FGF after TACE,7 theoretically, the combination of TACE and lenvatinib may achieve better therapeutic synergistic efficacy in real-world practice.
Thus, we conducted this retrospective study to compare the efficacy and safety of TACE-lenvatinib with TACE alone for patients with unresectable HCC.
Methods
This retrospective study was approved by our institutional ethics review board, and the procedures followed in this study were in accordance with the guidelines of the World Medical Association Declaration of Helsinki. The study protocol was also approved by the Ethics Committee of the First Affiliated Hospital with Nanjing Medical University (Ethical review no. 2021-SR-013). Based on the nature of this retrospective study, the requirement for informed consent was waived. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patients
Between June 2019 and September 2021, unresectable HCC patients who were treated with TACE plus lenvatinib (n = 53) or TACE alone (n = 162) at our institution were retrospectively reviewed. HCC was confirmed by pathology or α-fetoprotein (AFP), contrast-enhanced computed tomography (CT), or magnetic resonance imaging (MRI) examination according to the Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China.19 HCC was diagnosed pathologically based on biopsy (n = 11) or a prior history of resected HCC (n = 53). The patients were enrolled based on the following criteria: (1) Barcelona Clinic Liver Cancer (BCLC) stage B or C without extrahepatic metastasis; (2) Eastern Cooperative Group (ECOG) performance status score ≤ 1; and (3) Child–Pugh ≤ B7. The exclusion criteria included the following: (1) patients who received lenvatinib for less than 4 weeks; (2) patients with other primary malignancies; (3) patients who received chemotherapy before the initial TACE procedure; and (4) patients with incomplete data or who were lost to follow-up.
The baseline data collected from the medical record system included age, sex, etiology, ECOG performance status score, Child–Pugh class, AFP level, number of tumors, maximum tumor diameter, and presence of portal vein tumor thrombus (PVTT). Treatment options were determined mainly based on physicians’ discretion, patients’ consent, and economic situation of the patients. The final treatment decision was consented with each patient.
TACE Protocol
For the TACE procedure, a 5-F catheter was introduced via the femoral artery, and angiography was conducted to identify the tumor-feeding artery. Afterward, the chemotherapeutic agent (lobaplatin, 30–50 mg) was infused into the proper hepatic artery. Then, chemoembolization via a microcatheter either selectively or superselectively was performed with an emulsion of epirubicin (10 mg) and iodized oil (10 mL), followed by a gelatin sponge or particles. The dose of emulsion was 5–20 mL. Post-embolization syndromes were documented. Liver function was rechecked within 1 week after each session.
Lenvatinib
Lenvatinib was ordered one to two weeks following the TACE procedure. Lenvatinib was prescribed at a dose of either 12 mg/day for patients with a bodyweight of ≥ 60 kg or 8 mg/day for patients with a bodyweight of < 60 kg. Treatment discontinuation and dose reductions (from 8 mg to 4 mg/day) were allowed based on lenvatinib-related toxicity, patient tolerance, and clinician decisions.
Follow-Up and Assessments
During the follow-up visit, tumor marker tests and blood tests, including blood cell count, liver function, and renal function, were performed at approximately one-month intervals to evaluate adverse events (AEs). Contrast-enhanced CT or MRI was performed at 2–3 month intervals. When a residual tumor or new mass was confirmed, the patients were treated based on their liver function, general condition and tumor status after multidisciplinary discussion. All patients were followed up until the patient died or the end of the study (January 2022).
For both groups, OS was defined as the time from the initial TACE procedure to death from any cause. PFS was defined as the time from the initial TACE procedure to progression or death from any cause. The objective response rate (ORR) was calculated as the proportion of patients with the best overall tumor response of complete response (CR) or partial response (PR) based on the Modified Response Evaluation Criteria for Solid Tumors (mRECIST),20 and the disease control rate (DCR) was calculated as the proportion of patients with the best overall tumor response of CR, PR, or stable disease (SD). Safety assessments consisted of monitoring and recording treatment-related AEs based on the Common Terminology Criteria for Adverse Events version 5.21
Statistical Analysis
To minimize potential bias, propensity score matching (PSM) analysis was conducted between the TACE-lenvatinib group and the TACE group (1:2 ratio). The following variables were included in the model: age, sex, Child–Pugh class, AFP, number of tumors, tumor size, and presence of PVTT.
Continuous data are presented as the mean and range and were compared between the two groups using Student’s t test. Categorical data are expressed as frequencies and percentages and were compared between the two groups using the χ2 test or Fisher’s exact test when appropriate. PFS and OS were estimated using the Kaplan–Meier method and compared between the two groups using the Log rank test. Univariate and multivariate analyses based on the Cox regression model were used to evaluate predictors associated with PFS and OS. Factors with a P value < 0.10 in the univariable analysis were selected for multivariable analysis. A two-tailed P value < 0.05 was considered statistically significant. Data analysis was conducted using IBM SPSS statistics, version 24 (IBM Corp, New York, USA) and GraphPad Software (Prism 8.0.1, San Diego, California).
Results
Patient Characteristics
A total of 39 patients treated with TACE-lenvatinib and 148 patients treated with TACE alone were enrolled in this study. The clinical characteristics of all the patients (n = 187) are presented in Table 1. After the PSM analysis, 34 patients who received TACE-lenvatinib and 68 patients who received TACE alone were matched (Figure 1). No significant difference was observed between the two groups in any of the clinical variables after PSM. The median duration of lenvatinib treatment was 7.3 months (range: 2.5–30.8 months) in the TACE-lenvatinib group. The median number of TACE sessions was 2.9 (range: 1–10) in the TACE-lenvatinib group compared with 3.3 (range: 1–13) in the TACE group (P = 0.394).
 |
Table 1 Baseline Patient Characteristics Before and After Propensity Score Matching |
 |
Figure 1 Flowchart of patient enrollment. Abbreviations: HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; BCLC, Barcelona Clinic Liver Cancer. |
PFS
During the follow-up, 162 patients (86.6%) had disease progression, comprising 26 of the 39 (66.7%) patients in the TACE-lenvatinib group and 136 of the 148 (91.9%) patients in the TACE group. The median PFS was 8.3 months in the TACE-lenvatinib group compared with 4.4 months in the TACE alone group before PSM (P < 0.001) (Figure 2A). After PSM, the corresponding median PFS times were 8.3 months and 4.6 months (P = 0.008) (Figure 2B). Univariate and multivariate analyses of PFS are summarized in Table 2. The multivariate analysis showed that tumor number (HR = 2.01; 95% CI, 1.21–3.35; P = 0.007) and treatment modality (HR = 0.45; 95% CI, 0.27–0.75; P = 0.002) were independent prognostic factors for PFS.
 |
Table 2 Predictive Factor Analysis for Progression-Free Survival (After Matching) |
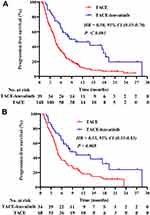 |
Figure 2 (A) Kaplan–Meier survival curves for progression-free survival before PSM. (B) Kaplan–Meier survival curves for progression-free survival after PSM. |
OS
During a median follow-up period of 11.7 months, 88 of the 187 (47.1%) patients died, including 9 patients in the TACE-lenvatinib group and 79 patients in the TACE group. The median OS was 27.7 months in the TACE-lenvatinib group compared with 15.9 months in the TACE alone group before PSM (P = 0.002) (Figure 3A). After PSM, the corresponding median OS times were 27.7 months and 18.4 months (P = 0.043) (Figure 3B). Univariate and multivariate analyses of OS are summarized in Table 3. The multivariate analysis showed that AFP level (HR = 2.17; 95% CI, 1.06–4.45; P = 0.034), tumor number (HR = 5.07; 95% CI, 2.21–11.61; P < 0.001), and treatment modality (HR = 0.38; 95% CI, 0.17–0.85; P = 0.018) were independent prognostic factors for OS.
 |
Table 3 Predictive Factor Analysis for Overall Survival (After Matching) |
 |
Figure 3 (A) Kaplan–Meier survival curves for overall survival before PSM. (B) Kaplan–Meier survival curves for overall survival after PSM. |
Tumor Response
The tumor responses are shown in Table 4. According to the mRECIST criteria, the ORR was significantly higher in the TACE-lenvatinib group than in the TACE alone group, regardless of the unmatched (64.1% vs 36.5%, P = 0.002) and matched cohorts (67.6% vs 39.7%, P = 0.008). Furthermore, the disease control rate had a higher trend in the TACE-lenvatinib group than in the TACE alone group (79.5% vs 67.6%, P = 0.148 before PSM; 79.4% vs 69.1%, P = 0.272 after PSM).
 |
Table 4 Tumor Response Based on Modified Response Evaluation Criteria in Solid Tumors |
AEs (Grade 3 or 4)
Treatment-related AEs (grade 3 or 4) are listed in Table 5. Grade 3 or 4 AEs were observed in 21 of the 34 (58.8%) patients in the TACE-lenvatinib group and 29 of the 68 (42.6%) patients in the TACE alone group, and there was no significant difference in the grade 3 or 4 AEs between the two groups (P = 0.069). Grade 3 or 4 abnormal liver function was the most common AE in both the TACE-lenvatinib group and the TACE alone group. No treatment-related deaths occurred.
 |
Table 5 Treatment-Related Adverse Events (Grade 3 or 4) |
In addition, 6 of the 34 patients (17.6%) reduced the dose of lenvatinib in the TACE-lenvatinib group. After dose reduction and conservative treatment, all AEs were manageable or eliminated. Finally, one patient in the TACE-lenvatinib group discontinued lenvatinib because of uncontrolled hypertension.
Discussion
TACE is the most feasible treatment for intermediate or locally advanced HCC in many countries.3,4 However, TACE usually makes it difficult to complete tumor necrosis and then creates a secondary hypoxic environment within the residual lesion. Hypoxia stimulates the expression of VEGF, FGF and other angiogenic factors, which induce tumor progression, recurrence and metastasis.7,8 Lenvatinib is a relatively broad-spectrum molecule tyrosine kinase inhibitor that inhibits VEGF receptors (VEGFR1-3), FGF receptors (FGFR1-4), KIT and RET.22–24 Based on this, we hypothesized that the combination of TACE and lenvatinib therapy for patients with unresectable HCC could work synergistically while eliminating tumor burden and preventing tumor relapse. Our study also further confirmed that TACE-lenvatinib therapy could significantly prolong PFS and OS and result in a better tumor control rate compared with TACE alone.
In the past decade, intermittent trials, eg, the post-TACE trial, SPACE trial, and TACE-2 trial, have not shown a significant clinical benefit regarding TACE plus sorafenib compared with TACE alone for patients with unresectable HCC.9–11 Recently, a positive result was reported by Kudo et al in the TACTICS trial.13 The reason for this switched result might be that time-to-untreated progression (TTUP) was proposed as a new primary endpoint, which embodied a longer treatment duration of sorafenib administration than before. Meanwhile, these different results also indicated that the efficacy of TACE-based combination therapy for unresectable HCC is still undefined.25 Our study tried to reveal the efficacy and safety of TACE-lenvatinib therapy and used PSM analysis to maintain confidence in our results. We found that the combination group achieved better efficacy results with a median PFS of 8.3 months and a median OS of 27.7 months than the TACE group (PFS, 4.6 months; OS, 18.4 months). In addition, people in the TACE-lenvatinib group had an ORR of 67.6%, which was dramatically better than that in the TACE group (39.7%). Moreover, after matching, multivariate analysis showed that TACE-lenvatinib therapy was an independent favorable predictor for PFS and OS.
To date, few studies have reported the clinical outcomes of TACE-lenvatinib versus TACE alone for the treatment of HCC patients. In 2021, Fu et al compared the efficacy and safety of combined treatment with lenvatinib TACE versus TACE only for the treatment of unresectable HCC.26 The main differences between the present study and the previous study are as follows: (1) the target population in our study and that in previous study is different. The target population in our study was patients with intermediate-advanced stage HCC and without extrahepatic metastasis, while previous study included 5 patients (4.2%) of BCLC A stage and 18 patients (15.0%) with extrahepatic metastasis. In Fu et al’ study, TACE-lenvatinib contributed to a prolonged OS benefit in the overall population. However, the subgroup analysis failed to confirm the survival benefit for TACE-lenvatinib treatment in the remained study cohort (BCLC stages B or C stage HCC). In contrast, our study results seemed to contribute to extend this treatment modality for possible beneficial population. (2) the statistical methods used in the present study are different from those used previously. A major strength of this study is the use of propensity score adjustment. In our study, PSM was used to balance the clinical variables and to control for the confounder bias, which might minimize the limitations of retrospective study. Moreover, we further validated the results via univariate and multivariate Cox regression analyses. Thus, combined with the results of previous studies, our results furtherly suggested that TACE-lenvatinib promised to be a potential combination therapy for unresectable HCC, which were informative for clinical decision making. However, because of the research limitations, these results should be interpreted as preliminary with thought provoking and require replication. Studies focusing on the TACE-based combination therapy modality is also worthy of investigation by further randomized controlled trials.
The explanation of these benefits might be largely due to the following potential mechanisms. First, lenvatinib not only suppresses the activity of factors involved in tumor angiogenesis but also suppresses proliferation signals from FGF to VEGF receptors. Second, FGF synergistically augments VEGF-mediated HCC development and angiogenesis.27 Moreover, other studies showed that targeting FGF inhibition is an attractive potential therapeutic strategy for HCC.28–31 Third, the safety profiles in our study also suggested that patients had high tolerability for taking lenvatinib, and only a small proportion of patients had to reduce the dose or discontinue treatment.24 Therefore, given these properties of lenvatinib, the synergistic effects of TACE-lenvatinib combination therapy could maximize the treatment effect.
In general, the AEs in this study were acceptable. The AEs were more frequent in the TACE-lenvatinib group than that in the TACE alone group. These AEs, including hypertension, diarrhea, decreased appetite, etc., were more likely attributed to lenvatinib. However, these AEs were predominantly graded 1 or 2 and could be relieved or eliminated after symptomatic treatment, sometimes with dose adjustment. Therefore, we believe that TACE-lenvatinib therapy for these patients was feasible and acceptable.
The current study also had some limitations. First, there was bias due to its retrospective nature. Although PSM was conducted, this limitation was unable to fully be avoided. Second, the number of patients in the TACE-lenvatinib treatment group was limited. Third, the data of this study came from a single center. Further prospective studies are warranted to further confirm the results.
In conclusion, TACE plus lenvatinib therapy seemed to be superior to TACE alone for patients with unresectable HCC in terms of PFS, OS, and tumor control. However, considering of limitations of this study, these results should be interpreted as preliminary and warrant further confirmation.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
This retrospective study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional ethics committee of the First Affiliated Hospital with Nanjing Medical University (Ethical review no. 2021-SR-013). Considering that patient medical data was analysed retrospectively, all informed consents were waived by the ethics committee. Of note, no patients-identifiable information was utilised.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
3. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
4. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi:10.21037/hbsn-20-480
5. Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl. 1):22–31. doi:10.1159/000368142
6. Zhong BY, Wang WS, Zhang S, et al. Re-evaluating transarterial chemoembolization failure/refractoriness: a survey by Chinese College of Interventionalists. J Clin Transl Hepatol. 2021;9(4):521–527. doi:10.14218/JCTH.2021.00049
7. Sergio A, Cristofori C, Cardin R, et al. Transcatheter Arterial Chemoembolization (TACE) in Hepatocellular Carcinoma (HCC): the Role of Angiogenesis and Invasiveness. Am J Gastroenterol. 2008;103(4):914–921. doi:10.1111/j.1572-0241.2007.01712.x
8. Shim JH, Park JW, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99(10):2037–2044. doi:10.1111/j.1349-7006.2008.00909.x
9. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47(14):2117–2127. doi:10.1016/j.ejca.2011.05.007
10. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64(5):1090–1098. doi:10.1016/j.jhep.2016.01.012
11. Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, Phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–575. doi:10.1016/S2468-1253(17)30156-5
12. Kudo M. Proposal of primary endpoints for TACE combination trials with systemic therapy: lessons learned from 5 negative trials and the positive TACTICS Trial. Liver Cancer. 2018;7(3):225–234. doi:10.1159/000492535
13. Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi:10.1136/gutjnl-2019-318934
14. Okamoto K, Ikemori-Kawada M, Jestel A, et al. Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. ACS Med Chem Lett. 2015;6(1):89–94. doi:10.1021/ml500394m
15. Kudo M. Lenvatinib may drastically change the treatment landscape of hepatocellular carcinoma. Liver Cancer. 2018;7(1):1–19. doi:10.1159/000487148
16. Kato Y, Tabata K, Kimura T, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14(2):e0212513. doi:10.1371/journal.pone.0212513
17. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
18. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi:10.1038/nrclinonc.2018.29
19. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
20. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72(2):288–306. doi:10.1016/j.jhep.2019.09.026
21. Common Terminology Criteria for Adverse Events (CTCAE) Version 5. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; November 27, 2017.
22. Matsuki M, Hoshi T, Yamamoto Y, et al. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7(6):2641–2653. doi:10.1002/cam4.1517
23. Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6(1):18. doi:10.1186/2045-824X-6-18
24. Suyama K, Iwase H. Lenvatinib: a promising molecular targeted agent for multiple cancers. Cancer Control. 2018;25(1):1073274818789361. doi:10.1177/1073274818789361
25. Lu J, Zhao M, Arai Y, et al. Clinical practice of transarterial chemoembolization for hepatocellular carcinoma: consensus statement from an international expert panel of International Society of Multidisciplinary Interventional Oncology (ISMIO). Hepatobiliary Surg Nutr. 2021;10(5):661–671. doi:10.21037/hbsn-21-260
26. Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15(3):663–675. doi:10.1007/s12072-021-10184-9
27. Yoshiji H, Kuriyama S, Yoshii J, et al. Synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in murine hepatocellular carcinoma: synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in murine hepatocellular carcinoma. Hepatology. 2002;35(4):834–842. doi:10.1053/jhep.2002.32541
28. Schmidt B, Wei L, DePeralta DK, et al. Molecular subclasses of hepatocellular carcinoma predict sensitivity to fibroblast growth factor receptor inhibition. Int J Cancer. 2016;138(6):1494–1505. doi:10.1002/ijc.29893
29. Wang L, Park H, Chhim S, et al. A novel monoclonal antibody to fibroblast growth factor 2 effectively inhibits growth of hepatocellular carcinoma xenografts. Mol Cancer Ther. 2012;11(4):864–872. doi:10.1158/1535-7163.MCT-11-0813
30. Miura S, Mitsuhashi N, Shimizu H, et al. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer. 2012;12(1):56. doi:10.1186/1471-2407-12-56
31. Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16(2):159–178. doi:10.1016/j.cytogfr.2005.01.004
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
