Back to Journals » Vascular Health and Risk Management » Volume 11
Comparison of the BPLab® sphygmomanometer for ambulatory blood pressure monitoring with mercury sphygmomanometry in pregnant women: validation study according to the British Hypertension Society protocol
Authors Dorogova IV, Panina ES
Received 7 February 2015
Accepted for publication 12 March 2015
Published 13 April 2015 Volume 2015:11 Pages 245—249
DOI https://doi.org/10.2147/VHRM.S82381
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Daniel Duprez
This paper has been retracted.
Inna V Dorogova, Elena S Panina
Penza Institute of Advanced Training for Physicians, Penza, Russia
Abstract: The purpose of this study was to validate the automated BPLab® sphygmomanometer for ambulatory blood pressure monitoring (ABPM) in pregnant women according to Part II of the 1993 British Hypertension Society protocol. Pregnant women attending the antenatal clinic were randomly asked to participate (n=30). The BPLab sphygmomanometer was tested on pregnant women in this study and achieved A/A ratings according to the BHS protocol when compared with the “gold” standard of mercury sphygmomanometry. The device can therefore be recommended for use in pregnancy.
Keywords: pregnancy, BPLab, blood pressure measuring
Introduction
Ambulatory blood pressure monitoring (ABPM) has been used in pregnancy for just over a quarter of a century now and is generally accepted in many countries.1,2 One purpose of ABPM has been to determine whether there is a significant “white coat” effect among hypertensive pregnant women who appear to have either preeclampsia or gestational hypertension.1 ABPM is a better predictor than conventional blood pressure (BP) for the development of preeclampsia and adverse pregnancy outcome.2–5
It is well-known that hemodynamics in pregnancy is different from in the nonpregnant condition. Circulatory blood volume is increased, which may affect the pulse wave. Perhaps this is one of the reasons that despite the great importance of the ABPM in pregnancy, only a small number of devices have been validated for use in pregnant women according to the British Hypertension Society (BHS) standards.6 In 2011, the results of validation of the BPLab® monitor (Petr Telegin LLC, Nizhny Novgorod, Russia) for ABPM in the general population were published. According to the obtained results, the BPLab monitor was assigned to the “A/A” accuracy class for systolic and diastolic BP.7 We consider those results as a Part I of the evaluation program according to the BHS protocol, and our study as Part II. Thus, the purpose of this study was to validate the device in pregnant women, using mercury sphygmomanometry as the “gold” standard.
Materials and methods
The tested device
The manufacturer supplied one test device and confirmed that it had been selected from a normal production line.
The sensor of the device is designed to record pulse waves and to measure pressure in the range 0–300 mmHg and pulse rates in the range 20–200 beats/min. The machine inflates the air in the cuff with an automatic pumping system and deflates stepwise (8 mmHg at each step) with an automatic pressure release valve. Four cuffs are available to be used with the device: small, standard, large, and extralarge size. The unit can be powered by two 1.5 V nickel metal hydride (NiMH) 2700-mAh accumulator batteries or two 1.5 V alkaline batteries (type AA). It weighs 180 g and measures 105×85×33 mm. Communication with the personal computer (PC) is provided via Universal Serial Bus (USB) or Bluetooth.
The device was designed for office (“Office” mode) and ambulatory (“ABPM” mode) use. The Office mode was used in this study.
Study design
This was validation study of the device using the BHS protocol.8 The study was approved by the Penza Institute of Advanced Training for Physicians Ethics Committee. Participants were considered to be recruited to the trial if they had provided the signed consent. Recruitment was ad hoc from a population of patients attending routine antenatal clinics at Penza town hospitals. The criteria for exclusion from the study were arrhythmia and sufficiently weak Korotkoff sounds that made acceptable auscultation impossible.
The validation procedure was performed by two medically qualified experts who had been trained using the Compact Disc, read-only-memory (CD-ROM) tutorial detailed on the BHS website.9
Data collection
The measurements were taken in the morning in comfortable settings (ambient temperature 22°C–25°C, no stimulatory sights or sounds, etc). The women were seated for at least 5 minutes before taking the measurements. Within this period, the participants were asked their age, height, and weight for the record. Arm circumference was measured at the approximate midpoint of the upper arm to establish the correct size cuff to be used. If the circumference was <31.5 cm, the standard size cuff was used, and for a circumference between 31.5 and 42 cm, a large cuff was used.
Same-arm, sequential BP measurements were taken alternating between use of a mercury sphygmomanometer (FC-110DELUXE; Focal, Tokyo, Japan) with binaural stethoscope readings (by both observers) and the tested device. The effect of venous congestion and variability of BP was minimized by allowing >30 seconds but <1 minute between measurements.
The observers were blinded for each other’s and the BPLab measurements by positioning the reference device so that each observer could view only the scale of their own device and by ensuring they completed separate forms when writing the results.
The BP of each patient was measured nine times, alternating between the reference measurements and the test device measurements according to the schedule described in the BHS protocol.8
Data analysis
The BHS protocol specifies accuracy criteria for interobserver agreement, as follows: for the device to receive an A classification, at least 60% of the differences must show less than or equal to a 5 mmHg difference from the standard device reading, 85% must show less than or equal to a 10 mmHg difference, and 95% must show less than or equal to a 15 mmHg difference.8
As specified in the BHS protocol, the first and the last recordings from each device were not included, and the remaining seven recordings were used in the analysis.
The BHS protocol dictates that the smaller of the differences between the mercury sphygmomanometer readings at either side of the study device readings are recorded (in mmHg), ignoring the direction of difference. We used for each patient (for systolic BP and diastolic BP, separately) the following formulae:

where BP is the BP measurement result corresponding to the measurement number n.
These differences in readings between the mercury and test devices were categorized into the percentages of BP differences, within 5, 10, and 15 mmHg.
The data were entered into a Microsoft Excel® spreadsheet, and this program was used for the analysis. Bland–Altman plots were used to determine the limits of agreement between the readings using the mercury and the test devices, and to ensure there was no bias in the direction of differences between the mercury and the test device.10
Results
Participants
Pregnant women attending the antenatal clinic were randomly asked to participate. Recruitment continued until all protocol-specified requirements were filled, requiring 33 participants. Three women were excluded because they met criteria for exclusion, leaving the required 30 participants for the analysis. The characteristics of the study group are shown in Table 1.
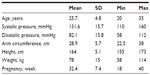  | Table 1 Characteristics of the participants (n=30) |
In the BHS protocol, the number of subjects required for the validation procedure was reduced from 85 for the main validation test in the Part I to 30 in the Part II.8 Thus, the number of the participants was sufficient to provide adequate results of the study. Additionally, there was the minimum number of subjects (five) required by BHS protocol within specified BP interval groups (systolic: 100–115,116–130, 131–145, and 146–160 mmHg; diastolic: 70–80, 81–90, and 91–105 mmHg).8
Observer agreement
The agreement between observers was well within these limits, and the results are presented in Table 2.
The BHS protocol specifies that for the device to receive an A classification, at least 60% of the differences must be in the less than or equal to 5 mmHg difference group, 85% in the less than or equal to 10 mmHg group, and 95% in the less than or equal to 15 mmHg category.8 As we can see, interobserver accuracy was excellent.
Observer-device agreement
The overall result of the validation for both observers is shown in Table 2. The BHS protocol allows the selection of the results from the best observer, in this case, observer 2. The “final grade” for each systolic and diastolic BP is the better of the grades obtained by the two observers. Thus, the BPLab device achieved an overall A rating (as shown in Table 2) for both systolic and diastolic.
Figure 1 shows the Bland–Altman plots corresponding to the better observer measurements for diastolic and systolic pressures. Here, the 90 values of the difference between the test device and the readings of the standard device by the better observer (y-axis, in mmHg) are plotted against the mean value of the test device and observer readings (x-axis, in mmHg). The graphs show a random scatter centered about the mean test device–observer difference and no unallowable trends in the data. These are both required features of the Bland–Altman plots for the results to be generalizable.8
Discussion
The oscillometric BPLab BP monitor has been validated in adult and pediatric population achieving similar results to those we have described.7,11 Moreover, the device was validated for measuring central aortic pressure and parameters of arterial stiffness.12–14 It provides normative data for arterial stiffness and central BP indices and allows studying the feasibility of BPLab indices in daily life dynamic conditions.15,16 We believe that this is an additional advantage of BPLab use for ABPM in the antenatal clinic, in the pregnancy day assessment unit, or when admitted to the antenatal ward for hypertension management.
Limitations of the study
There were no deviations from the standard procedure described by BHS protocol. However articles have discussed some of the limitations of the use of this protocol for the study of ABPM devices, and these should be noted.17,18
Conclusion
The automated BPLab sphygmomanometer for ABPM was tested on pregnant women in this study and achieved A/A ratings according to the BHS protocol when compared with the gold standard of mercury sphygmomanometry. The device can therefore be recommended for use in pregnancy.
Disclosure
The authors report no conflicts of interest in this work.
References
Brown MA. Is there a role for ambulatory blood pressure monitoring in pregnancy? Clin Exp Pharmacol Physiol. 2014;41(1):16–21. | |
Bartosh LF, Dorogova IV, Panina ES. The importance of office measurement and daily monitoring method of blood pressure for the estimation of efficiency of antihypertensive therapy received by pregnant women. Poster presented at: Internationaler Kongress Fachmesse: Moderne Aspekte der Prophylaxe, Behandlung und Rehabilitation; June 4–5; 2013; Hannover. | |
Krielessi V, Papantoniou N, Papageorgiou I, et al. Placental pathology and blood pressure’s level in women with hypertensive disorders in pregnancy. Obstet Gynecol Int. 2012;2012:684083. | |
Tranquilli AL, Giannubilo SR. Blood pressure is elevated in normotensive pregnant women with intrauterine growth restriction. Eur J Obstet Gynecol Reprod Biol. 2005;122(1):45–48. | |
Hermida RC, Ayala DE. Prognostic value of office and ambulatory blood pressure measurements in pregnancy. Hypertension. 2002;40(3):298–303. | |
dableducational.org [homepage on the Internet]. Sphygmomanometers for ambulatory blood pressure measurement. Devices for ABPM. Dabl Educational Trust; 2014. Available from: http://dableducational.org/sphygmomanometers/devices_3_abpm.html#AbpmTable. Accessed March 14, 2015. | |
Koudryavtcev SA, Lazarev VM. Validation of the BPLab(®) 24-hour blood pressure monitoring system according to the European standard BS EN 1060-4:2004 and British Hypertension Society protocol. Med Devices (Auckl). 2011;4:193–196. | |
O’Brien E, Petrie J, Littler W, et al. The British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11(Suppl 2):S43–S62. | |
bhsoc.org [homepage on the Internet]. How to measure blood pressure. British Hypertension Society; 2012 [updated August 3, 2012]. Available from: http://www.bhsoc.org/latest-guidelines/how-to-measure-blood-pressure/. Accessed March 14, 2015. | |
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;i: 307–310. | |
Ledyaev MY, Stepanova OV, Ledyaeva AM. Validation of the BPLab(®) 24-hour blood pressure monitoring system in a pediatric population according to the 1993 British Hypertension Society protocol. Med Devices (Auckl). 2015;8:115–118. | |
Rogoza AN, Kuznetsov AA. Central aortic blood pressure and augmentation index: comparison between Vasotens® and SphygmoCor® technology. Research Reports in Clinical Cardiology. 2012;3:27–33. | |
Posokhov IN. Pulse wave velocity 24-hour monitoring with one-site measurements by oscillometry. Med Devices (Auckl). 2013;6:11–15. | |
Kotovskaya YV, Kobalava ZD, Orlov AV. Validation of the integration of technology that measures additional “vascular” indices into an ambulatory blood pressure monitoring system. Med Devices (Auckl). 2014;7:91–97. | |
Kuznetsova TY, Korneva VA, Bryantseva EN, et al; BPLab-Vasotens Registry Collaborators. The 24-hour pulse wave velocity, aortic augmentation index, and central blood pressure in normotensive volunteers. Vasc Health Risk Manag. 2014;10:247–251. | |
Omboni S, Posokhov IN, Rogoza AN. Evaluation of 24-hour arterial stiffness indices and central hemodynamics in healthy normotensive subjects versus treated or untreated hypertensive patients: A feasibility study. Int J Hypertens. 2015;2015:601812. | |
Koenen SV, Franx A, Oosting H, Bonsel GJ, Bruinse HW, Visser HA. Within-subject variability of differences between conventional and automated blood pressure measurements in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1998;80(1):79–84. | |
Mancia G, Parati G. Commentary on the revised British Hypertension Society protocol for evaluation of blood pressure measuring devices: a critique of aspects related to 24-hour ambulatory blood pressure measurement. J Hypertens. 1993;11(6):595–597. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


