Back to Journals » Infection and Drug Resistance » Volume 13
Comparison of the Clinical Characteristics and Severity of Influenza and Non-influenza Respiratory Virus-Related Pneumonia in China: A Multicenter, Real-World Study
Authors Chen L , Han XD, Li YL, Zhang CX, Xing XQ
Received 24 June 2020
Accepted for publication 15 September 2020
Published 8 October 2020 Volume 2020:13 Pages 3513—3523
DOI https://doi.org/10.2147/IDR.S267102
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Liang Chen,1 Xiu-Di Han,2 Yan-Li Li,3 Chun-Xiao Zhang,4 Xi-Qian Xing5
1Department of Infectious Diseases, Beijing Jishuitan Hospital, 4th Medical College of Peking University, Beijing, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, Qingdao Municipal Hospital, Qingdao City, Shandong Province, People’s Republic of China; 3Department of Infectious Diseases and Clinical Microbiology, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 4Department of Pulmonary and Critical Care Medicine, Beijing Huimin Hospital, Beijing, People’s Republic of China; 5Department of Pulmonary and Critical Care Medicine, The 2nd People’s Hospital of Yunnan Province, Kunming City, Yunnan Province, People’s Republic of China
Correspondence: Liang Chen Tel +8613810438037
Email [email protected]
Purpose: Respiratory viruses are important etiologies of community-acquired pneumonia (CAP). However, the impact of different RVs on the outcomes of CAP is not well elucidated. This study aims to compare the clinical features and severity of influenza (Flu-p) and non-influenza respiratory viruses-related pneumonia (NIRVs-p) onset in the community among immunocompetent adults.
Methods: The data of the patients hospitalized with laboratory-confirmed RVs-p were retrospectively reviewed from five teaching hospitals in China from January 2013 to May 2019. Univariate and multivariate logistic regressions were performed to compare the clinical characteristics and outcomes between Flu-p and NIRVs-p.
Results: A total of 1079 patients with Flu-p and 341 patients with NIRVs-p were included in this study. A multivariate logistic regression model revealed chronic pulmonary disease [odd ratio (OR) 0.341, 95% confidence interval (CI) 0.225– 0.515, p < 0.001], solid malignant tumor (OR 0.330, 95% CI 0.163– 0.668, p = 0.002), myalgia (OR 1.697, 95% CI 1.236– 2.330, p < 0.001), lymphocytes < 0.8× 109/L (OR 10.811, 95% CI 6.949– 16.818, p < 0.001) and blood albumin < 35 g/L (OR 0.327, 95% CI 0.242– 0.442, p < 0.001) were predictors for Flu-p. After adjusting for confounders, the multivariate logistic regression analysis confirmed that influenza B-related pneumonia (FluB-p) (OR 0.419, 95% CI 0.272– 0.646, p < 0.001) and NIRVs-p (OR 0.260, 95% CI 0.158– 0.467, p < 0.001) were associated with a decreased risk of 30-day mortality compared with the influenza A-related pneumonia (FluA-p).
Conclusion: Our results showed that patients with FluA-p experience a more severe disease than those with FluB-p and NIRVs-p. Some clinical features are helpful to distinguish between NIRVs-p and Flu-p.
Keywords: influenza, respiratory virus, pneumonia, clinical characteristics, severity
Introduction
Community-acquired pneumonia (CAP) is a common infectious disease, which considerably contributes to the morbidities and mortalities despite the advances in the medical technology and progression of the economy.1 Its annual incidence ranges from 2.7 to 10 per 1000 persons in European countries and 2.67 to 12 per 1000 persons in the US.2 It is reported by the world health organization (WHO) that CAP is the leading cause of death among infectious diseases.1,2
With the development of the molecular diagnostic techniques and wide clinical applications, respiratory viruses (RVs), including the influenza virus (IFV), human rhinovirus (hRV), respiratory syncytial virus (RSV), human metapneumovirus (hMPV), human parainfluenza virus (hPIV), human adenovirus (hAdV), enterovirus (EV) and human coronaviruses (hCoV), have been increasingly recognized to play an important role in the occurrence of CAP.3 Previous research reported the prevalence of RVs in CAP to be about 20–50%,4,5 sometimes even higher than that of Streptococcus pneumoniae,6 which was regarded as the most common pathogen in the patients with CAP. A meta-analysis including 31 studies indicated that the pooled proportion of patients with viral infection was 24.5% (95% confidence interval (CI) 21.5–27.5%); while the studies that obtained lower respiratory samples in >50% of the patients reported the proportion to be 44.2% (95% CI 35.1–53.3%).7 In the patients with severe CAP that were admitted to the intensive care unit (ICU), the overall prevalence of RVs was even as high as 55%.8 However, except for the influenza virus, the clinical characteristics and outcomes of other non-influenza respiratory viruses-related pneumonia (NIRVs-p) are still not clear. The majority of studies were not designed for pneumonia patients, or just focused on immunocompromised hosts, pediatrics and patients resident in healthcare institutions.9,10 An improved knowledge of the potential role of RVs in immunocompetent adults with pneumonia is crucial for the treatment and prevention of respiratory viruses-related pneumonia (RVs-p).
In this work, we conducted a multicenter, retrospective study aiming to 1) explore the possibility of clinical recognition of Flu-p and NIRVs-p, by comparing their clinical features, and 2) evaluate the impact of the virus type on the illness severity and outcomes in immunocompetent adults hospitalized with community-onset pneumonia in China.
Materials and Methods
Study Design and Participants
Hospitalized patients that tested positive for the nucleic acid of nine respiratory viruses (influenza virus, human rhinovirus, respiratory syncytial virus, human metapneumovirus, parainfluenza virus, human adenovirus, human enterovirus, human coronavirus and human bocavirus) at the microbiology labs of five teaching hospitals in China (the details of the participating centers are listed in Supplementary material 1) in the period from January 1, 2013 to May 31, 2019 were screened. The patients with laboratory-confirmed RVs-p were included. The exclusion criteria were as follows: 1) patients aged less than 18 years; 2) patients not classified as community-onset pneumonia (pneumonia onset ≥48 h post-admission and hospitalized within the last 28 days),11 since it was difficult to confirm the association between RVs and nosocomial pneumonia; 3) immunocompromised patients, since the clinical characteristics and outcomes of immunocompromised patients with RVs-p might be different from that of immunocompetent hosts;12 4) patients coinfected with ≥2 respiratory viruses, to assess the impact of each kind of respiratory viruses on the outcomes and disease severity of pneumonia.
Disease and Treatment Definitions
The patients with RVs-p were defined as the patients that were positive for the nucleic acids of RVs using the polymerase chain reaction (PCR) from respiratory specimens (i.e., nasal/nasopharyngeal swabs, sputum, bronchial aspirates or bronchoalveolar lavage fluid), and manifested with respiratory symptoms together with newly emerging pulmonary infiltrates on the chest radiographs. The systemic corticosteroid use was defined as at least one dose of any systemic corticosteroid administrated during hospitalization. The community-acquired respiratory coinfected pathogens were defined as any pathogen identified within the first 48 hours after admission using the standard microbiologic procedures (the microbiological criteria of coinfection are shown in Supplementary material 2).13 The antiviral treatment was defined as the administration of neuraminidase inhibitor in influenza patients, since no antiviral medications are approved in adults with other respiratory viruses’ infections.14
Data Collection
The following information was retrospectively collected from the medical records using a standardized data collection form, including the demographic conditions, underlying disease (comorbidities are defined in Supplementary material 3), clinical symptoms, vital signs, laboratory and radiological findings, CURB-65 score (mental confusion, urea, respiratory rate, blood pressure, age ≥65 years) and pneumonia severity index (PSI) at admission, community-acquired respiratory coinfections, management and outcomes (the administration of neuraminidase inhibitors, antibiotics, systemic corticosteroids and vasopressor agents, invasive and non-invasive mechanical ventilation, complications during hospitalization, admittance to the ICU, length of hospital stay and 30-day mortality). The patients with a hospital stay <30 days were followed up by a phone call to determine the survival status.
Statistical Analysis
The data were analyzed for normality using the Kolmogorov–Smirnov test. The measurement data with a normal distribution are shown as the mean ± standard deviation, while those with a non-normal distribution are expressed as the median. The categorical variables were analyzed using the Chi-square or Fisher’s exact test, while the continuous variables were analyzed using the Student’s t-test or Mann–Whitney U-test. A p-value ≤0.05 was considered to be statistically significant. All the probability tests were two-tailed.
The demographic and baseline clinical features between the patients with Flu-p and those with NIRVs-p were compared. The variables with a p-value ≤0.05 in the univariate analysis were entered into the multivariate logistic regression to identify the predictors for Flu-p.
A multivariate logistic regression analysis was conducted to evaluate the impact of the virus type on the outcomes (invasive ventilation, ICU admission and 30-day mortality) of pneumonia, after adjustment for the factors of age, sex, duration from illness onset to admission, comorbidities, pregnancy, obesity, smoking history, systemic corticosteroid use, antiviral treatment and coinfection with other pathogens. These risk factors were previously reported to be associated with the clinical outcomes in patients with influenza or other respiratory viruses’ infections and served as confounders.
According to the survival status within 30 days after admission, all the RVs-p patients were divided into the deceased group and the survival group, and the baseline characteristics of the patients were then compared between the two groups. In order to explore the risk factors for 30-day mortality in RVs-p patients, the variables with a p-value ≤0.05 in the univariate analysis were entered into the multivariate logistic regression analysis. All the analyses were performed using the Statistical Package for Social Science 22.0 (SPSS, Chicago, IL, USA).
Results
Screening Process
We screened 4150 patients with nucleic acids that were positive for respiratory viruses. A total of 1420 laboratory-confirmed RVs-p patients were recruited, including 693 patients with influenza A-related pneumonia (FluA-p), 386 patients with influenza B-related pneumonia (FluB-p), 127 patients with RSV-related pneumonia (RSV-p), 66 patients with hRV-related pneumonia (hRV-p), 42 patients with hPIV-related pneumonia (hPIV-p), 55 patients with hMPV-related pneumonia (hMPV-p) and 51 patients with hAdV-related pneumonia (hAdV-p) (Figure 1). Among the FluA-p patients, 38.1% (264/693) were infected with A (H1N1) pdm09, 11.0% (76/693) were infected with A (H3N2), and 50.9% (353/693) of the patients were infected with an unclassified subtype.
 |
Figure 1 Screening algorithm of patients hospitalized with RVs-p. |
Distribution of the Patients with RVs-p by Months
The distribution of the patients with RVs-p by months in our study is showed in Supplementary Figure 1. The cases of FluA-p, FluB-p, RSV-p and hMPV-p generally had a similar seasonality covering the period from October to May, and the peak was during December through February. Meanwhile, the cases of hPIV-p covered the period from October to June, with the peak during January to April. The cases of hRV-p and hAdv-p were relatively equally distributed.
Overview of the Demographic and Clinical Features of Patients with Flu-p and NIRVs-p
In total, 54.1% (584/1079) of the Flu-p patients were males, and the median age was 61.0 years old. The top three chronic underlying conditions were the cardiovascular disease (24.0%, 259/1079), diabetes mellitus (11.8%, 27/1079) and cerebrovascular disease (9.0%, 97/1079). Twenty-nine percent (313/1079) of the patients had a history of smoking. The most frequent symptoms at admission were cough (98.2%, 1060/1079), sputum production (79.1%, 854/1079) and fever (75.4%, 814/1079). PO2/FiO2 <250 mmHg and multilobar infiltrates on the chest radiology could be seen in 30.2% (310/1025) and 73.6% (794/1079) of the Flu-p patients, respectively. In total, 71.0% (760/1071) and 51.3% (436/850) of Flu-p patients were classified as CURB-65 score 0 ~ 1 and PSI risk classI~II, respectively (Table 1).
 |
Table 1 Comparison of Clinical and Radiologic Features Between Patients with Flu-p and NIRVs-p |
Thirty-four percent (367/1079) of the Flu-p patients were coinfected with other community-acquired pathogens. The most common etiology was Klebsiella pneumoniae (31.6%, 116/367), followed by Streptococcus pneumoniae (29.7%, 109/367) and Staphylococcus aureus (19.3%, 71/367) (Supplementary material 4).
Among the NIRVs-p patients, 54.3% (185/341) were males, and the median age was 60.0 years old. The top three underlying diseases were the cardiovascular disease (25.2%, 86/341), chronic pulmonary disease (COPD) (18.5%, 63/341) and cerebrovascular disease (12.3%, 42/341). The prevalence of obesity was 18.5% (63/341), and 33.4% (114/341) of the patients had a smoking history. The most common symptoms were cough (97.4%, 332/341), fever (67.2%, 229/341) and dyspnea (54.8%, 187/341). The frequencies of confusion and respiratory rates >30 beats/min were 6.7% (23/341) and 13.8% (47/341), respectively. The rates of lymphocytes <0.8×109/L, blood albumin <35 g/L and PO2/FiO2 <250 mmHg were observed in 7.3% (25/341), 38.7% (132/341) and 26.1% (89/341) of the NIRVs-p patients, respectively. The proportion of multilobar infiltrates and pleural effusion on the chest radiology were 68.2% (234/341) and 28.4% (97/341), respectively. And, 71.3% (243/341) and 55.1% (188/341) of the patients with NIRVs-p were identified as CURB-65 score 0 ~ 1 and PSI risk classⅠ~Ⅱ, respectively (Table 1).
The detailed clinical characteristics and outcomes of the patients with each specific RV-p are shown in Supplementary material 4 and Supplementary material 5.
A coinfection with other community-acquired pathogens was identified in 30.5% (104/341) of the NIRVs-p patients, with the top three etiologies being Klebsiella pneumoniae (37.5%, 39/104), Staphylococcus aureus (18.3%, 19/104) and Haemophilus influenzae (16.3%, 17/104) (Supplementary material 6).
Overview of the Management and Clinical Outcomes of Patients with Flu-p and NIRVs-p
Antibiotics and neuraminidase inhibitors were administrated to all the Flu-p patients after admission. In total, 24.3% (262/1079) of the Flu-p patients received systemic corticosteroids during hospitalization, while 23.1% (249/1079), 24.6% (265/1079) and 8.2% (89/1079) developed respiratory failure, heart failure and septic shock, respectively. In total, 17.9% (193/1079) of the Flu-p patients received invasive ventilation and 22.4% (242/1079) were admitted to the ICU. The 30-day mortality of the Flu-p patients was 19.3% (208/1079), as shown in Table 2.
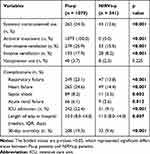 |
Table 2 Comparison of Clinical Management and Outcomes Between Patients with Flu-p and NIRVs-p |
All the NIRVs-p patients received antibiotics, while none of them received approved antiviral agents. Non-invasive ventilation and invasive ventilation were conducted in 15.5% (53/341) and 8.2% (28/341) of the patients, respectively. The most common complications during hospitalization were heart failure (14.4%, 49/341), respiratory failure (13.8%, 47/341) and septic shock (3.2%, 11/341). Also, 9.1% (31/341) of the NIRVs-p patients were admitted to the ICU, and the 30-day mortality was 9.4% (32/341) (Table 2).
Predictors for Flu-p
A multivariate logistic regression model revealed that the factors of COPD (odd ratio (OR) 0.341, 95% confidence interval (CI) 0.225–0.515, p < 0.001), solid malignant tumor (OR 0.330, 95% CI 0.163–0.668, p = 0.002), myalgia (OR 1.697, 95% CI 1.236–2.330, p < 0.001), lymphocytes <0.8×109/L (OR 10.811, 95% CI 6.949–16.818, p < 0.001) and blood albumin <35 g/L (OR 0.327, 95% CI 0.242–0.442, p < 0.001) were independent predictors for Flu-p (Figure 2).
 |
Figure 2 Forest plot of predictors for Flu-p. |
Impact of the Virus Type on the Clinical Outcomes
Compared with FluA-p, a univariate logistic regression suggested that FluB-p was associated with a decreased risk for invasive ventilation (OR 0.338, 95% CI 0.229–0.499, p < 0.001) and ICU admission (OR 0.606, 95% CI 0.442–0.831, p = 0.002), but not for the 30-day mortality (OR 0.939, 95% CI 0.684–1.290, p = 0.698). On the other hand, NIRVs-p was associated with a decreased risk for invasive ventilation (OR 0.303, 95% CI 0.198–0.464, p < 0.001), ICU admission (OR 0.304, 95% CI 0.203–0.455, p < 0.001) and 30-day mortality (OR 0.424, 95% CI 0.282–0.639, p < 0.001); this also applies to each specific non-influenza virus type (Table 3).
 |
Table 3 Impact of Viruses Types on Clinical Outcomes of Patients with RVs-p |
After adjusting for the factors of age, sex, comorbidities, obesity, smoking history, pregnancy, antiviral treatment, systemic corticosteroids use and coinfections, the multivariate logistic regression analysis confirmed that, compared with FluA-p, FluB-p was related to a decreased risk for invasive ventilation (OR 0.200, 95% CI 0.127–0.315, p < 0.001), admittance to the ICU (OR 0.546, 95% CI 0.363–0.820, p = 0.004) and 30-day mortality (OR 0.419, 95% CI 0.272–0.646, p < 0.001), while NIRVs-p was associated with a decreased risk for invasive ventilation (OR 0.273, 95% CI 0.175–0.425, p < 0.001), ICU admission (OR 0.325, 95% CI 0.212–0.425, p < 0.001) and 30-day mortality (OR 0.260, 95% CI 0.158–0.467, p < 0.001). The same associations were seen in all the specific non-influenza viruses, except for the risk for ICU admission (OR 0.477, 95% CI 0.190–1.196, p = 0.114) in hMPV-p that was similar to that of FluA-p (Table 3).
The survival curves showed the 30-day mortality of the FluA-p patients was significantly higher than that of FluB-p and the patients of each NRIV-p after being adjusted for confounders (Supplementary Figure 2).
Risk Factors for the 30-Day Mortality in RVs-p Patients
Compared with the survived patients, the deceased patients showed older age (median: 68.0 years vs 59.0 years, p = 0.001), longer duration from illness to admission (median: 4.0 days vs 3.0 days, p = 0.029) and more frequent FluA-p (56.7% vs 47.2%, p = 0.008). The proportion of cardiovascular disease (38.8% vs 21.4%, p < 0.001), COPD (18.3% vs 9.3%, p < 0.001) and chronic kidney disease (7.9% vs 2.2%, p < 0.001) were higher in the deceased patients than the survived ones. Confusion (35.4% vs 9.1%, p < 0.001), lymphocytes <0.8×109/L (75.0% vs 27.9%, p < 0.001), hemoglobin <100 g/L (41.3% vs 17.4%, p < 0.001), blood urea nitrogen >7 mmol/L (74.2% vs 32.5%, p < 0.001) and PO2/FiO2 <250 mmHg (30.8% vs 28.1%, p = 0.044) were more frequent in the deceased patients, while obesity (5.8% vs 10.6%, p = 0.024) and blood albumin <35 g/L (16.3% vs 24.9%, p = 0.004) was less frequent. More deceased patients were given systemic corticosteroids (49.6% vs 15.8%, p < 0.001) and antiviral medications (86.7% vs 73.8%, p < 0.001) than the survived patients (Supplementary material 7).
A multivariate logistic regression model revealed that the factors of age (OR 1.021, 95% CI 1.009–1.033, p = 0.001), FluA-p (OR 3.556, 95% CI 2.274–5.559, p < 0.001), COPD (OR 1.766, 95% CI 1.067–2.925, p = 0.027), chronic kidney disease (OR 3.899, 95% CI 1.817–8.363, p < 0.001), smoking history (OR 6.488, 95% CI 4.043–10.412, p < 0.001), confusion (OR 2.914, 95% CI 1.746–4.862, p < 0.001), lymphocytes <0.8×109/L (OR 4.471, 95% CI 2.820–7.091, p < 0.001), blood urea nitrogen >7 mmol/L (OR 5.398, 95% CI 3.430–8.495, p < 0.001) and PO2/FiO2 <250 mmHg (OR 1.504, 95% CI 1.041–2.172, p = 0.030) were independent risk factors for the 30-day mortality among the RVs-p patients (Table 4).
 |
Table 4 Risk Factors for 30-Day Mortality in Patients with RVs-p |
Discussion
The presented multicenter, real-world study with a relatively large sample had two important findings: 1) although Flu-p and NIRVs-p showed similar clinical presentations in general, some clinical features could serve as useful indicators of the differential diagnosis; and 2) the specific respiratory virus types had a different impact on pneumonia. The clinical outcomes of FluA-p were significantly worse than those of FluB-p and NIRVs-p in Chinese patients.
In accordance with most previous reports,4,7,8 in our study, the influenza virus was the most frequently identified etiology among the patients with RVs-p. A prospective research from China suggested that the influenza virus, RSV and hMPV presented the same seasonal pattern with the peaks being during winter to spring, while hPIV peaked in spring to early summer. On the other hand, the seasonalities of hRV and hAdV were not obvious.15 This was also observed in our study.
Although the RVs-p showed similar symptoms, we found that some clinical characteristics could actually be used in the differential diagnosis. In our study, myalgia and lymphocytes <0.8×109/L were proven to be associated with an increased risk for Flu-p, while the presence of chronic pulmonary disease (COPD), solid malignant and blood albumin <35 g/L favored NIRVs-p. Previous studies suggested that the severe infection of RSV, hPIV and hMPV is more likely to occur in patients with older age, malnutrition, systemic underlying disease and immunocompromising factors.16,17 Kim et al found hRV was the most common pathogen among viral pneumonia adult patients with cancer in Korea.18 In the study by Jin, solid cancer (OR 3.85, 95% CI 1.65–9.02) was independently associated with RSV pneumonia, which suggested that even young adults could suffer from severe RSV infection.19 Bénézit et al20 investigated 1421 patients with influenza-like illness (ILL) during three influenza seasons. They found that, compared with the patients with influenza, the patients with non-influenza respiratory viruses’ infection were more frequently diagnosed with cancer and chronic respiratory disease, and the chronic respiratory disease (OR 1.5, 95% CI 1.1–2.0) was confirmed to be associated with an increased detection of a non-influenza viruses’ infection by a multivariate analysis. Additionally, several studies suggested that COPD was a risk factor for an infection by non-influenza respiratory viruses,21,22 these viruses were proven to be common triggers for the acute exacerbation of COPD.23,24 Jennings et al25 found that the symptom of myalgia was common in viral pneumonias (OR 3.62, 95% CI 1.29–10.12). However, it is more likely associated with influenza pneumonia (OR 190.72, 95% CI 3.68–9891.91) compared with non-viral one. The study by Pedersen26 also suggested that myalgia was a clinical predictor that is positively associated with influenza compared with other the infections of respiratory viruses in the patients with ILL. In addition to the above-discussed key findings, we found that having lymphocytes <0.8×109/L could effectively discriminate between NIRVs-p and Flu-p. This finding appears to be novel and has not been previously reported. Lymphopenia was very common in severe influenza with an incidence rate of 50–100%,27 and was associated with reduced T lymphocytes in the peripheral blood.28 Previous research suggested that lymphopenia was an early and reliable laboratory finding in adults with influenza A infection. In the study by Merekoulias et al,29 lymphopenia appeared to be a marker for the A (H1N1) virus infection and could be used as a screening tool for the influenza infection differentiating it from ILL caused by other respiratory viruses. Cunha30 reviewed 37 patients with ILL and found that lymphopenia with monocytosis was a surrogate marker for influenza A infection compared with the infections of hRV, hMPV and RSV. When persisting for >3 days, it was powerful to differentiate the diagnosis from hPIV infection. It was noteworthy that previous studies suggested that lymphopenia was not only a marker for influenza virus infection but also a predictor for poor outcomes in severe influenza and other non-influenza viral pneumonia,31,32 which was also confirmed by our study.
The clinical outcomes of infectious diseases are related to many factors, such as the hosts, pathogens and environment. Although some studies investigated the severity of influenza and other non-influenza viruses’ infections, the results were too inconsistent due to the study settings, populations, sample size and the ability to control potential confounders. For example, Lee et al16 retrospectively reviewed 607 patients with RSV infection and 547 patients with seasonal influenza that were admitted to three acute care general hospitals in Hong Kong. They found that the overall outcomes of survival and length of stay were not significantly different between the patients with RSV and those with the influenza infection. However, in their study, only 42.3% and 36.7% of the patients with RSV and influenza infection had evidences of pneumonia, respectively. As a result, their conclusions were unsuitable for the respiratory viruses-associated pneumonia. The study by Bjarnason et al33 only directly compared the outcomes of CAP patients with influenza and non-influenza viruses and did not control any confounders. Similarly, in the research by Zhou et al,34 only few confounders were adjusted in the multivariate regression model as no differences in the severe outcomes were found.
In our study, the large sample size allowed us to control and adjust for as many potential confounders as possible. In order to further minimize the bias, we used two methodologies to control for the confounders. Both sets of results confirmed the association between FluA-p and increased risk for mortality. Our study revealed the direct effect of the virus types on the outcomes and disease severity of pneumonia, which was in accordance with some previous reports. Katsurada et al35 conducted a prospective study that included 2617 patients with pneumonia. After adjusting for the factors of age, study site, comorbidity status, duration of symptoms, month of diagnosis, antibiotic use and presence of bacteria, they found that the influenza infection was associated with an increased risk for in-hospital mortality (relative risk (RR) 1.13, 95% CI 0.60–2.13), while the paramyxovirus (RSV/hMPV/hPIV) was related to a decreased risk (RR 0.29, 95% CI 0.12–0.71) compared with the case of no virus infection. The prospective study by Qu et al36 also confirmed that influenza A (H1N1) pneumonia was recognized with an elevated pneumonia severity index compared with influenza B and other respiratory viral pneumonia.
Our study had some limitations. First, the retrospective nature meant some unavoidable selection bias. For example, the nucleic acid tests were performed by the subjective judgement of the attending physicians. It was possible that more severe (or milder) patients were inclined to be tested; thus, not all respiratory cases were eligible for swabbing and there was some kind of selection. Specifically, patients infected with human enterovirus, human coronavirus or human bocavirus were not included, so they could not be compared with patients with FluA-p in this study. Second, due to the retrospective design, the impact of vaccination on the disease severity could not be evaluated, and the incomplete data might have lowered the accuracy of our results. Third, there exists some evidence indicating the different severity of respiratory virus subtypes.37,38 However, most patients were not tested for subtypes in our study. Further work needs to be focused on the comparison of the clinical features by different subtypes. Finally, the population of our study consisted of immunocompetent and adult hospitalized patients. The conclusions should be prudently assessed prior to be considered for immunocompromised and pediatric patients.
Conclusions
Our study showed that the disease severity of FluA-p is worse than that of FluB-p and NIRVs-p in Chinese patients. Although some clinical features are helpful to discriminate the pneumonia caused by influenza and other respiratory viruses, the differences in the outcomes highlight the importance of the virus strains testing in the clinical management of viral pneumonia. Additionally, our results provide a theoretical basis for the development of antiviral medications and optimizing the strategy of vaccination in public health.
Ethical Approval
The study design was approved by the Ethics Committee of Beijing Jishuitan Hospital (No. 201,911-15). Given the retrospective nature of the study, the Ethics Committee determined that an informed consent was not required. The data of the patients used in the study were anonymized or maintained with confidentiality. The patient data accessed complied with relevant data protection and privacy regulations.
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided.
Author Contributions
All the authors contributed to the data analysis, drafting or revising of the article, have on the journal to which the article will be submitted, gave the final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding
This study was funded by Beijing JST research (ZR-201921). The funding agencies had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclosure
The authors declare they have no conflicts of interest for this work.
References
1. Mandell LA. Community-acquired pneumonia: an overview. Postgrad Med. 2015;127(6):607–615. doi:10.1080/00325481.2015.1074030
2. Waterer GW. Community-acquired pneumonia: a global perspective. Semin Respir Crit Care Med. 2016;37(6):799–805. doi:10.1055/s-0036-1592313
3. Saldías Peñafiel F, Ortega Gutiérrez M, Fuentes López G, et al. Importance of respiratory virus in immunocompetent adult patients hospitalized with community-acquired pneumonia. Rev Med Chil. 2016;144(12):1513–1522. doi:10.4067/S0034-98872016001200002
4. Ruiz Carmona M. Viral etiology in community-acquired pneumonia. Rev Med Chil. 2016;144(12):1511–1512. doi:10.4067/S0034-98872016001200001
5. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. Adults N Engl J Med. 2015;373(5):415–427. doi:10.1056/NEJMoa1500245
6. Gadsby NJ, Russell CD, McHugh MP, et al. Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin Infect Dis. 2016;62(7):817–823. doi:10.1093/cid/civ1214
7. Alimi Y, Lim WS, Lansbury L, Leonardi-Bee J, Nguyen-Van-Tam JS. Systematic review of respiratory viral pathogens identified in adults with community-acquired pneumonia in Europe. J Clin Virol. 2017;95:26–35. doi:10.1016/j.jcv.2017.07.019
8. Piralla A, Mariani B, Rovida F, Baldanti F. Frequency of respiratory viruses among patients admitted to 26 intensive care units in seven consecutive winter-spring seasons (2009–2016) in Northern Italy. J Clin Virol. 2017;92:48–51. doi:10.1016/j.jcv.2017.05.004
9. Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev. 2017;30(1):277–319. doi:10.1128/CMR.00010-16
10. Koo HJ, Lee HN, Choi SH, et al. Human metapneumovirus infection: pneumonia risk factors in patients with solid organ transplantation and computed tomography findings. Transplantation. 2018;102(4):699–706. doi:10.1097/TP.0000000000001965
11. Chen L, Zhou F, Li H, et al. Disease characteristics and management of hospitalised adolescents and adults with community-acquired pneumonia in China: a retrospective multicentre survey. BMJ Open. 2018;8(2):e018709. doi:10.1136/bmjopen-2017-018709
12. Chandler TM, Leipsic J, Nicolaou S, et al. Confirmed swine-origin influenza A(H1N1) viral pneumonia: computed tomographic findings in the immunocompetent and the immunocompromised. J Comput Assist Tomogr. 2011;35(5):602–607. doi:10.1097/RCT.0b013e31822c56f1
13. Ku Y-H, Chan K-S, Yang -C-C, Tan C-K, Chuang Y-C, Yu W-L. Higher mortality of severe influenza patients with probable aspergillosis than those with and without other coinfections. J Formos Med Assoc. 2017;116(9):660–670. doi:10.1016/j.jfma.2017.06.002
14. Brendish NJ, Clark TW. Antiviral treatment of severe non-influenza respiratory virus infection. Curr Opin Infect Dis. 2017;30(6):573–578. doi:10.1097/QCO.0000000000000410
15. Wu X, Wang Q, Wang M, et al. Incidence of respiratory viral infections detected by PCR and real-time PCR in adult patients with community-acquired pneumonia: a meta-analysis. Respiration. 2015;89(4):343–352. doi:10.1159/000369561
16. Lee N, Lui GC, Wong KT, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis. 2013;57(8):1069–1077. doi:10.1159/000369561
17. Walter JM, Wunderink RG. Severe respiratory viral infections: new evidence and changing paradigms. Infect Dis Clin North Am. 2017;31(3):455–474. doi:10.1016/j.idc.2017.05.004
18. Kim Y-J, Lee ES, Lee Y-S. High mortality from viral pneumonia in patients with cancer. Infect Dis (Lond). 2019;51(7):502–509. doi:10.1080/23744235.2019.1592217
19. Yoon JG, Noh JY, Choi WS, et al. Clinical characteristics and disease burden of respiratory syncytial virus infection among hospitalized adults. Sci Rep. 2020;10(1):12106. doi:10.1038/s41598-020-69017-8
20. Bénézit F, Loubet P, Galtier F, et al. Non-influenza respiratory viruses in adult patients admitted with influenza-like illness: a 3-year prospective multicenter study. Infection. 2020:1–7. doi:10.1007/s15010-019-01388-1
21. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi:10.1056/NEJMoa043951
22. Shih H-I, Wang H-C, Su I-J, et al. Viral respiratory tract infections in adult patients attending outpatient and emergency departments, Taiwan, 2012–2013. Medicine (Baltimore). 2015;94(38):e1545. doi:10.1097/MD.0000000000001545
23. Waans WA, Mallia P, van Winden ME, Rohde GG. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease—a systematic review. J Clin Virol. 2014;61(2):181–188. doi:10.1016/j.jcv.2014.06.025
24. Mallia P, Message SD, Gielen V, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183(6):734–742. doi:10.1164/rccm.201006-0833OC
25. Jennings LC, Anderson TP, Beynon KA, et al. Incidence and characteristics of viral community acquired pneumonia in adults. Thorax. 2008;63(1):42–48. doi:10.1136/thx.2006.075077
26. Pedersen CJ, Quinn JV, Rogan DT, Yang S. Factors associated with influenza in an emergency department setting. J Emerg Med. 2019;56(5):478–483. doi:10.1016/j.jemermed.2018.12.012
27. Lalueza A, Folgueira D, Díaz-Pedroche C, et al. Severe lymphopenia in hospitalized patients with influenza virus infection as a marker of a poor outcome. Infect Dis (Lond). 2019;51(7):543–546. doi:10.1080/23744235.2019.1598572
28. Fox A, Le NM, Horby P, et al. Severe pandemic H1N1 2009 infection is associated with transient NK and T deficiency and aberrant CD8 responses. PLoS One. 2012;7(2):e31535. doi:10.1371/journal.pone.0031535
29. Merekoulias G, Alexopoulos EC, Belezos T, Panagiotopoulou E, Jelastopulu DM. Lymphocyte to monocyte ratio as a screening tool for influenza. PLoS Curr. 2010;2:RRN1154. doi:10.1371/currents.rrn1154
30. Cunha BA, Connolly JJ, Irshad N. The clinical usefulness of lymphocyte: monocyte ratios in differentiating influenza from viral non-influenza-like illnesses in hospitalized adults during the 2015 influenza A (H3N2) epidemic: the uniqueness of HPIV-3 mimicking influenza A. Eur J Clin Microbiol Infect Dis. 2016;35(1):155–158. doi:10.1007/s10096-015-2521-8
31. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi:10.3389/fmicb.2019.02752
32. Shi SJ, Li H, Liu M, et al. Mortality prediction to hospitalised patients with influenza pneumonia: PO2/FiO2 combined lymphocyte count is the answer. Clin Respir J. 2017;11(3):352–360. doi:10.1111/crj.12346
33. Bjarnason A, Westin J, Lindh M, et al. Incidence, etiology, and outcomes of community-acquired pneumonia: a population-based study. Open Forum Infect Dis. 2018;5:ofy010. doi:10.1093/ofid/ofy010
34. Zhou F, Wang Y, Liu Y, et al. Disease severity and clinical outcomes of community-acquired pneumonia caused by non-influenza respiratory viruses in adults: a multicentre prospective registry study from the CAP-China network. Eur Respir J. 2019;54(2):1802406. doi:10.1183/13993003.02406-2018
35. Katsurada N, Suzuki M, Aoshima M, et al. The impact of virus infections on pneumonia mortality is complex in adults: a prospective multicentre observational study. BMC Infect Dis. 2017;17(1):755. doi:10.1186/s12879-017-2858-y
36. Qu JX, Gu L, Pu ZH, et al. Viral etiology of community-acquired pneumonia among adolescents and adults with mild or moderate severity and its relation to age and severity. BMC Infect Dis. 2015;15:89. doi:10.1186/s12879-015-0808-0
37. Minney-Smith CA, Selvey LA, Levy A, Smith DW. Post-pandemic influenza A/H1N1pdm09 is associated with more severe outcomes than A/H3N2 and other respiratory viruses in adult hospitalisations. Epidemiol Infect. 2019;147:e310. doi:10.1017/S095026881900195X
38. Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus–a comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331–379. doi:10.1007/s12016-013-8368-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
