Back to Journals » OncoTargets and Therapy » Volume 8
Comparison of single-agent chemotherapy and targeted therapy to first-line treatment in patients aged 80 years and older with advanced non-small-cell lung cancer
Authors Zhang Q, Wang Z, Guo J, Liu L, Han X, Li M, Fang S, Bi X, Tang N, Liu Y
Received 29 January 2015
Accepted for publication 11 March 2015
Published 20 April 2015 Volume 2015:8 Pages 893—898
DOI https://doi.org/10.2147/OTT.S81837
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Daniele Santini
Qianqian Zhang,1 Zhehai Wang,2 Jun Guo,2 Liyan Liu,2 Xiao Han,2 Minmin Li,1 Shu Fang,1 Xiang Bi,1 Ning Tang,1 Yang Liu1
1School of Medicine and Life Sciences, Shandong Academy of Medical Sciences, University of Jinan, 2Department of Oncology, Shandong Cancer Hospital, Jinan, People’s Republic of China
Purpose: The aim of this study was to compare single-agent chemotherapy with targeted therapy in initial treatment and to explore a better choice of treatment for patients aged 80 years and older with advanced non-small-cell lung cancer (NSCLC).
Patients and methods: A retrospective chart review was conducted for 136 patients aged 80 years and older who were cytopathologically diagnosed and staged as advanced (stage IIIB or IV) NSCLC. The patient population was divided into two treatment groups: 78 patients were allocated to the chemotherapy group (group A, pemetrexed or gemcitabine or docetaxel as a single agent), and 60 patients were allocated to another group and received epidermal growth factor-receptor tyrosine-kinase inhibitors (group B, erlotinib or gefitinib as a single agent). The primary end points were overall survival (OS) and progression-free survival (PFS), and the secondary end points were response rate, disease-control rate, safety, and quality of life.
Results: In group A and group B, respectively, the median PFS was 2 versus 4 months (P=0.013), and the median OS was 8 versus 16 months (P=0.025). The 1- and 2-year survival rates of the two groups were 23.7% (group A, 18 of 76) versus 76.7% (group B, 46 of 60) and 13.2% (group A, ten of 76) versus 10% (group B, six of 60), respectively. The response rate and disease-control rate were 28.9% versus 36.7% (P=0.39) and 57.9% versus 76.7% (P=0.022) in group A and group B, respectively.
Conclusion: Elders aged 80 years and over with advanced NSCLC in group B had longer PFS and OS compared with group A. It was well tolerated in group B because of the mild adverse effects. Targeted therapy can be considered primarily for patients aged 80 years and older with advanced NSCLC who cannot tolerate chemotherapy or radiotherapy.
Keywords: non-small-cell lung cancer, elderly, 80 years old, first-line, chemotherapy, targeted therapy
Introduction
Lung cancer is one of the most common malignant tumors in both Western and Asian countries, and remains the major cause of cancer-related death in the world.1 Accounting for about 80% of the total number of lung cancers is non-small-cell lung cancer (NSCLC).2 However, approximately 40%–50% of these patients present with incurable metastatic (stage IV) disease.3
As the population ages, elderly lung cancer cases show an increasing trend. It is estimated that more than half of 500,000 patients with lung cancer diagnosed each year are older than 70 years.4 Obviously, lung cancer in elders is an increasingly common problem faced by the oncologist. Elders present particularity with a variety of chronic diseases, and their status score is decreased; another feature is the loss of organ-function reserve, which will lead to tolerance differences on treatment.5 A number of retrospective analyses of trials have shown a survival benefit with platinum-based combination chemotherapy in patients aged 70 years and over.6 A study for unfit elderly patients over 70 years with advanced NSCLC indicated that single-agent chemotherapy should be considered the standard treatment, and such targeted therapies as epidermal growth-factor receptor (EGFR) tyrosine-kinase inhibitors (TKIs), if approved for use in the future, also in the first-line treatment of this group of patients.7 In the NEJ 003 study,8 Maemondo et al demonstrated that first-line gefitinib may be preferable to standard chemotherapy for patients aged 75 years or older with NSCLC harboring EGFR mutations.
It is still not clear whether these studies mentioned in the previous paragraph apply to patients aged 80 years and over with advanced NSCLC. People aged from 80 to 89 years are known as octogenarians. With prolongation of life expectancy, the incidence of octogenarians with lung cancer is increasing year by year. The life expectancies of 80-year-old men and women are 87.3 years and 89.0 years, respectively, but NSCLC may rob them of many years of meaningful life in addition to causing morbidity.9 Seeking a suitable treatment for this age group of patients is of great significance to prolong survival and improve quality of life. However, because of octogenarians’ poor physical condition, too few elderly patients aged over 80 years were included in previous clinical trials to make any conclusions regarding this group of patients. In this instance, studies specifically focusing on these elderly patients are required. A total of 136 patients aged 80 years and older diagnosed with advanced NSCLC in Shandong Cancer Hospital were enrolled and retrospectively analyzed.
Patients and methods
Patients
A total of 136 patients who had been cytopathologically diagnosed with advanced NSCLC and treated at Shandong Cancer Hospital from January 2010 to January 2013 were enrolled in this study and analyzed retrospectively. Eligibility criteria were: male or female with cytopathologically diagnosed stage IIIB or IV NSCLC aged 80 years or older, chemotherapy-naïve for this stage of the disease, at least one measurable lesion by computed tomography according to World Health Organization criteria,10 an Eastern Collaborative Oncology Group (ECOG) performance status of 0–2, and life expectancy of at least 4 months.
Assessment
The primary end points of the study were progression-free survival (PFS) and overall survival (OS). PFS was refers to the time from patients receiving the first-line therapy to disease progression or death. OS was measured from the first-line treatment-decision day to the day of death or the day of last follow-up. We did follow-up on all patients, and the last one was in January 2015. Secondary efficacy analyses were response rate (RR) and disease-control rate (DCR), determined by the Response Evaluation Criteria in Solid Tumors:11 complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). RR was defined as CR plus PR, and DCR was defined as CR plus PR and SD. Toxicities were graduated according to the National Cancer Institute Common Toxicity Criteria 3.0.
Statistical analysis
We analyzed the statistics with SPSS 17.0 software (SPSS, Chicago, IL, USA). All analyses were two-sided with a 5% significance level. The primary object was to assess the OS and PFS between the two groups in this analysis. For the OS and PFS analysis, we used Kaplan–Meier curves and the log-rank test for comparisons. RR and DCR were compared between the two groups with the χ2 test. A Cox proportional hazard analysis was done to calculate hazard ratios (95% confidence interval [CI]). Covariates included sex, histology, stage, ECOG performance status, weight loss, and smoking status.
Results
Patient characteristics
Between January 2010 and January 2013, a total of 136 patients were enrolled in the research. The likelihood of patients aged 80 years and older being treated with single-agent chemotherapy compared with patients treated by EGFR TKIs is presented here. The median age was 82 (range 80–92) years, with 30.9% being female. Baseline characteristics were well balanced between the two 80-and-older groups from this study except histology (Table 1). A total of 78 cases of the total 136 patients received single-agent chemotherapy in group A, with gemcitabine or single pemetrexed or docetaxel regimens administered in 38 (50.0%), 26 (34.2%), and 12 (15.8%) patients as first-line treatment, respectively. The general course was for four to six cycles. Patients with EGFR mutations in group B received gefitinib (76.7%) or erlotinib (23.3%) as first-line therapy, administered until progressive disease.
  | Table 1 Patient characteristics |
Efficacy
All 136 patients with advanced NSCLC were able to be evaluated for efficacy. Responses to treatment for patients aged 80 years and over are displayed in Table 2. RR was similar in group A and group B (RR 28.9% versus 36.7%, P=0.399). Disease control was obtained in 44 (57.8%) versus 46 (76.7%) of patients in group A and group B (P=0.022), respectively. Patients who received EGFR TKIs had significantly improved DCR (P=0.022). There were no differences in RR or DCR when gemcitabine, pemetrexed and docetaxel treatment were compared (RR 26.3% versus 30.8%, 33.3%, P=0.868; DCR 63.2% versus 61.5%, 50%, P=0.713, respectively), and similar results were obtained when erlotinib and gefitinib were compared in subgroup analysis (RR 33.3% versus 44.4%, P=0.413; DCR 76.2% versus 77.8%, P=0.894; respectively). In a subgroup analysis, patients with adenocarcinoma in the two groups had no differences in RR (P=0.965) or DCR (P=0.313).
Survival
Median PFS was 2 months (95% CI 1.49–2.515 months) with chemotherapy versus 4 months (95% CI 4.3–9.5 months) with EGFR TKIs (P=0.013). When we compared OS between the two groups, this was 8 months (95% CI 7.10–8.89 months) in group A and 16 months (95% CI 13.17–18.83 months) in group B (P=0.025). The 1- and 2-year survival rates of the two groups were 23.7% in group A versus 76.7% in group B (P<0.001), and 13.2% in group A versus 10% in group B (P=0.605), respectively. In subgroup analysis, patients with adenocarcinoma in group B had a higher 1-year survival rate compared with group A (29.4% in group A versus 66.7% in group B, P=0.001); however, the 2 year survival rate showed no significant differences (11.7% in group A versus 8.3% in group B, P=0.89). We used Cox multivariate regression analysis to determine factors influencing survival. The results showed that ECOG performance status was an independent prognostic factor (Table 3). The PFS OS curves of the two groups are shown in Figures 1 and 2, respectively. The efficacy of the first-line treatment is shown in Table 4.
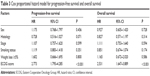  | Table 3 Cox proportional hazard model for progression-free survival and overall survival |
  | Figure 1 Progression-free survival (PFS) in first-line treatment. |
  | Figure 2 Overall survival (OS) in first-line treatment. |
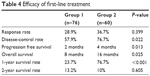  | Table 4 Efficacy of first-line treatment |
Safety
In general, patients were well tolerated in the two groups. The most common adverse events were hematology toxicity (61 [80%] of 76 patients at grade 1–2) and rash (20 [47%] of 60 patients at grade 1–2) in group A and group B, respectively. Grade 3/4 hematologic toxicity and rash were documented in eight (10%) versus two (3%) patients and 0 versus 3 (5%) patients in group A and group B, respectively. One patient in group A had grade 4 hematologic toxicity. Detailed adverse effects are displayed in Table 5.
  | Table 5 Common adverse events in the safety population, n (%) |
Discussion
There has been increasing interest in the treatment of elderly patients aged 80 years and older with advanced NSCLC. However, the clinical data regarding this group of patients have not been fully available, because such patients are not usually included in retrospective analysis and clinical trials, and there is still no standard treatment for patients aged 80 years and older. The present study evaluated the efficacy, survival, and feasibility of first-line EGFR TKIs and single-agent chemotherapy treatment for elderly patients aged 80 years and older. It is of great significance to show that EGFR-TKIs can prolong survival and improve quality of life.
Patients treated with EGFR TKIs had higher DCR, longer PFS and OS, and milder side effects than patients who were treated with single-agent chemotherapy in this study. Molecularly targeted therapy changed the specific characteristics of the tumor cells, but showed more potent antitumor activity and reduced the side effects on normal cells. About 40%–85% of lung cancer tissue specimens in patients with NSCLC were detected in EGFR expression or high expression, and lesions in patients with high expression of EGFR progress more quickly and not sensitive to chemotherapy or radiotherapy as well as show poor prognosis.12 At present, gefitinib and erlotinib are the most common EGFR TKIs and are widely used in the treatment of patients with advanced NSCLC with mild adverse effects, and can significantly improve the quality of life. Considering that a large portion of elderly patients aged 80 years and older with advanced NSCLC often refuse chemotherapy,13 molecularly targeted drugs have become a new choice for this group of patients. A number of findings have shown benefit for EGFR TKIs in both Asian and European populations.14–16 Recent data suggest that patients with EGFR mutations should use erlotinib as first-line systemic therapy before first-line chemotherapy.17,18 The OPTIMAL trial demonstrated that erlotinib as first-line treatment in patients harboring EGFR mutations with advanced NSCLC can significantly prolong PFS regardless of age, sex, ECOG status, stage, histological type, or smoking status, and quality of life is better than those receiving chemotherapy.19 In our study, elderly patients in the targeted therapy group achieved median PFS and OS of 4 and 16 months, respectively, which were longer when compared with the chemotherapy group (PFS 4 versus 2 months, P=0.013; OS 16 versus 8 months, P=0.025). The 1-year survival rate was higher (23.7% versus 76.7%, P<0.001) in group B, but something interesting was that the 2-year survival rate showed no significant difference (13.2% versus 10%, P=0.605), perhaps because of insufficient cases. The number of patients with adenocarcinoma in the targeted group was larger than in the chemotherapy group. Because the enrolled cases were so few, the compared groups could not be completely randomized or controlled. Another reason is that EGFR TKIs are suitable for patients with adenocarcinoma who harbor EGFR mutations. A subgroup analysis showed that patients with adenocarcinoma in the two groups showed no differences in RR (P=0.965) or DCR (P=0.313). Patients had a higher 1-year survival rate in group B compared with group A (29.4% in group A versus 66.7% in group B, P=0.001); however, the 2-year survival rate showed no significant difference (11.7% in group A versus 8.3% in group B, P=0.89). Therefore, patients aged 80 years and older with adenocarcinoma benefit from EGFR-TKI than chemotherapy. In group B, the main adverse effect was rash and mostly were grade I–II. Three patients (5%) got a grade III rash which patients were well tolerated.
In most patients aged 70–79 years with advanced NSCLC, single-agent chemotherapy is the standard treatment,20 but data on patients aged 80 years and over were not mentioned. The joint SWOG0027 and LUN6 trials studied elderly patients aged 80 years and older with advanced NSCLC receiving chemotherapy, and the results showed encouraging disease efficiency (RR 53%) and the treatment was well tolerated.21 In our study, the RR (28.9%) in the chemotherapy group was lower than in the trial, and the OS of 8 months with single-agent chemotherapy also shorter than data previously reported for patients aged below 80 years with advanced NSCLC.22 Maybe the reason for single-agent chemotherapy reduced the curative effects,23 and for the loss of organ reserve function made poor tolerance to chemotherapy. Although the results of chemotherapy-treated elderly patients with advanced NSCLC have been unsatisfactory, chemotherapy can prolong survival and improve quality of life when compared with best supportive care in previous research.24 The adverse reactions of patients in group A were akin to reported data of patients aged below 80 years,25 and the application of single-agent chemotherapy reduced toxicity of drugs probably. Patients treated with single-agent chemotherapy showed good tolerance.
In this study, we noted differences in OS, PFS, and DCR between the two groups. Multifactorial analysis revealed that performance status was strongly related to survival, and this is consistent with the results of previous research.26 We recommend that elderly patients with ECOG status ≤2 receive medical treatment. The study results reinforce the feasibility of upfront genotyping of patients and the improved results attained with therapy directed against a known target. By comparing the data of the two groups, we propose that for patients aged 80 years and older diagnosed with NSCLC, the feasibility of application of TKIs should be considered first. Then, gene detection is suggested for this group of patients. We suggest that patients with activating EGFR mutations should receive EGFR-TKIs. Quality of life can be obviously improved with EGFR-TKIs due to the mild side-effects. EGFR-TKI is more suitable than chemotherapy for patients aged 80 years and older with NSCLC who harboring EGFR mutations. For patients with wild-type EGFR with good ECOG status (<2), age should not become an obstacle to receiving chemotherapy. In order to avoid toxicities and disease control for this group of patients, we suggest the application of single-agent chemotherapy starting with a small dose, and for doses to be altered according to specific circumstances.
Disclosure
The authors report no conflicts of interest in this work.
References
Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with tg4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011;12:1125–1133. | ||
Le Péchoux C. Role of postoperative radiotherapy in resected non-small cell lung cancer: a reassessment based on new data. Oncologist. 2011;16:672–681. | ||
Ihde DC. Chemotherapy of lung cancer. N Engl J Med. 1992;327:1434–1441. | ||
Frusch N, Bosquee L, Louis R. lung cancer epidemiology and etiologic factors. Rev Med Liege. 2007;62(9):548–553. | ||
Weiss J, Stinchcombe TE. Treatment of elderly patients with stage IV non-small-cell lung cancer. Expert Rev Anticancer Ther. 2012;12:111–120. | ||
Gridelli C, Aapro M, Ardizzoni A, et al. Treatment of advanced non-small-cell lung cancer in the elderly: results of an international expert panel. J Clin Oncol. 2005;23:3125–3137. | ||
Gridelli C, Maione P, Rossi A, Palazzolo G, Colantuoni G, Rossi E. Management of unfit older patients with advanced NSCLC. Cancer Treat Rev. 2009;35:517–521. | ||
Maemondo M, Minegishi Y, Inoue A, et al. First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J Thorac Oncol. 2012;7:1417–1422. | ||
Altundag O, Stewart DJ, Fossella FV, et al. Many patients 80 years and older with advanced non-small cell lung cancer (NSCLC) can tolerate chemotherapy. J Thorac Oncol. 2007;2:141–146. | ||
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. | ||
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. | ||
Haber DA, Bell DW, Sordella R, et al. Molecular targeted therapy of lung cancer: EGFR mutations and response to EGFR inhibitors. Cold Spring Harb Symp Quant Biol. 2005;70:419–426. | ||
Wang S, Wong ML, Hamilton N, Davoren JB, Jahan TM, Walter LC. Impact of age and comorbidity on non-small-cell lung cancer treatment in older veterans. J Clin Oncol. 2012;30:1447–1455. | ||
Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. | ||
Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. | ||
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. | ||
Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. | ||
Zhou C, Wu YL, Chen G, et al. Updated efficacy and quality-of-life (QoL) analyses in OPTIMAL, a phase III, randomized, open-label study of first-line erlotinib versus gemcitabine/carboplatin in patients with EGFR-activating mutation-positive (EGFR Act Mut+) advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2011;29 Suppl 15:7520. | ||
Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. | ||
Socinski MA, Crowell R, Hensing TE, et al. Treatment of non-small cell lung cancer, stage IV: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:277S–289S. | ||
Hesketh PJ, Lilenbaum RC, Chansky K, et al. Chemotherapy in patients ≥80 with advanced non-small cell lung cancer: combined results from SWOG 0027 and LUN 6. J Thorac Oncol. 2007;2:494–498. | ||
Semrau S, Zettl H, Hildebrandt G, Klautke G, Fietkau R. Older patients with inoperable non-small cell lung cancer: long-term survival after concurrent chemoradiotherapy. Strahlenther Onkol. 2014;190:1125–1132. | ||
Qi WX, Tang LN, He AN, Shen Z, Lin F, Yao Y. Doublet versus single cytotoxic agent as first-line treatment for elderly patients with advanced non-small-cell lung cancer: a systematic review and meta-analysis. Lung. 2012;190:477–485. | ||
Goto Y, Sekine I, Yamada K, et al. Influence of previous chemotherapy on the efficacy of subsequent docetaxel therapy in advanced non-small-cell lung cancer patients. J Thorac Oncol. 2008;3:412–416. | ||
Guckenberger M, Kavanagh A, Partridge M. Combining advanced radiotherapy technologies to maximize safety and tumor control probability in stage III non-small cell lung cancer. Strahlenther Onkol. 2012;188:894–900. | ||
Breen D, Barlesi F, Zemerli M, et al. Results and impact of routine assessment of comorbidity in elderly patients with non-small-cell lung cancer aged >80 years. Clin Lung Cancer. 2007;8:331–334. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

