Back to Journals » OncoTargets and Therapy » Volume 9
Comparison of the effectiveness and toxicity of neoadjuvant chemotherapy regimens, capecitabine/epirubicin/cyclophosphamide vs 5-fluorouracil/epirubicin/cyclophosphamide, followed by adjuvant, capecitabine/docetaxel vs docetaxel, in patients with operable breast cancer
Authors Zhang M, Wei W, Liu J, Yang H, Jiang Y , Tang W, Li Q, Liao X
Received 17 January 2016
Accepted for publication 8 April 2016
Published 8 June 2016 Volume 2016:9 Pages 3443—3450
DOI https://doi.org/10.2147/OTT.S104431
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Li
Minmin Zhang,* Wei Wei,* Jianlun Liu, Huawei Yang, Yi Jiang, Wei Tang, Qiuyun Li, Xiaoming Liao
Department of Breast Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China
*These authors contributed equally to this work
Abstract: The aim of this study was to compare the effectiveness and toxicity of neoadjuvant chemotherapy regimens, xeloda/epirubicin/cyclophosphamide (XEC) vs 5-fluorouracil/epirubicin/cyclophosphamide (FEC), followed by adjuvant chemotherapy regimens, capecitabine/taxotere (XT) vs taxotere (T), in axillary lymph node (LN)-positive early-stage breast cancer. In this randomized, Phase III trial, 137 patients with operable primary breast cancer (T2-0, N0-1) who were tested axillary LN positive through aspiration biopsy of axillary LNs were randomized (1:1) to four 3-weekly cycles of XEC or FEC. Patients underwent surgery within 4–6 weeks after the fourth cycle, followed by four adjuvant cycles of 3-weekly XT or T. The primary end point was tumor pathological complete response. Toxicity profiles were secondary objectives. In total, 131 patients had clinical and radiological evaluation of response and underwent surgery. Treatment with XEC led to an increased rate of pathological complete response in primary tumor (18% vs 6%, respectively, P=0.027) and objective remission rate (87% vs 73%, P=0.048) compared to FEC. Clinical complete response occurred in 20% and 7% for XEC and FEC, respectively. Compared to FEC, XEC was associated with more hand-foot syndrome (57% vs 11%, P<0.001) and 3/4 grade nausea/vomiting/diarrhea (30% vs 14%, P=0.034) but less phlebitis (3% vs 14%, P=0.035). XT and T adjuvant chemotherapy regimens were well tolerated: treatment-related 3/4 grade adverse events occurred in 28% and 17% of patients receiving XT and T, respectively.
Keywords: breast cancer, capecitabine, docetaxel, neoadjuvant chemotherapy, curative effect, toxic side effects
Introduction
Breast cancer is a type of most commonly highly metastatic malignant tumor among females.1 Neoadjuvant chemotherapy was first used as induced chemotherapy or primary chemotherapy to treat locally advanced and inoperable breast cancer. Patients with breast cancer with pathological complete response (pCR) after neoadjuvant chemotherapy were able to achieve better overall survival rate.2,3 Therefore, pursuing the pCR becomes the primary goal in neoadjuvant chemotherapy.
Neoadjuvant chemotherapy can be used to evaluate the therapeutic effect of tumors, and the evaluation of neoadjuvant chemotherapy has advantages in providing prognosis and adjusting treatment strategy compared to other treatments. Therefore, with the development of research in neoadjuvant chemotherapy, its application has expanded from locally advanced to early and medium stage in breast cancer.4 Neoadjuvant chemotherapy is the best in vivo chemosensitivity test because an effective follow-up plan can be made according to the reaction of tumor during chemotherapy treatment, and it is an important method of achieving individualized treatment in operable breast cancer.5,6 The advantage of neoadjuvant chemotherapy in operable breast cancer before operation can not only decrease the size of large tumors and clinical staging but also narrow the area of operative surgical resection and kill small metastasized micrometastases prior to surgery. An objective evaluation of resistance to chemotherapy in cancer and prognosis by clinical and pathological response is another advantage.7 The pCR rate can be mainly used to predict the long-term outcomes of neoadjuvant chemotherapy and also as a surrogate end point in clinical trials.8
The best preoperative treatment should achieve a high pCR rate by neoadjuvant chemotherapy. The effect of the chemotherapy regimen, capecitabine/epirubicin/cyclophosphamide (XEC), as a neoadjuvant chemotherapy regimen has been reported to be better than that of 5-fluorouracil (5-FU)/epirubicin/cyclophosphamide (FEC), with a good tolerance; thus, it is advisable to use XEC instead of FEC during neoadjuvant or adjuvant chemotherapy treatment.9 Lee et al10 claimed that the efficacy of capecitabine (Xeloda®, F. Hoffmann La-Roche, Basel, Switzerland)/ docetaxel (Taxotere®, Sanofi-Aventis, Paris, France) (XT) neoadjuvant chemotherapy was better than doxorubicin/cyclophosphamide (AC), because the pCR rate of the former was significantly improved and treatment-related G3/4 adverse events were relatively lower. Other data11,22 proved that capecitabine which treated patients with metastatic breast cancer was a valid single reagent and well tolerated. A meta-analysis also reported that capecitabine should be used in neoadjuvant chemotherapy, and treatment with XT was the first-line chemotherapy regimen with an effective rate of 42%.13 Another meta-analysis also reported that treatment with XT can effectively improve the efficacy of adjuvant chemotherapy and is highly likely to become a new adjuvant chemotherapy regimen.14
The primary objective of this study was to compare the tumor pCR rate achieved with four 3-weekly cycles of XEC vs FEC when used as neoadjuvant chemotherapy for patients with axillary lymph node (LN)-positive stage II/III and operable breast cancer.
Patients and methods
Study design
This was a randomized, open-label, single-center clinical trial comparing XEC with FEC as a neoadjuvant chemotherapy for patients with axillary LN-positive stage II/III and operable breast cancer. All the patients provided written informed consent before enrollment. Furthermore, all the procedures performed in this study involving human participants followed the ethical standards of the Medical ethics committee of the Affiliated Cancer Hospital of Guangxi Medical University who approved the study and followed the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
The trial profile is shown in Figure 1. Patients were randomized to one of two treatment arms: capecitabine (Xeloda®; Shanghai Roche Pharmaceuticals Ltd., Shanghai, People’s Republic of China) 1,000 mg/m2 orally twice daily on days 1–14, plus epirubicin (Pfizer, Inc., New York, NY, USA) 100 mg/m2 infusion on day 1, plus cyclophosphamide (Endoxan®; Baxter Oncology GmbH, Halle, Germany) 500 mg/m2 infusion on day 1 every 3 weeks for four cycles, or 5-FU (Shanghai Xudong Haipu Pharmaceutical Co. Ltd., Shanghai, People’s Republic of China) 500 mg/m2 infusion on day 1, plus epirubicin 100 mg/m2 infusion on day 1, plus cyclophosphamide 500 mg/m2 infusion on day 1 every 3 weeks for four cycles before surgery. The first assessment of curative effect was performed within 24–48 hours before the third cycle of chemotherapy when the second cycle was over, and the second assessment was performed within 24–48 hours after the fourth cycle. If the evaluation was considered invalid, then the patients were categorized as invalid group and the others underwent surgery after the fourth cycle of neoadjuvant chemotherapy and then crossed over to receive the other treatment regimens as adjuvant therapy. In the XEC arm, four-cycle adjuvant chemotherapy of XT was given: capecitabine 1,000 mg/m2 orally twice daily on days 1–14, plus docetaxel (Jiangsu Hengrui Medicine Co. Ltd., Lianyungang, People’s Republic of China) 75 mg/m2 infusion on day 1 every 3 weeks. In the FEC arm, four-cycle adjuvant chemotherapy of T was given: docetaxel 75 mg/m2 infusion on day 1 every 3 weeks. All patients positive for human epidermal growth factor receptor 2 (HER2) were not using trastuzumab while receiving adjuvant chemotherapy because it might increase the cardiac toxicity of chemotherapy drugs. Upon completion of the adjuvant chemotherapy, all patients received radiotherapy and were concurrently treated with tamoxifen or letrozole when hormone receptor (HR) was positive.
  | Figure 1 Trial profile. |
Eligibility criteria
To be included in the study, patients should have biopsy-proven, newly diagnosed stage II/III, and operable breast cancer with axillary LN involved. Operable breast cancer was defined as a tumor with a diameter of >1 cm diagnosed by ultrasonography or magnetic resonance imaging (MRI). The axillary LN positivity was determined by fine-needle aspiration cytology of axillary LN. Further, the eligibility criteria included patients aged 18–70 years, with an Eastern Cooperative Oncology Group (ECOG) performance status of ≤1 and adequate hematologic (absolute neutrophil count ≥1,500/mm3, platelet count ≥10,000/mm3, hemoglobin ≥10 g/dL), renal (serum creatinine ≤1.5 mg/dL), cardiac (confirmed by normal or nonspecific ECG or multigated acquisition scan taken within 1 month of enrollment), and hepatic (total bilirubin ≤1.5 mg/dL, aspartate aminotransferase, alanine transaminase, alkaline phosphatase ≤2.5× upper normal limit) functions. Patients were excluded if they had undergone prior surgery, hormonal treatment, chemotherapy, or radiotherapy, or had a history of cancer except for in situ uterine cervical cancer or non-melanotic skin cancer, any distant metastasis, or any serious concomitant systemic disorder.
Patient evaluations
The three-dimensional size of the primary breast tumor was measured by physical examination, ultrasound, and molybdenum target or MRI. These measuring methods started within 1–2 days before the first cycle of neoadjuvant chemotherapy, before the third cycle, and after the fourth cycle.
Clinical response was assessed using the response evaluation criteria in solid tumors15 and was categorized into invalid, partial response, clinical complete response (cCR), and pCR. In this study, pCR was defined such that invasive carcinoma was not found in both primary site and axillary node; however, pCR was considered if the ductal carcinoma remained in situ in the primary site.16
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0.
Statistical methods
The primary objective of this study was to compare pCR rates between the XEC and FEC groups. Chi-square test was used in the comparison of clinical and pathological response rates and toxicities in the two treatment groups, while Fisher’s exact test was used when the cell expectation was less than six. Breslow–Day test for homogeneity was performed to determine whether significant treatment by subgroup interactions existed with respect to pCR. All statistical analyses were performed using SPSS Version 20.0 (IBM Corporation, Armonk, NY, USA) with significance determined at P<0.05.
Results
Patient characteristics
A total of 137 patients were enrolled in this study between January 2011 and December 2013. Of these, 131 patients had clinical and radiological evaluation of response and completed surgery and were randomly assigned to the XEC (n=61) and FEC (n=70) groups. The reasons why the six subjects were withdrawn from the study were as follows: three patients refused further chemotherapy or surgery after the third cycle of FEC, one patient refused surgery after the fourth cycle of FEC, and two patients refused any further therapy after the first cycle of XEC.
Patients’ baseline characteristics were balanced between the two treatment arms (Table 1). Patients in each treatment arm were well matched for age, ECOG performance status, clinical T-stage, pathological pattern, clinical N-stage, estrogen receptor, progesterone receptor, and HER2 status. Invasive ductal carcinoma (n=47, 77%) was the most common pathological pattern. All patients had an ECOG performance status of 0 or 1. HR and HER2 were positive in 64% (XEC vs FEC: 64% vs 64%) and 29% (XEC vs FEC: 28% vs 30%) of patients, respectively.
Efficacy
Of the 131 patients evaluable for response, one patient in the XEC arm and two patients in the FEC arm did not complete treatment due to tumor progression or stable disease and received alternative chemotherapy prior to surgery. Treatment with XEC led to an increased rate of pCR (18% vs 6%, P=0.027), cCR (20% vs 7%, P=0.033), and objective remission rate (ORR; 87% vs 73%, P=0.048) compared with FEC (Table 2). No patient progressed in the XEC arm, whereas eight patients progressed in the FEC arm (13%, P=0.004).
Tumor ORRs were not significantly different among the various molecular types of breast cancer between the two arms (P>0.05) as shown in Table 3. The ORR was greater in XEC than in FEC in triple-negative breast cancer (TNBC) without statistical significance (92% vs 73%, P=0.571).
An exploratory analysis of pCR rate according to major subgroups (age, T-stage, N-stage, HR status, HER2 status, molecular type, and clinical response) was performed (Table 4). Although the subset analysis might not have enough power to detect the differences, XEC appeared more effective than FEC, particularly in patients with TNBC (83% vs 22%; P=0.009; interaction P=0.028).
Safety
Safety was assessed in all 131 patients. The tolerance in XEC and FEC regimens was good (Table 5). In terms of 3/4 grade treatment-related clinical adverse events, there were more cases of hand-foot syndrome (HFS; 57% vs 11%, P<0.001) and 3/4 grade nausea/vomiting/diarrhea (30% vs 14%, P=0.034) but less cases of phlebitis (3% vs 14%, P=0.035) in XEC compared with FEC. The incidence of 3/4 grade leukopenia (18% vs 16%, P=0.723), arthralgia/myalgia (5% vs 8%, P=0.502), alopecia (85% vs 89%, P=0.572), and 3/4 grade mucositis (8% vs 10%, P=0.721) was lower in XEC than in FEC, but the difference between the two groups was not statistically significant.
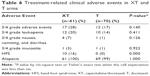
XT and T adjuvant chemotherapy regimens were well tolerated (Table 6). In terms of 3/4 grade treatment-related clinical adverse events, there were more cases of HFS (16% vs 0%, P<0.001) in XT compared to T, and the treatment-related 3/4 grade adverse events occurred in 28% and 17% of patients receiving XT and T, respectively. XT and T were associated with a similar rate of 3/4 grade leukopenia (20% vs 14%, P=0.411), 3/4 grade nausea/vomiting/diarrhea (7% vs 1%, P=0.126), 3/4 grade mucositis (3% vs 1%, P=0.922), and alopecia (100% vs 99%, P=1.000).
Discussion
The incidence of breast cancer has a rising tendency in developed cities in the People’s Republic of China and becomes the first morbidity of malignant tumor among females.17 The treatment is conventional surgery assisted with chemotherapy and radiotherapy, while distant metastasis and local relapse are the main reasons for mortality in breast carcinoma.
Neoadjuvant chemotherapy before surgery is one of the main treatments for locally advanced breast cancer. It not only narrows the primary lesion and regional LNs, degrades clinical staging, increases the chances in patients who could not be operated before, and improves the resection rate and the breast conserving rate but also controls subclinical lesions effectively, reduces tumor loading, and improves the prognosis. Furthermore, it could reduce the chances of distant metastasis by destroying distantly potential micrometastasis and provide evidence for sequential treatment through judging the sensitivity of tumors to chemotherapy.18,19 Currently, neoadjuvant chemotherapy has become a standard treatment for invasive breast cancer in stage II/III, but there is no unified standard in the specific treatment including cyclophosphamide/methotrexate/5-FU, 5-FU/adriamycin/cyclophosphamide, FEC, and docetaxel/doxorubicin/cyclophosphamide.20,21
Neoadjuvant chemotherapy has provided an opportunity for studying the biological effects of systemic treatment in breast cancer and can be used to choose effective index for predicting the prognosis in clinical settings. Early systemic chemotherapy can induce apoptosis of tumor cells and reduce the metastasis rate in surgery.22 The pathological response in the primary tumor after neoadjuvant chemotherapy is closely related to prognosis. Patients with pCR have an 86% 5-year survival rate and achieve an obvious survival benefit. Therefore, we can evaluate the effect of chemotherapy through pathological response condition of the primary tumor and predict the prognosis.18,20,23,24 Patients who responded well to neoadjuvant chemotherapy especially those with pCR, have a significantly improved disease-free survival rate.25
Capecitabine, as an antimetabolite, is a new-generation oral derivative of FU. After ingestion, it is rapidly nearly completely absorbed as an active compound in the gastrointestinal tract and is converted into 5-FU via a three-step enzymatic pathway.26,27 After two intermediate steps involving carboxylesterase in the liver and cytidine deaminase in the liver and/or tumor tissue, the final metabolite is converted into 5-FU by thymidine phosphorylase. Thymidine phosphorylase as the last enzyme in the activation of capecitabine has a higher concentration in malignant cells compared to healthy tissue, especially in breast cancer and gastric carcinoma causing DNA dyssynthesis in cancer tissue to achieve an antitumor effect, thus having selective and targeted antitumor effect.28 Therefore, cancer tissue can convert more capecitabine into 5-FU, with less concentration in healthy tissue, so that 5-FU does not injure the healthy tissue and has less toxic and side effects. In conclusion, capecitabine is an oral chemotherapy drug with less toxicity and has a unique advantage in the treatment of breast cancer.
Patients with stage II/III breast cancer were treated prior to surgery by neoadjuvant chemotherapy, including treatment regimens XEC and FEC where both were compared for efficacy and adverse events in this study. It was concluded from the results that compared to the FEC arm, XEC significantly increased both the tumor pCR rate (18% vs 6%, P=0.027) and the clinical response rate (87% vs 73%, P=0.048). A large-scale, multiple-center clinical research reported that patients with primary or metastatic breast cancer who could not be treated with anthracycline- and/or taxol-containing regimens can be treated with capecitabine. The effective rate of single-drug first-line treatment was found to be 15%–37%, and the median progression-free survival was from 3 to 5 months.29 In the study by Kamal et al,30 257 patients with metastatic breast cancer were treated with capecitabine or taxol. The results showed that the overall survival rate and tumor specificity in patients treated with capecitabine or taxol were similar, and there was no significant difference in the survival benefit. In addition, the results from a multiple-center, randomized Phase III clinical trial showed that the effect of capecitabine was as good as the combination of vinorelbine plus gemcitabine, when compared between these drugs in patients who were treated ineffectively with anthracycline- and/or taxol-containing regimens.31 Therefore, capecitabine played an important role in both single drug and combination therapy. Capecitabine monotherapy or capecitabine-containing regimens can be used not only as first-line treatment in advanced breast cancer but also as second- or third-line treatment in locally advanced breast cancer due to its good tolerance to side effects.32–35 After success with combination therapy, sequential treatment until single-drug maintenance could prolong the survival time in advanced breast cancer patients.
One research36 showed that TNBC was more sensitive to capecitabine and could achieve better pCR and cCR as neoadjuvant chemotherapy compared to non-TNBC. Other studies37,38 suggested that HR-positive tumors show resistance to chemotherapeutic drugs and were difficult to achieve pCR. In this study, the fact that 92% of TNBC achieved ORR in XEC while only 73% in FEC implied that treatment with XEC would have better curative effect to TNBC than FEC. Since there was no statistically significant difference, the result may change if the number of subjects was increased. In addition, XEC achieved more tumor pCR (ten patients, 83%) compared with FEC (two patients, 22%) in TNBC (P=0.009; interaction P=0.028). At the same time, XEC had a trend toward increased pCR in patients with HR negative and HER2 negative/unknown. Similarly, due to small sample, the result may change after increasing the number of subjects, although there was no statistically significant difference.
In addition to superior efficacy, the tolerability of XEC was also compared to that of FEC. From the comparison of adverse events, the incidence rate of HFS in the XEC arm was higher (57% vs 11%, P<0.001) compared to the FEC arm. Several studies showed that the incidence rate of HFS in capecitabine-containing chemotherapy regimens was higher because of the characteristics of the drug, which was consistent with the results in this study.39,40 Severe HFS may lead to reduction in drug dosage; therefore, it is important to prevent and reduce the incidence rate and degree of this syndrome, and it is known that vitamin B6 can relieve the symptom of HFS caused by 5-FU. In this study, it is reported that oral capecitabine with a large dose of vitamin B6 (300 mg/d) can reduce the incidence rate and symptom of HFS. In addition, a retrospective study suggested that cyclooxygenase-specific inhibitor might have the same effect as the combination of capecitabine and vitamin B6, as well.41 There were more patients with 3/4 grade nausea/vomiting/diarrhea in the XEC arm than in the FEC arm (30% vs 14%; P=0.034), because capecitabine was absorbed in the gastrointestinal tract and needs to be taken orally for 2 weeks.
The side effects such as phlebitis, arthralgia/myalgia, alopecia, leukopenia, and 3/4 grade mucositis were lower in XEC than in FEC. Among them, there was a statistically significant difference in phlebitis. XT and T adjuvant chemotherapy regimens were well tolerated: treatment-related 3/4 grade adverse events occurred in 28% and 17% of patients receiving XT and T, without statistical significance, respectively. This indicated that the XEC followed by XT regimen can be safely implemented because of less side effects and toxicity.
Conclusion
This study showed that the XEC regimen was effective and had similar curative effect compared to the FEC regimen. Due to its good tolerability and low incidence and severity of preventable adverse events, XEC regimen should be promoted and implemented in clinical practice.
Disclosure
The authors report no conflicts of interest in this work.
References
Anderson BO, Yip CH, Smith RA, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer. 2008;113(8 suppl):2221–2243. | ||
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;(30):96–102. | ||
Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24(13):2019–2027. | ||
Mieog JS, van de Veld CJ. Neoadjuvant chemotherapy for early breast cancer. Expert Opin Pharmacother. 2009;10(9):1423–1434. | ||
Specht J, Gralow JR. Neoadjuvant chemotherapy for locally advanced breast cancer. Semin Radiat Oncol. 2009;19(4):222–228. | ||
Mathew J, Asgeirsson KS, Cheung KL, Chan S, Dahda A, Robertson JF. Neoadjuvant chemotherapy for locally advanced breast cancer: a review of the literature and future directions. Eur J Surg Oncol. 2009;35(2):113–122. | ||
Thomas E, Holmes FA, Smith TL, et al. The use of alternate, noncross- resistant adjuvant chemotherapy on the basis of pathologic response to a neoadjuvant doxorubicin-based regimen in women with operable breast cancer: long-term results from a prospective randomized trial. J Clin Oncol. 2004;22(12):2294–2302. | ||
Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–3685. | ||
Berton-Rigaud D, Roché H, Penault-Llorca F, et al. Benefit of neoadjuvant capecitabine + epirubicin + cyclophosphamide (CEX) versus 5-FU + epirubicin + cyclophosphamide (FEC) for operable breast cancer (BC) followed by adjuvant docetaxel (T). J Clin Oncol. 2008;26(15s):598. | ||
Lee KS, Ro J, Nam BH, et al. A randomized phase-III trial of docetaxel/capecitabine versus doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Res Treat. 2008;109(3):481–489. | ||
Fumoleau P, Largillier R, Clippe C, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40(4):536–542. | ||
Talbot DC, Moiseyenko V, Van Belle S, et al. Randomised, phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. Br J Cancer. 2002;86(9):1367–1372. | ||
O’Shaughnessy J, Miles D, Vukelija S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast. J Clin Oncol. 2002;20(12):2812–2823. | ||
Jiang Y, Yin W, Zhou L, et al. First efficacy results of capecitabine with anthracycline- and taxane-based adjuvant therapy in high-risk early breast cancer: a meta-analysis. PLoS One. 2012;7(3):e32474. | ||
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. | ||
García-Martínez E, Gil GL, Benito AC, et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014;16(6):488. | ||
Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. | ||
Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95(4):681–695. | ||
Fitzgibbons PL, Page DL, Weaver D, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124(7):966–978. | ||
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. | ||
Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005;16(10):1569–1583. | ||
Cleator SJ, Makris A, Ashley SE, Lal R, Powles TJ. Good clinical response of breast cancers to neoadjuvant chemoendocrine therapy is associated with improved overall survival. Ann Oncol. 2005;16(2):267–272. | ||
Kong X, Moran MS, Zhang N, Haffty B, Yang Q. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer. 2011;47(14):2084–2090. | ||
Spanheimer PM, Carr JC, Thomas A, et al. The response to neoadjuvant chemotherapy predicts clinical outcome and increases breast conservation in advanced breast cancer. Am J Surg. 2013;206(1):2–7. | ||
Ye M, Zhu Z, Fu Q, Sun Q, Mao F. Tissue penetration of capecitabine and its tumor-selective delivery of 5-FU in advanced breast cancer patients. J Chin Pharm Sci. 2006;15(3):131–138. | ||
Judson IR, Beale PJ, Trigo JM, et al. A human capecitabine excretion balance and pharmacokinetic study after administration of a single dose of 14C-labelled drug. Invest New Drugs. 1999;17(1):49–56. | ||
Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40(2):85–104. | ||
Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001;92(7):1759–1768. | ||
Zielinski C, Gralow J, Martin M. Optimising the dose of capecitabine in metastatic breast cancer: confused, clarified or confirmed? Ann Oncol. 2010;21(11):2145–2152. | ||
Kamal AH, Camacho F, Anderson R, Wei W, Balkrishnan R, Kimmick G. Similar survival with single-agent capecitabine or taxane in first-line therapy for metastatic breast cancer. Breast Cancer Res Treat. 2012;134(1):371–378. | ||
Pallis AG, Boukovinas I, Ardavanis A, et al. A multicenter randomized phase III trial of vinorelbine/gemcitabine doublet versus capecitabine monotherapy in anthracycline- and taxane-pretreated women with metastatic breast cancer. Ann Oncol. 2012;23(5):1164–1169. | ||
Stockler MR, Harvey VJ, Francis PA, et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol. 2011;29(34):4498–4504. | ||
Lam SW, de Groot SM, Honkoop AH, et al. Paclitaxel and bevacizumab with or without capecitabine as first-line treatment for HER2-negative locally recurrent or metastatic breast cancer: a multicentre, open-label, randomised phase 2 trial. Eur J Cancer. 2014;50(18):3077–3088. | ||
Lück HJ, Du Bois A, Loibl S, et al. Capecitabine plus paclitaxel versus epirubicin plus paclitaxel as first-line treatment for metastatic breast cancer: efficacy and safety results of a randomized, phase III trial by the AGO Breast Cancer Study Group. Breast Cancer Res Treat. 2013;139(3):779–787. | ||
Bachelot T, Bajard A, Ray-Coquard I, et al. Final results of ERASME-4: a randomized trial of first-line docetaxel plus either capecitabine or epirubicin for metastatic breast cancer. Oncology. 2011;80(3–4):262–268. | ||
Zhang M, Song M, Qu SX, et al. Short-term curative effects of docetaxel combined with capecitabine in neoadjuvant chemotherapy of triple negative breast cancer and non triple-negative breast cancer. Med Pharm J Chin Peoples Liberation Army. 2015;27(3):55–62. | ||
Guarneri V, Broglio K, Kau SW, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24(7):1037–1044. | ||
Colleoni M, Viale G, Zahrieh D, et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res. 2004;10(19):6622–6628. | ||
Yang B, Yang JL, Shi WW, et al. Clinical paired study of comparing docetaxel plus capecitabine versus docetaxel plus epirubicin as first-line treatment in women with HER-2 negative advanced breast cancer. Zhonghua Yi Xue Za Zhi. 2013;93(18):1397–1400. | ||
Huang H, Jiang Z, Wang T, et al. Single-agent capecitabine maintenance therapy after response to capecitabine-based combination chemotherapy in patients with metastatic breast cancer. Anticancer Drugs. 2012;23(7):718–723. | ||
Lin E, Morris JS, Ayers GD. Effect of celecoxib on capecitabine-induced hand-foot syndrome and antitumor activity. Oncology (Williston Park). 2002;16(12 suppl No 14):31–37. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.