Back to Journals » OncoTargets and Therapy » Volume 9
Comparison of the clinical features and hematopoietic stem cell transplantation outcomes of mediastinal malignant germ cell tumors with nonmediastinal extragonadal placements
Authors Ocal N , Yildiz B, Karadurmus N, Dogan D, Ozaydin S, Ocal R, Ozturk M, Arpaci F, Bilgic H
Received 6 March 2016
Accepted for publication 21 July 2016
Published 9 December 2016 Volume 2016:9 Pages 7445—7450
DOI https://doi.org/10.2147/OTT.S107899
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Min Li
Nesrin Ocal,1 Birol Yildiz,2 Nuri Karadurmus,2 Deniz Dogan,1 Sukru Ozaydin,2 Ramazan Ocal,3 Mustafa Ozturk,2 Fikret Arpaci,4 Hayati Bilgic1
1Department of Chest Diseases, 2Department of Oncology, Gulhane Military Medical Faculty, Ankara, Turkey; 3Department of Hematology, Medical Faculty, Gazi University, Ankara, Turkey; 4Department of Oncology, Liv Hospital, Ankara, Turkey
Objective: Even though the primary mediastinal extragonadal germ cell tumors (EGCTs) are rare, they are noteworthy in the differential diagnosis of mediastinal masses. In this study, we aimed to identify the clinical features of mediastinal malignant GCTs and compare the results of hematopoietic stem cell transplantation between mediastinal and nonmediastinal malignant EGCTs.
Method: Data of the patients with EGCT who were treated and underwent hematopoietic stem cell transplantation at our hospital between 1988 and 2015 were retrieved retrospectively. Results were compared between mediastinal and nonmediastinal EGCTs.
Results: Data of 65 patients diagnosed with EGCT (37 [56.92%] cases with mediastinal EGCT and 28 [43.07%] cases with nonmediastinal EGCT) were assessed. The clinical stages, frequency of pretransplant status, mean pretransplant time, and mean number of chemotherapy lines before hematopoietic stem cell transplantation were not significantly different between groups. Although the overall survival did not significantly differ between groups, the 5-year survival was significantly higher in mediastinal EGCTs (P=0.02). Yolk sac tumor was significantly more common in mediastinal EGCTs (P=0.05). Mortality rates were higher in seminomas and yolk sac tumors in all cases, higher in embryonal carcinomas in mediastinal EGCT group and higher in yolk sac tumors in nonmediastinal EGCT group. While choriocarcinomas had more aggressive courses in mediastinal EGCTs, seminomas and yolk sac tumors had poorer prognosis in nonmediastinal EGCTs. Short pretransplant time and persistence of elevated posttransplant βhCG and AFP levels were the significant mortality risk factors both in mediastinal and nonmediastinal EGCTs.
Conclusion: Mediastinal placement of EGCT was not a poor prognostic factor; furthermore, the 5-year survival was significantly higher in mediastinal EGCTs. According to our knowledge, this is the first study that compares the clinical outcomes of hematopoietic stem cell transplantation of mediastinal and nonmediastinal malignant EGCTs.
Keywords: mediastinum, extragonadal germ cell tumor, hematopoietic stem cell transplantation, survival, tumor marker, βhCG, α-fetoprotein
Introduction
Mediastinal masses, which belong to the group of thoracic tumors, have a wide histopathological spectrum. The most common lesions encountered in the mediastinum are germ cell neoplasms and neurogenic tumors in childhood and young adults, whereas lymphomas, thyroid masses, and thymic neoplasms are the most common lesions in adults. Anterior mediastinal lesions account for 50% of all mediastinal masses. Teratomas and other germ cell tumors (GCTs) are the frequently encountered lesions of the anterior mediastinum.1,2
Extragonadal germ cell tumors (EGCTs), described as GCTs with no evidence of a primary tumor in the testes or ovaries, represent approximately the 2%–5% of all germ-cell malignancies. They are thought to represent the malignant transformation of germinal elements with no gonadal focus.3 The locations of EGCTs vary with age. They tend to emerge in the midline of the anterior mediastinum, retroperitoneal region, and the suprasellar and pineal regions in adults.2,4 Major histopathological types of EGCTs are seminomas (dysgerminomas in females), nonseminomatous GCTs (nondysgerminomas in females), and teratomas (mature and immature teratomas).3,4 Considering the fact that EGCTs represent a unique entity, the need for a specialized management is obvious.
Even though the primary mediastinal GCTs constitute a rare group of thorax tumors and account for approximately 10%–20% of all mediastinal neoplasms, they are noteworthy entities for pulmonologists in the differential diagnosis of mediastinal masses.1,5 However, the number of the studies focused on the mediastinal malignant GCTs is limited, and usually consists of only case series. Therefore, there is still a lack in the current literature on this subject. The clinical follow-up and treatment modalities are also specified in EGCTs. Besides surgery and high-dose chemotherapy, single autologous hematopoietic stem cell transplantation is considered necessary as a treatment of EGCTs in cases that show one of the followings after standard chemotherapy: a partial response, refractory GCTs, or relapsed disease.2,4,6 Although the identification of clinical features of mediastinal malignant EGCTs would improve the treatment strategies such as hematopoietic stem cell transplantation that may enhance the survival, we have not identified any study that compares the clinical outcomes of hematopoietic stem cell transplantation of mediastinal and nonmediastinal malignant extragonadal GCTs in the current literature. It has still not been exactly demonstrated whether the mediastinal and nonmediastinal extragonadal involvements of GCTs play significant role in terms of clinical outcomes, prognosis, and survival after hematopoietic stem cell transplantation. In this study, we aimed to identify the clinical features of mediastinal malignant EGCTs and compare the results of hematopoietic stem cell transplantation between mediastinal and nonmediastinal malignant EGCTs.
Method
This study is a retrospective evaluation of mediastinal malignant EGCT cases in comparison with nonmediastinal malignant EGCT cases. The Medical Ethics Committee of Gulhane Military Medical Faculty (Ankara) approved the study. Due to the retrospective design of the study, The Medical Ethics Committee of Gulhane Military Medical Faculty (Ankara) confirmed informed consent was not necessary. Patients with EGCT, who were treated and underwent hematopoietic stem cell transplantation at Gulhane Medical Faculty Hospital between 1988 and 2015, were included in the study. Data on age, sex, tumor localization, histopathological type of EGCT, stage of cancer, number of chemotherapy lines, pretransplant time, pretransplant condition, pretransplant and posttransplant (third month of posttransplantation period) serum βsubunit of human chorionic gonadotropin (βhCG) and α-fetoprotein (AFP) levels, mortality rates, and survival times were retrieved. The number of chemotherapy lines was defined as the number of chemotherapy protocols performed on the patient before the hematopoietic stem cell transplantation. Pretransplant time was the number of the months between diagnosis and hematopoietic stem cell transplantation time. Pretransplant condition, the reason for the decision of hematopoietic stem cell transplantation, was classified into three groups, namely, status 1: complete response + third-line treatment; status 2: increase in tumor markers; and status 3: increase in tumor markers + metastasis.
Relationships between investigated parameters were evaluated statistically. SPSS software (SPSS Inc., Chicago, IL, USA) was used for statistical evaluation. Frequencies and percentages for discrete data and means ± standard deviations for continuous variables were used for descriptive statistics. The Mann–Whitney U-test was used for comparing differences between groups. Probability (P)-values less than 0.05 were considered statistically significant.
Results
A total of 65 patients (63 males and two females) were diagnosed with EGCT. The mean age of the patients was 26±7.6 (12–54) years. Thirty-seven (56.92%) cases had mediastinal EGCT and 28 (43.07%) cases had nonmediastinal EGCT.
Mediastinal EGCTs vs nonmediastinal EGCTs
The tumor was in anterior mediastinum in all mediastinal EGCTs and in retroperitoneal region in all nonmediastinal EGCTs. Mean age of patients in the mediastinal EGCTs group was 26±5 (12–54) years and in the retroperitoneal EGCTs group was 27±7 (18–46) years. All patients were diagnosed by surgical procedures: surgical excision of the mass in mediastinal EGCTs and retroperitoneal lymph node dissection in retroperitoneal EGCTs. While all patients had primary EGCTs, orchiectomy had not been performed in any cases. During the clinical course, totally 17 patients (26.1%) underwent metastasectomy before the hematopoietic stem cell transplantation: lung metastasectomy in nine patients (13.8%), liver metastasectomy in three patients (4.6%), and retroperitoneal lymph node dissection in five patients (7.7%). Among the nine patients who underwent lung metastasectomy, one patient had mediastinal EGCTs and eight patients had retroperitoneal EGCTs. All patients who underwent liver metastasectomy had retroperitoneal EGCTs, and all patients who underwent retroperitoneal lymph node dissection due to metastasis were primary mediastinal EGCT cases. The most histopathological type was mixed GCT in both mediastinal and nonmediastinal groups (29.7% and 46.4%, respectively). On the other hand, yolk sac tumor was significantly more common in mediastinal EGCTs (P=0.05). There were only two female subjects, one with seminoma and one with choriocarcinoma. According to clinical stage, similar ratios of Stage II and III tumors were observed in both groups. Mean number of chemotherapy lines before hematopoietic stem cell transplantation was four in both groups (2–6 lines in mediastinal EGCTs and 2–5 lines in retroperitoneal EGCTs). Mean pretransplant time was 24±37 (0–189) months in mediastinal EGCTs and 18±14 (4–65) months in nonmediastinal EGCTs. Pretransplant conditions did not show a significant difference between groups. While status 1 was the most common condition in mediastinal cases, status 2 was the most common one in nonmediastinal cases. Increased serum βhCG and AFP levels before hematopoietic stem cell transplantation were identified in 14 (37.8%) and 15 (40.5%) cases of mediastinal EGCTs and in 14 (50%) and 10 (35.7%) cases of nonmediastinal EGCTs, respectively. Increased serum βhCG and AFP values at month 3 posttransplantation were identified in 5 (13.5%) and 4 (10.8%) cases of mediastinal EGCTs and in 8 (28.6%) and 3 (10.7%) cases of nonmediastinal EGCTs, respectively. Combined βhCG and AFP increase was observed in 11 (29.7%) pretransplant mediastinal EGCT cases and 2 (5.4%) posttransplant cases. Although combined βhCG and AFP increase was observed in eight (28.6%) pretransplant nonmediastinal EGCTs, it was not observed in any of the posttransplant cases. Mortality rates and overall survival rates of mediastinal and nonmediastinal EGCTs were 29.7%, 35.7%, and 70.3%, 64.3%, respectively. Five-year survival rates of the mediastinal and nonmediastinal EGCTs were 64.9% and 35.7%. Five-year survival rate was significantly higher in mediastinal EGCTs (P=0.02; Figure 1 and Table 1).
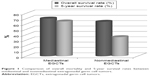  | Figure 1 Comparison of overall mortality and 5-year survival rates between mediastinal and nonmediastinal extragonadal germ cell tumors. |
Mortality assessment
In the clinical follow-up, 21 (32.3%) patients died and 44 (67.7%) are alive to date. Overall mortality rate of EGCTs was 32.3%. Mean survival time was 7.3±5.4 years in the death group. The 5-year survival of all patients was 52.3% (64.9% in mediastinal EGCTs and 35.7% in nonmediastinal EGCTs) and was significantly higher in mediastinal EGCTs (P=0.02; Figure 1 and Table 1). Mediastinum was the most common extragonadal localization in both the death and survival groups. Mixed GCTs constituted the first order of frequency in both groups. Mortality rate was higher in seminomas and yolk sac tumors in all cases. The mean age, stage, mean number of chemotherapy lines before hematopoietic stem cell transplantation, and the distribution of pretransplant status did not show significant differences between death and survival groups. However, mean pretransplant time was significantly shorter in the death group (P=0.01). Frequency of mediastinal and retroperitoneal localization did not significantly differ between the death and survival groups.
Tumor markers
While there was a significant difference in pre- and posttransplant βhCG levels in mediastinal and nonmediastinal EGCTs (P=0.005 and P=0.002, respectively), any significant difference was observed in AFP in both groups (P=0.283 and P=0.284, respectively). The rate of increase in AFP was highest in mixed GCTs and yolk sac tumors, respectively, in both pre- and posttransplant periods. The rate of increase in βhCG was highest in choriocarcinomas and mixed GCTs, respectively, in both pre- and posttransplant periods. Although βhCG levels decreased after the transplantation in both Stage II and Stage III cases, the decrease was statistically significant only in Stage III patients (P<0.001). Mortality rates were significantly higher in patients who had increased posttransplant serum βhCG and AFP values (mortality rates: 61.5% and 57.1%) than cases with normal posttransplant βhCG and AFP values (mortality rates: 30.9% and 29.3%; P=0.001 and P=0.05, respectively). While the difference between pre- and posttransplant increase in AFP in survival group was statistically significant (P=0.05), there was no significant difference in the death group (P=0.618). Although posttransplant AFP levels were lower in survival group, a decrease in elevated AFP levels did not certainly indicate a good prognosis. However, persistence of elevated posttransplant AFP levels was a predictor of poor prognosis in general. Elevated pretransplant βhCG levels also significantly decreased after the hematopoietic stem cell transplantation in survival group as an indicator of well response to the treatment (P=0.011). In the death group, the increase in βhCG levels persisted in the posttransplant period in all patients with increased pretransplant levels except for one. The relationship between pre- and posttransplant βhCG was significant in this group (P<0.001), and elevated βhCG was a good predictor of nonresponsiveness to treatment. Although the rate of increased pretransplant βhCG and AFP did not show significant difference between mortality groups, the rate of elevated posttransplant βhCG and AFP was significantly higher in death group (P=0.001 and P=0.05, respectively).
Overall assessment
Mediastinum was the most common extragonadal localization of EGCTs in all histopathological groups. Seminomas and yolk sac tumors tended to locate in mediastinum, whereas mixed GCTs tended to locate in retroperitoneal region. The overall survival did not significantly differ between mediastinal and nonmediastinal EGCTs, but 5-year survival was significantly higher in mediastinal EGCTs (P=0.02; Figure 1 and Table 1). Mortality rates were higher in seminomas and yolk sac tumors in all cases, higher in embryonal carcinomas in mediastinal EGCT group and higher in yolk sac tumors in nonmediastinal EGCT group (Table 2). While choriocarcinomas had more aggressive courses in mediastinal EGCTs, seminomas and yolk sac tumors had poorer prognosis in nonmediastinal EGCTs. Mean pretransplant time was higher in mediastinal EGCTs than nonmediastinal EGCTs, but the difference was not statistically significant (P=0.319). There was no significant relationship between the number of chemotherapy lines and mortality. While the status 1 (complete response + third-line treatment) was the most common pretransplant condition in mediastinal EGCTs, status 2 (increase in tumor markers) was the most common pretransplant condition in nonmediastinal EGCTs. Persistence of elevated levels of posttransplant βhCG and AFP was assigned as a mortality risk factor both in mediastinal and nonmediastinal EGCTs.
  | Table 2 Mortality rates of mediastinal and nonmediastinal extragonadal germ cell tumors |
Discussion
Primary mediastinal GCTs account for 10%–20% of mediastinal neoplasms. The general histological and clinical characteristics of mediastinal malignant EGCTs are similar to those of gonadal GCTs.3,5 However, mediastinal EGCTs have been reported to have a poorer prognosis than gonadal GCTs by International Germ Cell Cancer Collaborative Group.7,8 Considering the results of some studies suggested that mediastinal EGCTs arise from a different embryological origin than testicular GCTs, the differences in clinical features of mediastinal EGCTs may be the result of histological characteristics. While the 5-year survival rate is 64% in gonadal GCTs, it is usually shorter in EGCTs, especially in mediastinal EGCTs.9 Some researchers have suggested that the poor prognosis of mediastinal EGCTs may be related to the fact that they are usually diagnosed at more advanced stages than gonadal GCTs.10 However, the absolute etiological reason for these differences is still unknown and needs to be clarified and improved by new consequences. Additionally, in a partial discordance with previous knowledge, our results showed that mortality rate of mediastinal malignant EGCTs is similar to that of nonmediastinal EGCTs. Furthermore, the 5-year survival was significantly higher in mediastinal EGCTs. On the other hand, sex can also affect the risk of EGCTs. Male sex has been reported to be a risk factor for malignant transformation of EGCTs. Considering that our subjects all had malignant EGCTs and were treated with hematopoietic stem cell transplantation, it is not surprising that the majority of our cases were males. Nevertheless, we could not demonstrate the effect of sex on the disease due to the small number of female cases, which is a limitation of the study.
The practical prognostic factors have not been identified to date maybe because of the low incidence of mediastinal EGCTs.11 One of the most remarkable single-institutional experiences is the study of Kesler et al12 who demonstrated two significant prognostic factors: the positivity of serum tumor markers (βhCG and AFP) and the presence of viable cancer in tumor residue after surgery. In another international study on 635 cases with EGCTs (241 patients with mediastinal EGCTs), the presence of metastasis at diagnosis time was shown to be a poor prognostic factor.13 Short pretransplant time and persistence of elevated posttransplant βhCG and AFP levels were the significant mortality risk factors according to our results. Histological type is another factor that has been claimed to play a role in the prognosis. Several reports have confirmed that pure seminomas have better prognosis than nonseminomatous GCTs.14–17 In this regard, the Southeastern Cancer Study Group reported sustained remissions with first-line chemotherapy in cases with mediastinal seminomas.18 The 5-year survival rate of seminomatous mediastinal EGCTs was 100% in Sakurai et al’s19 study. On the other hand, GCTs do not always have a pure histology and could have a mixed histological component of both seminomatous and nonseminomatous ingredients.20 In contrast to the previous reports, in this study, mortality rates were higher in seminomas and yolk sac tumors in all cases, higher in embryonal carcinomas in mediastinal EGCT group and higher in yolk sac tumors in nonmediastinal EGCT group. While choriocarcinomas had more aggressive courses in mediastinal EGCTs, seminomas and yolk sac tumors had poorer prognosis in nonmediastinal EGCTs.
Elevated serum tumor markers have been demonstrated to be useful prognostic markers in EGCTs. Persistence of increase in βhCG and AFP after chemotherapy is considered to be a predictor of poor prognosis of the disease.21–24 Sakurai et al19 have demonstrated that patients with normal preoperative tumor marker levels had significantly better survival rates than patients with elevated preoperative tumor marker levels. Increased AFP levels may also help to identify the type of GCT, by indicating whether the tumor is a pure seminoma or mixed with nonseminoma, since AFP is not produced by seminomas.19,22,25 In this study, the rate of increase in AFP was highest in mixed GCTs and yolk sac tumors, respectively, in both pre- and posttransplant periods. The rate of increase in pre- and posttransplant βhCG was highest in choriocarcinomas and mixed GCTs. In accordance with previous results, the persistence of elevated posttransplant βhCG and AFP levels was a predictor of poor prognosis in EGCTs.21–24
Conclusion
The mean pretransplant time, mean number of chemotherapy lines before hematopoietic stem cell transplantation, clinical stages, and the frequency of pretransplant status did not show significant differences between mediastinal and nonmediastinal EGCTs. The overall survival also did not significantly differ between mediastinal and nonmediastinal EGCTs, but 5-year survival was significantly higher in case of mediastinal EGCTs. Short pretransplant time and persistence of elevated posttransplant βhCG and AFP levels were the significant mortality risk factors both in mediastinal and nonmediastinal EGCTs. To the best of our knowledge, this is the first study in which a comparison was performed between the clinical outcomes of hematopoietic stem cell transplantation of mediastinal and nonmediastinal malignant EGCTs. The retrospective nature and the disproportionate distribution of the sexes are the limitation of our study, and so further prospective studies are needed to confirm and improve our results.
Disclosure
The authors report no conflicts of interests in this work.
References
Liu TZ, Zhang DS, Liang Y, et al. Treatment strategies and prognostic factors of patients with primary germ cell tumors in the mediastinum. J Cancer Res Clin Oncol. 2011;137(11):1607–1612. | ||
Rivera C, Arame A, Jougon J, et al. Prognostic factors in patients with primary mediastinal germ cell tumors, a surgical multicenter retrospective study. Interact Cardiovasc Thorac Surg. 2010;11(5):585–589. | ||
Takeda S, Miyoshi S, Ohta M, Minami M, Masaoka A, Matsuda H. Primary germ cell tumors in the mediastinum: a 50-year experience at a single Japanese institution. Cancer. 2003;97(2):367–376. | ||
Caposole MZ, Aruca-Bustillo V, Mitchell M, Nam B. Benign metachronous bilateral ovarian and mediastinal teratomas with an elevated alpha-fetoprotein. Ann Thorac Surg. 2015;99(3):1073–1075. | ||
Sarkaria IS, Bains MS, Sood S, et al. Resection of primary mediastinal non-seminomatous germ cell tumors: a 28-year experience at memorial sloan-kettering cancer center. J Thorac Oncol. 2011;6(7):1236–1241. | ||
Bokemeyer C, Nichols CR, Droz JP, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol. 2002;20(7):1864–1873. | ||
International Germ Cell Cancer Collaborative Group. International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15(2):594–603. | ||
Mead GM, Stenning SP. The International Germ Cell Consensus Classification: a new prognostic factor-based staging classification for metastatic germ cell tumours. Clin Oncol (R Coll Radiol). 1997;9(4):207–209. | ||
Yokoyama Y, Chen F, Date H. Surgical resection of a giant mediastinal teratoma occupying the entire left hemithorax. Gen Thorac Cardiovasc Surg. 2014;62(4):255–257. | ||
Ganjoo KN, Rieger KM, Kesler KA, Sharma M, Heilman DK, Einhorn LH. Results of modern therapy for patients with mediastinal nonseminomatous germ cell tumors. Cancer. 2000;88(5):1051–1056. | ||
Walsh GL, Taylor GD, Nesbitt JC, Amato RJ. Intensive chemotherapy and radical resections for primary nonseminomatous mediastinal germ cell tumors. Ann Thorac Surg. 2000;69(2):337–343. | ||
Kesler KA, Rieger KM, Hammoud ZT, et al. A 25-year single institution experience with surgery for primary mediastinal nonseminomatous germ cell tumors. Ann Thorac Surg. 2008;85(2):371–378. | ||
Hartmann JT, Nichols CR, Droz JP, et al. Prognostic variables for response and outcome in patients with extragonadal germ-cell tumors. Ann Oncol. 2002;13(7):1017–1028. | ||
Vuky J, Bains M, Bacik J, et al. Role of postchemotherapy adjunctive surgery in the management of patients with nonseminoma arising from the mediastinum. J Clin Oncol. 2001;19(3):682–688. | ||
Kang CH, Kim YT, Jheon SH, Sung SW, Kim JH. Surgical treatment of malignant mediastinal nonseminomatous germ cell tumor. Ann Thorac Surg. 2008;85(2):379–384. | ||
Moran CA, Suster S, Przygodzki RM, Koss MN. Primary germ cell tumors of the mediastinum: II. Mediastinal seminomas – a clinicopathologic and immunohistochemical study of 120 cases. Cancer. 1997;80(4):691–698. | ||
Wright CD, Kesler KA, Nichols CR, et al. Primary mediastinal nonseminomatous germ cell tumors. Results of a multimodality approach. J Thorac Cardiovasc Surg. 1990;99(2):210–217. | ||
Loehrer PJ Sr, Birch R, Williams SD, Greco FA, Einhorn LH. Chemotherapy of metastatic seminoma: the Southeastern Cancer Study Group experience. J Clin Oncol. 1987;5(8):1212–1220. | ||
Sakurai H, Asamura H, Suzuki K, Watanabe SI, Tsuchiya R. Management of primary malignant germ cell tumor of the mediastinum. Jpn J Clin Oncol. 2004;34(7):386–392. | ||
Greif J, Staroselsky AN, Gernjac M, et al. Percutaneous core needle biopsy in the diagnosis of mediastinal tumors. Lung Cancer. 1999;25(3):169–173. | ||
Toner GC, Motzer RJ. Poor prognosis germ cell tumors: current status and future directions. Semin Oncol. 1998;25(2):194–202. | ||
Radaideh SM, Cook VC, Kesler KA, Einhorn LH. Outcome following resection for patients with primary mediastinal nonseminomatous germcell tumors and rising serum tumor markers post-chemotherapy. Ann Oncol. 2010;21(4):804–807. | ||
Massard C, Kramar A, Beyer J, et al. Tumor marker kinetics predict outcome in patients with relapsed disseminated non-seminomatous germ-cell tumors. Ann Oncol. 2013;24(2):322–328. | ||
Stang A, Trabert B, Wentzensen N, et al. Gonadal and extragonadal germ cell tumours in the United States, 1973–2007. Int J Androl. 2012;35(4):616–625. | ||
Albany C, Einhorn LH. Extragonadal germ cell tumors: clinical presentation and management. Curr Opin Oncol. 2013;25(3):261–265. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

