Back to Journals » International Journal of General Medicine » Volume 15
Comparison of the Accuracy of Two Different Molecular Tests for the Diagnosis of Tuberculous Lymphadenitis Using Core Needle Biopsy Specimens: A Diagnostic Accuracy Study
Authors Yao L , Xu X, Chen G, Shen Y , Jiang W
Received 26 March 2022
Accepted for publication 13 May 2022
Published 27 May 2022 Volume 2022:15 Pages 5237—5246
DOI https://doi.org/10.2147/IJGM.S367127
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Liwei Yao,1 Xudong Xu,2 Gang Chen,2 Yanqin Shen,2,* Weixian Jiang1,*
1Department of Nursing, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Zhejiang Tuberculosis Diagnosis and Treatment Center, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yanqin Shen, Zhejiang Tuberculosis Diagnosis and Treatment Center, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, No. 208 East Huancheng Road, Hangzhou, Zhejiang, People’s Republic of China, Email [email protected] Weixian Jiang, Department of Nursing, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, No. 208 East Huancheng Road, Hangzhou, Zhejiang, People’s Republic of China, Email [email protected]
Purpose: To evaluate the diagnostic accuracy of the CapitalBio Mycobacterium real-time polymerase chain reaction assay (CapitalBio test) testing of core needle biopsy (CNB) specimens for tuberculous lymphadenitis (TBL) and to compare it with Xpert MTB/RIF.
Methods: We retrospectively analyzed the medical data on patients with suspected peripheral TBL. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) of the CapitalBio test, Xpert MTB/RIF, and parallel test (positive result for either of these two tests) were calculated to evaluate their diagnostic efficacy compared with the final clinical diagnosis.
Results: The study included 114 patients. For diagnosing TBL using CNB samples, the sensitivity, specificity, PPV, NPV, and AUC were 65.0%, 100.0%, 100.0%, 28.6%, and 0.83, respectively, for the CapitalBio test; 72.0%, 100.0%, 100.0%, 33.3%, and 0.86, respectively, for Xpert MTB/RIF; and 82.0%, 100.0%, 100.0%, 43.8%, and 0.91, respectively, for the parallel test.
Conclusion: The accuracy of the CapitalBio test and Xpert MTB/RIF for diagnosing TBL using CNB specimens was moderate, while the sensitivity and NPV of these two tests were relatively low. The diagnostic accuracy of the CapitalBio test was slightly lower than that of Xpert MTB/RIF, but the difference between the two was not statistically significant. Parallel test might improve the diagnostic accuracy but not substantially over a single test.
Keywords: CapitalBio test, Xpert, tuberculous lymphadenitis, core needle biopsy, diagnostic accuracy
Introduction
Human infection with Mycobacterium tuberculosis (Mtb) can lead to tuberculosis (TB), an ancient disease that is still a major threat to human health.1 Depending on the MTB infection site, TB is divided into two main categories: pulmonary TB (PTB) and extrapulmonary TB (EPTB),2 the latter of which includes TB of tissues and organs other than the lungs. Tuberculous lymphadenitis (TBL) is one of the most common types of EPTB.3 TBL is one of the severe forms of TB,4 and delayed treatment can lead to significant lymph node enlargement and even pustule rupture, affecting the patient’s appearance and social life, or worse, causing social isolation and seriously affecting their quality of life.5 Early diagnosis is a prerequisite for early treatment; however, the early diagnosis of TBL is still challenging.6 Due to the confined nature of lymph node sites, the acquisition of lymph node specimens generally requires unpleasant and invasive procedures,7 with lymph node puncture being the most common procedure.8 The procedure is less invasive, is highly acceptable to patients, and can be divided into fine needle aspiration (FNA) and core needle biopsy (CNB).9 The accuracy of FNA for diagnosing TBL is low due to the small amount of specimen obtained.10 More specimens can be obtained by CNB than FNA, which is more favorable for the diagnosis of TBL.9 The CNB is currently used more often in clinical practice. The diagnostic accuracy of acid-fast bacilli (AFB) smear testing using CNB specimens is still low, and culture does not meet the need for early and rapid diagnosis for its time-consuming nature.11 Culture of AFB and CNB specimens still has limitations for early TBL diagnosis.
The rapid development of molecular tests has enabled the early and rapid diagnosis of infectious diseases, including TB. The Xpert MTB/RIF (Xpert, Cepheid, Sunnyvale, CA, USA) has greatly contributed to the early and rapid diagnosis of TB and has been recommended by the World Health Organization for diagnosing TBL.12,13 Other molecular tests have emerged, including the CapitalBio Mycobacterium real-time polymerase chain reaction test (CapitalBio test, CapitalBio Technology Inc., Beijing, China), which can detect both TB and nontuberculous mycobacterial infections.14 In PTB and EPTB, the CapitalBio test exhibited similar diagnostic accuracy to Xpert MTB/RIF.15,16 However, the diagnostic accuracy of the CapitalBio test using CNB specimens in TBL is still unknown and requires further investigation. To this end, we conducted a study to evaluate the diagnostic accuracy of the CapitalBio test using CNB specimens for TBL and to compare it with Xpert MTB/RIF to provide clinicians with more information for decision-making.
Materials and Methods
Study Design
We conducted a retrospective study at the Tuberculosis Diagnosis and Treatment Center of Zhejiang Province, China.
Participants
Patients with suspected peripheral TBL were hospitalized in our center between January 2018 and December 2020. We retrospectively analyzed the medical data of these patients.
Inclusion and Exclusion Criteria
An enlarged peripheral lymph node with TB symptoms such as low-grade fever, night sweats, positive TB-related immunological tests (gamma interferon release test and tuberculin purified protein derivative test), and the presence of other TB sites were considered to be suspected TBL. The study included patients who underwent ultrasound-guided CNB and had a concurrent Mycobacterium culture, CapitalBio test, and Xpert MTB/RIF test using CNB specimens. Patients who did not undergo ultrasound-guided CNB did not perform relevant tests or had incomplete data, and inconclusive test results were excluded.
Reference Standard
The final clinical diagnosis was considered as the reference standard for this study, according to this reference standard,17 including clinical symptoms, radiological examinations, laboratory tests, pathology findings, and follow-up findings, the patients were categorized into the following three groups: 1) confirmed TBL. MTB was detected in the CNB specimens by Mycobacterium culture; 2) probable TBL. TB-related clinical symptoms, radiological examinations, positive pathology findings and immunological tests, and satisfactory results of anti-TB therapy, which were indicative of TBL; and 3) non-TBL. Negative bacteriological evidence, ineffective anti-TB therapy, definite infection with other pathogens, and effective corresponding therapy. Confirmed and probable TBL were ultimately diagnosed clinically as TBL.
Ethics Approval and Consent to Participate
All patients or their guardians signed written consent forms. The present study complied with the Declaration of Helsinki and was approved by The Human Research Ethics Committee of the Affiliated Hangzhou Chest Hospital Zhejiang University School of Medicine.
Test Methods
Mycobacterium Culture
Solid (Lowenstein–Jensen medium) and/or liquid (BACTEC MGIT 960 Mycobacteria Culture System [BD Diagnostic Systems, Sparks, MD]) medium were used for the Mycobacterium culture using fresh CNB specimen, which was conducted according to the manufacturer’s instructions.
CapitalBio Test
Buffer was added to the fresh CNB specimen, and a homogeneous 1-mL suspension was obtained by grinding the specimen. To prepare the liquefied specimen, a 2:1 ratio was achieved by adding 1 mL of the specimen to 2 mL of the specimen preparation solution. We then centrifuged 1 mL of the liquefied specimen to obtain a sediment, which then underwent nucleic acid extraction. The extracted nucleic acids were then employed in the next step of the assay, according to the manufacturer’s instructions. The MTB IS6110 targeted gene was amplified and detected using a fluorescence quantification real-time polymerase chain reaction instrument (SLAN-96S Real-Time PCR System ZEESAN Xiamen CN), obtaining the results within 3 hours.18 The MTB detection limit of this test was 5000 colony-forming units (CFU)/mL.16
Xpert MTB/RIF
After grinding the CNB specimen, a volume of sample reagent twice that of the CNB specimen was added to the crushed CNB. The resulting mixture was stirred for 20s and then incubated for 15 min at room temperature. A 2-mL aliquot of the incubated mixture was added to the first-generation Xpert MTB/RIF reaction cartridge and placed into the reaction module for automated detection, with the instrument automatically reporting the results within 2 hours.19 The detection limit of Xpert MTB/RIF was 131 CFU/mL.16
Data Processing and Statistical Analysis
Microsoft Excel 2019 was employed to manage the clinical data. Using SPSS 24.0 (IBM Corp., Armonk, NY), we calculated the four values of the cross-tabulation: true positive, false positive, false negative, and true negative. We calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) with 95% confidence interval (CI) of the CapitalBio test and Xpert MTB/RIF based on the true positive, false positive, false negative, and true negative values using MedCalc Statistical v15.2.2 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org). The final clinical diagnosis was considered as the diagnostic reference standard to evaluate the diagnostic efficacy of the relevant assays. A positive parallel test result meant a positive test for either the CapitalBio test or Xpert MTB/RIF, and a negative parallel test result meant that both the CapitalBio test and Xpert MTB/RIF results were negative. We used McNemar’s test to compare the differences between the paired data, the chi-squared test or Fisher’s exact test to compare the differences between proportions, and Z test to compare the differences between AUCs. We used the jvenn interactive tool (http://jvenn.toulouse.inra.fr/app/index.html) to generate a Venn diagram.20 Statistical P-values <0.05 suggested that the differences are statistically significant.
Results
After screening the clinical data, 25 patients were excluded due to incomplete data, and 114 patients were ultimately included based on the inclusion criteria (Figure 1). The included patients’ mean age was 38.4 years, 50 were male, and 64 were female. All included patients underwent ultrasound-guided CNBs and contributed 1 CNB specimen per patient for a total of 114 CNB specimens. All patients underwent MTB culture, 26 of which were culture positive. All patients had definitive results for CapitalBio test and Xpert MTB/RIF. There were no inconclusive results. The patients’ clinical characteristics are summarized in Table 1. The final clinical diagnosis was TBL for 100 patients and non-TBL for 14 patients (Figure 1). The distribution and overlap of the positive findings resulting from the MTB culture, CapitalBio test, and Xpert MTB/RIF are shown in Figure 2.
 |
Table 1 Clinical Characteristics of the Included Patients |
 |
Figure 1 Participant screening flow diagram. |
 |
Figure 2 Venn diagram of positive tests for core needle biopsy specimens. |
Diagnostic Accuracy of CapitalBio Test, Xpert MTB/RIF, and Parallel Test
Of the 100 patients clinically diagnosed with TBL, 65, 72, and 82 had positive CapitalBio test, Xpert MTB/RIF, and parallel test, respectively. In the patients without TBL, there was no positive result for the relevant test. The sensitivity, specificity, PPV, NPV, and AUC in diagnosing TBL using CNB samples were 65.0% (54.8–74.3%), 100.0% (76.8–100.0%), 100.0% (94.5–100.0%), 28.6% (16.6–43.3%), and 0.83 (0.74–0.89), respectively, for the CapitalBio test; 72.0% (62.1–80.5%), 100.0% (76.8–100.0%), 100.0% (95.0–100.0%), 33.3% (19.6–49.6%), and 0.86 (0.78–0.92), respectively, for Xpert MTB/RIF; and 82.0% (73.1–89.0%), 100.0% (76.8–100.0%), 100.0% (95.6–100.0%), 43.8% (26.4–62.3%), and 0.91 (0.84–0.96), respectively, for the parallel test (Table 2). The ROC curves of CapitalBio test, Xpert MTB/RIF, and parallel test are shown in Figure 3.
 |
Table 2 Accuracy of Xpert MTB/RIF, CapitalBio Test, and Parallel Test for Tuberculous Lymphadenitis Using Core Needle Biopsy Sample |
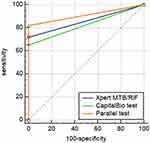 |
Figure 3 The ROC curves of CapitalBio test, Xpert MTB/RIF, and parallel test. |
Comparison of the Diagnostic Accuracy of the CapitalBio Test, Xpert MTB/RIF, and Parallel Test
The overall diagnostic accuracy of the CapitalBio test and Xpert MTB/RIF using CNB specimens was higher for Xpert MTB/RIF than for the CapitalBio test, but the difference between the two was not statistically significant (P > 0.05) (Table 3). The parallel test can achieve higher diagnostic accuracy than the individual tests, but there was no statistically significant improvement in overall diagnostic accuracy compared with the individual tests (either the CapitalBio test or Xpert MTB/RIF; P > 0.05) (Table 3). The result of the two-by-two comparison of these tests is presented in Table 3.
 |
Table 3 Comparison of the Diagnostic Accuracy Between Xpert MTB/RIF, the CapitalBio Test, and Parallel Test for Tuberculous Lymphadenitis Using Core Needle Biopsy Sample |
Discussion
Effective TBL treatment and improved patient prognoses depend on early and rapid diagnosis. Lymph node puncture is less invasive than lymph node biopsy, produces no incisional scars, and is more acceptable to patients, especially women.21 In clinical practice, lymph node puncture is therefore a routine procedure for obtaining specimens in cases of early lymph node enlargement. Ultrasound-guided CNB has played an increasing role in the etiological diagnosis of lymph node enlargement by obtaining more complete tissue specimens of the lesion than FNA.22 CNB can effectively identify lymph node neoplasms and non-neoplastic lesions.23 CNB is also the most common method for obtaining lymph node specimens in the diagnosis of TBL.24 Previous studies have shown that the accuracy of TBL diagnosis using CNB specimens and biopsy specimens is similar and that CNB specimens can be used instead of biopsy specimens for diagnosing TBL, which minimizes patient injury and provides patient cooperation and satisfaction.11 CNB specimens are still poorer than biopsy tissue specimens in terms of pathological diagnoses because their volume is still small.11 Therefore, CNB specimens are still not the first choice for diseases (such as lymphoma) that rely mainly on pathological diagnosis, and lymph node biopsy is still required.25 For diagnosing TB, pathology can provide supporting diagnostic information, but microbiological evidence is still crucial.
Classical microbiological evidence usually includes AFB smear and MTB culture. However, the positive rate of AFB smear and MTB culture is on the low side for EPTB, which is generally less bacterial in nature. The positive rate of MTB culture in TBL in this study was 26% (26/100), which was unsatisfactory and was related to the possible low MTB load in early TBL. On the other hand, MTB culture takes several weeks to show results and is not suitable for the early diagnosis of TBL.
The limitations of classical microbiological assays that do not meet the needs of TB diagnoses have stimulated the development of new TB microbiological assays. With the understanding of the molecular level of MTB, the detection of MTB at this level has seen major developments. Various molecular tests have been increasingly employed in TB and have achieved good diagnostic accuracy.26 Molecular tests are also gaining ground in TBL because of their rapid MTB discovery ability.27 The results of previous meta-analyses suggest that the diagnostic accuracy of molecular tests in FNA specimens might be superior to that of biopsy tissue specimens.7 In CNB specimens, the same results were observed, ie, the diagnostic efficacy of molecular tests was superior in CNB specimens than in biopsy tissue specimens.11 These results suggest that lymph node puncture specimens might be more suitable for molecular testing, possibly related to the fact that puncture specimens are easier to homogenize, whereas biopsy tissue specimens are more difficult to handle and have lower MTB loads within solid tissue specimens.7 Molecular testing using CNB specimens is widely used in diagnosing TBL, but the difference in diagnostic accuracy of the various types of molecular tests in CNB specimens has not been effectively evaluated, which was the purpose of this study.
In the present study, the CapitalBio test had lower sensitivity (65.0% vs 72.0%), NPV (28.6% vs 33.3%), and AUC (0.83 vs 0.86) than Xpert MTB/RIF. Xpert MTB/RIF detected 7 more patients with TBL than the CapitalBio test, which might be related to the fact that Xpert MTB/RIF is a fully automated test requiring no nucleic acid extraction, while the CapitalBio test requires the manual extraction of nucleic acid, which might induce inter-operator differences and increase the risk of contamination. The two tests also had different detection limits (131 CFU/mL for Xpert MTB/RIF and 5000 CFU/mL for CapitalBio test). The sensitivity and NPV of CapitalBio test, Xpert MTB/RIF using CNB specimens to diagnose TBL were low, while the specificity and PPV were very high, both at 100%. These results suggested that CapitalBio test and Xpert MTB/RIF were more prone to false negatives and that negative results needed to be treated with more caution and that repeat testing or combined testing might be beneficial. Positive results were likely to be more reliable because positive results require higher MTB levels, while low MTB levels can lead to negative results. Although the diagnostic accuracy of the CapitalBio test in diagnosing TBL is slightly lower than that of Xpert MTB/RIF, the difference between the two in clinical practice is not statistically significant, and it can be concluded that the diagnostic accuracy of these two molecular tests using CNB specimens is similar for TBL.
The diagnostic efficacy of the combination of multiple molecular tests has also not been effectively evaluated. In this study, we further evaluated the diagnostic accuracy of parallel test for TBL and compared it with different single tests to clarify the advantages of parallel test. The sensitivity, specificity, PPV, NPV, and AUC of parallel test were 82.0%, 100.0%, 100.0%, 43.8%, and 0.91, respectively. The sensitivity and NPV of parallel test were improved to varying degrees, suggesting that parallel test can be beneficial for patients with negative results of a single test by reducing false-negative results. The reason for this result is that each single test is different from each other and there are more or less differences, which makes the results of different single tests for the same specimen may vary; some specimens may be negative for CapitalBio test but positive for Xpert MTB/RIF and vice versa; for this part of the patients, the parallel test is positive, which leads to a better detection rate of parallel test. The diagnostic accuracy of parallel test was superior to that of a single test, but the advantage of parallel test over single tests did not reach statistical significance. This may be related to the limited number of patients included in this study, and studies with larger sample sizes are needed to further validate the accuracy of parallel test. Although the accuracy of parallel test was not significantly better than that of single tests, if conditions permit, parallel test was still meaningful to identify more patients with TBL; hence, these TBL patients could be treated early and improve their prognosis.
Limitations
The present study had several limitations. First, this retrospective study might have incurred a selection bias during patient inclusion. Second, this study used the final clinical diagnosis as the reference standard, which includes multiple factors, and not every patient was evaluated for the relevant factors, which might also have created bias. Third, the small number of patients without TBL included in this study might be related to the fact that our center is a TB diagnostic center, where TB is clustered and non-TB is relatively rare, which might be another source of patient selection bias. Lastly, the results of this study might only be applicable to endemic TB areas and might not be very informative for areas without endemic TB.
Conclusions
The accuracy of the CapitalBio test and Xpert MTB/RIF for diagnosing TBL using CNB specimens was moderate, while the sensitivity and NPV of these two tests was relatively low. The diagnostic accuracy of the CapitalBio test was slightly lower than that of Xpert MTB/RIF, but the difference was not statistically significant and can be considered similar for diagnosing TBL. Parallel testing might improve the diagnostic accuracy but not substantially over a single test.
Data Sharing Statement
Data will be made available on reasonable request.
Ethics Approval and Consent to Participate
All patients gave written informed consent and the study was approved by the Human Research Ethics Committee of Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine. This study complies with the Declaration of Helsinki.
Acknowledgments
We would like to express our feelings to the patients and colleagues in our department.
Funding
Liwei Yao, 2022ZB266, Administration of Traditional Chinese Medicine of Zhejiang Province. The funder does not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no conflict of interest.
References
1. World Health Organization. Global tuberculosis report 2021; 2021.
2. Natarajan A, Beena PM, Devnikar AV, Mali S. A systemic review on tuberculosis. Indian J Tuberc. 2020;67(3):295–311. doi:10.1016/j.ijtb.2020.02.005
3. Qian X, Albers AE, Nguyen DTM, et al. Head and neck tuberculosis: literature review and meta-analysis. Tuberculosis. 2019;116:S78–s88. doi:10.1016/j.tube.2019.04.014
4. Mekonnen D, Derbie A, Abeje A, et al. Epidemiology of tuberculous lymphadenitis in Africa: a systematic review and meta-analysis. PLoS One. 2019;14(4):e0215647. doi:10.1371/journal.pone.0215647
5. Chahed H, Hachicha H, Berriche A, et al. Paradoxical reaction associated with cervical lymph node tuberculosis: predictive factors and therapeutic management. Int J Infect Dis. 2017;54:4–7. doi:10.1016/j.ijid.2016.10.025
6. Ben Brahim H, Kooli I, Aouam A, et al. Diagnostique et thérapeutique de la tuberculose ganglionnaire en Tunisie. [Diagnostic and therapeutic management of lymph node tuberculosis in Tunisia]. Pan Afr Med J. 2014;19:211. French. doi:10.11604/pamj.2014.19.211.5213
7. Yu G, Zhong F, Ye B, Xu X, Chen D, Shen Y. Diagnostic accuracy of the Xpert MTB/RIF assay for lymph node tuberculosis: a systematic review and meta-analysis. Biomed Res Int. 2019;2019:4878240. doi:10.1155/2019/4878240
8. Wright CA, Warren RM, Marais BJ. Fine needle aspiration biopsy: an undervalued diagnostic modality in paediatric mycobacterial disease. Int J Tuberc Lung Dis. 2009;13(12):1467–1475.
9. Oh KH, Woo JS, Cho JG, Baek SK, Jung KY, Kwon SY. Efficacy of ultrasound-guided core needle gun biopsy in diagnosing cervical lymphadenopathy. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133(6):401–404. doi:10.1016/j.anorl.2016.01.013
10. Gouda K, Das U, Dhangadamajhi G. Utility of Fine Needle Aspiration Cytology (FNAC) in the diagnosis of tuberculous lymphadenitis compared to GeneXpert in a tertiary health care center in Northern Odisha, India. Indian J Tuberc. 2021;68(4):437–444. doi:10.1016/j.ijtb.2021.01.005
11. Shen Y, Fang L, Ye B, Xu X, Yu G, Zhou L. The role of core needle biopsy pathology combined with molecular tests in the diagnosis of lymph node tuberculosis. Infect Drug Resist. 2022;15:335–345. doi:10.2147/IDR.S350570
12. Park M, Kon OM. Use of Xpert MTB/RIF and Xpert ultra in extrapulmonary tuberculosis. Expert Rev Anti Infect Ther. 2021;19(1):65–77. doi:10.1080/14787210.2020.1810565
13. Li HH, He ZJ, Liang JQ, et al. Evaluation of Xpert MTB/RIF for the diagnosis of lymphatic tuberculosis. Biomed Res Int. 2020;2020:1968487. doi:10.1155/2020/1968487
14. Shen Y, Fang L, Xu X, Ye B, Yu G. CapitalBio Mycobacterium real-time polymerase chain reaction detection test: rapid diagnosis of Mycobacterium tuberculosis and nontuberculous mycobacterial infection. Int J Infect Dis. 2020;98:1–5. doi:10.1016/j.ijid.2020.06.042
15. Yu G, Shen Y, Ye B, Chen D, Xu K. Comparison of CapitalBio™ Mycobacterium nucleic acid detection test and Xpert MTB/RIF assay for rapid diagnosis of extrapulmonary tuberculosis. J Microbiol Methods. 2020;168:105780. doi:10.1016/j.mimet.2019.105780
16. Zheng H, Zhong F, Yu G, Shen Y. Comparison of the diagnostic efficacy of the CapitalBio Mycobacterium real-time polymerase chain reaction detection test and Xpert MTB/RIF in smear-negative pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis. 2021;40(5):969–977. doi:10.1007/s10096-020-04113-1
17. Sun W, Gu J, Bi K, et al. Clinical performance of Xpert MTB/RIF on contrast-enhanced ultrasound-guided core biopsy specimens for rapid diagnosis of superficial tuberculous lymphadenitis in high TB burden settings. Infection. 2021;49(4):653–660. doi:10.1007/s15010-021-01578-w
18. Sun L, Yao L, Fu G, Lin L, Zhu E, Huang J. A comparison of the accuracy of the CapitalBio Mycobacterium real-time polymerase chain reaction and the Xpert MTB/RIF assay for the diagnosis of tuberculous meningitis. Int J Infect Dis. 2021;104:92–96. doi:10.1016/j.ijid.2020.12.044
19. Yu G, Zhong F, Shen Y, Zheng H. Diagnostic accuracy of the Xpert MTB/RIF assay for tuberculous pericarditis: a systematic review and meta-analysis. PLoS One. 2021;16(9):e0257220. doi:10.1371/journal.pone.0257220
20. Yu G, Wang X, Zhu P, Shen Y, Zhao W, Zhou L. Comparison of the efficacy of metagenomic next-generation sequencing and Xpert MTB/RIF in the diagnosis of tuberculous meningitis. J Microbiol Methods. 2021;180:106124. doi:10.1016/j.mimet.2020.106124
21. Blaikley JF, Khalid S, Ormerod LP. Management of peripheral lymph node tuberculosis in routine practice: an unselected 10-year cohort. Int J Tuberc Lung Dis. 2011;15(3):375–378.
22. Balasubramanian I, Fleming CA, Corrigan MA, Redmond HP, Kerin MJ, Lowery AJ. Meta-analysis of the diagnostic accuracy of ultrasound-guided fine-needle aspiration and core needle biopsy in diagnosing axillary lymph node metastasis. Br J Surg. 2018;105(10):1244–1253. doi:10.1002/bjs.10920
23. Elahamdoust E, Motamedfar A, Gharibvand MM, Jazayeri SN. Investigation of the value of ultrasound-guided core needle biopsy from pathologic lymph nodes to the diagnosis of lymphoma. J Family Med Prim Care. 2020;9(6):2801–2805. doi:10.4103/jfmpc.jfmpc_1260_19
24. Zhao D, Shao YQ, Hu J, Liu D, Tang W, He N. Role of contrast-enhanced ultrasound guidance in core-needle biopsy for diagnosis of cervical tuberculous lymphadenitis. Clin Hemorheol Microcirc. 2021;77(4):381–389. doi:10.3233/CH-201038
25. Ji X, Li D, Gao D, et al. Value of ultrasound-guided biopsy in evaluating internal mammary lymph node metastases in breast cancer. Clin Breast Cancer. 2021;21(6):532–538. doi:10.1016/j.clbc.2021.04.016
26. Morel F, Jaffré J, Sougakoff W, Aubry A, Véziris N. Place de la biologie moléculaire dans le diagnostic de la tuberculose. [Molecular diagnosis of tuberculosis]. Rev Mal Respir. 2020;37(5):412–416. French. doi:10.1016/j.rmr.2019.09.004
27. Walzl G, McNerney R, du Plessis N, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis. 2018;18(7):e199–e210. doi:10.1016/S1473-3099(18)30111-7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
