Back to Journals » Medical Devices: Evidence and Research » Volume 13
Comparison of Silicone- and Porous-Plate Ahmed Glaucoma Valves
Authors Roa TM, Netland PA , Costa VP, Sarkisian Jr SR, Al-Aswad LA , Moster MR, Ahmed IIK
Received 16 April 2020
Accepted for publication 22 June 2020
Published 16 July 2020 Volume 2020:13 Pages 213—221
DOI https://doi.org/10.2147/MDER.S258498
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Tina M Roa,1 Peter A Netland,1 Vital P Costa,2 Steven R Sarkisian Jr,3 Lama A Al-Aswad,4 Marlene R Moster,5 Iqbal IK Ahmed6
1Department of Ophthalmology, University of Virginia School of Medicine, Charlottesville, VA 22908, USA; 2Department of Ophthalmology, University of Campinas, Campinas, Brazil; 3Oklahoma Eye Surgeons, PLLC, Oklahoma City, OK 73112, USA; 4Department of Ophthalmology, NYU Grossman School of Medicine, NYU Langone Health, New York, NY 10017, USA; 5Glaucoma Service, Wills Eye Hospital, Philadelphia, PA 19107, USA; 6Department of Ophthalmology and Vision Sciences, University of Toronto, Toronto, ON, Canada
Correspondence: Peter A Netland
University of Virginia School of Medicine, PO Box 800715, Charlottesville, VA 22908, USA
Tel +1 434 982-1086
Email [email protected]
Purpose: Our aim was to evaluate and compare the clinical outcomes after implantation of the silicone-plate (model FP7) and porous polyethylene-plate (model M4) Ahmed Glaucoma Valves.
Patients and Methods: This was a prospective, multicenter, comparative series. A total of 52 eyes (52 patients) were treated with either the silicone or porous plate Ahmed Glaucoma Valve implant. Hypertensive phase was defined as intraocular pressure > 21 mmHg during the first 3 months postoperatively. Success was defined as 5 mmHg ≤intraocular pressure ≤ 21 mmHg (with or without additional glaucoma medications), without loss of light perception and without additional glaucoma procedures. Patients were monitored for 1 year after surgery.
Results: The pre-operative intraocular pressure was 29.9 ± 6.6 mmHg and 33.8 ± 10.5 in the silicone-plate and porous-plate groups, respectively (P = 0.118). At 12 months after surgery, the mean intraocular pressure was 13.6 ± 4.7 mmHg in the silicone-plate group and 17.9 ± 10.9 mmHg in the porous-plate group (P = 0.141). The mean number of glaucoma medications at 12 months was 1.64 ± 1.40 mmHg and 1.89 ± 1.54 mmHg in the silicone- and porous-plate groups, respectively (P = 0.605). Hypertensive phase was not significantly different in the two groups (50.0% of the silicone-plate and 57.7% of the porous-plate groups, P = 0.578). At 12 months after surgery, the percent success for the silicone-plate and porous-plate groups was 88.5% and 53.8%, respectively (P = 0.005). Complications were similar in the two groups.
Conclusion: The porous-plate Ahmed Glaucoma Valve showed similar average intraocular pressure reduction compared with the silicone-plate model. At 12 months after surgery, there was a significantly lower success rate in the porous-plate compared with the silicone-plate group.
Keywords: Ahmed Glaucoma Valve, model FP7, model M4, glaucoma drainage implant, porous polyethylene, Medpor
Introduction
Glaucoma drainage implants lower intraocular pressure by draining aqueous through a long silicone tube to a plate implanted posterior to the limbus. The Ahmed Glaucoma Valve (New World Medical Inc., Rancho Cucamonga, CA) is a flow-restrictive glaucoma drainage device, which has a valve-like mechanism that reduces hypotony and associated complications in the early postoperative period.1 The utilization of glaucoma drainage implants, including the Ahmed Glaucoma Valve, has dramatically grown in recent years, increasing by 410% during the period from 1994 to 2012.2 Variables related to the plate have been found to influence clinical outcomes with glaucoma drainage implants,3 including surface area and plate material.4,5
The M4 model of the Ahmed Glaucoma Valve has a plate made of porous, high-density polyethylene (Medpor; Porex, Atlanta, GA; subsequently Stryker Corp., Kalamazoo, MI).6 The Ahmed Glaucoma Valve model M4 plate surface area is 191 mm2 (revised surface area after the initial release of the implant, personal communication, New World Medical Inc., Rancho Cucamonga, CA), which is approximately the same surface area as the standard silicone-plate model FP7 (184 mm2). The theoretical surface area of the model M4 is higher because of the porous nature of the material. The average pore size is greater than 100 μm and pore volume is in the 50% range, with pore interconnections that allow the potential for tissue ingrowth. The M4 model plate was developed to allow tissue integration, which could increase surface area for absorption of aqueous and reduce encapsulation of the device.
In animal models, the Ahmed Glaucoma Valve with a surrounding porous membrane of expanded polytetrafluoroethylene (ePTFE) improved the hydraulic conductivity of the capsule around the implant.7,8 In pre-clinical animal studies, the Ahmed Glaucoma Valve model M4 was associated with thinner and more vascular capsules with reduced outflow resistance compared with the Ahmed Glaucoma Valve model S2.9 Retrospective clinical studies of the Ahmed Glaucoma Valve model M4 have shown variable results.10–14 Although the model M4 porous-plate implant is approved by the Food and Drug Administration (FDA) for implantation in humans with intractable glaucoma, the production of the model M4 was voluntarily discontinued by the manufacturing company. Results regarding this implant are of interest for future development of glaucoma drainage implants. In this prospective, randomized, controlled study, we evaluated and compared the clinical outcomes after implantation of the Ahmed Glaucoma Valve model FP7 (silicone plate) and model M4 (porous polyethylene plate).
Patients and Methods
This was a multicenter, prospective, randomized, controlled trial that enrolled patients from six sites, with one surgeon per site (P.A.N., V.P.C., L.A.A., I.I.K.A., S.R.S. and M.R.M.). The study was approved by the University of Virginia Institutional Review Board (IRB) and conformed to the requirements of the Declaration of Helsinki and the United States Health Insurance Portability and Privacy Act (HIPPA). The study was approved by the institutional review board for each site. All patients signed the informed consent before the screening. The trial was registered with http://clinicaltrials.gov (identifier NCT01883856), with the study start date listed as the date of IRB approval at the sponsoring institution (University of Virginia) and the submission (registration) date listed after completion of IRB approval and site training completion of the other sites in this multicenter study.
Inclusion criteria were patients between ≥18 years and ≤80 years of age who were diagnosed with intractable glaucoma which has not responded to conventional medical and surgical therapy, mean elevated intraocular pressure of >21 mmHg in two consecutive measurements using Goldmann applanation tonometry as baseline pressure, candidates for glaucoma drainage device surgery, and signed informed consent. Exclusion criteria precluded patients diagnosed with secondary glaucoma from silicone oil tamponade, prior drainage implant surgery, and history of cyclophotocoagulation. Pregnant and imprisoned patients were also excluded.
Baseline data and examinations collected were demographic information, glaucoma diagnosis, medical and ocular history, systemic medications, ocular medications, manifest refraction, Snellen visual acuity, mean intraocular pressure of two consecutive measurements by Goldmann applanation tonometry, slit-lamp examination, gonioscopy, fundus exam, 30–2 or 24–2 SITA Standard Humphrey Visual Field, and negative urine or serum pregnancy test for women of child-bearing potential. If both eyes of one patient qualified, the study eye was identified as the eye that had the highest baseline intraocular pressure or worse baseline visual acuity. Surgery was scheduled within 60 days of screening date. The patients were randomized 1:1 between the M4 and FP7 groups, with a computer-generated randomization scheme using varying block sizes of 2 to 4. The subject surgical intervention arm assignment was placed in a sealed envelope, securely delivered to sites, and opened by the site study staff in the surgical admission suite immediately before administering anesthesia to the patient. Post-randomization, the assigned surgical intervention arm was unmasked to both patient and surgeon.
Implantation of both models followed the guidelines as described previously.6,15 After adequate anesthesia, a fornix-based incision was made through the conjunctiva and Tenon’s capsule. A pocket was formed in the quadrant between the rectus muscles by blunt dissection of Tenon’s capsule from the episclera. The implant was examined and primed by injecting a balanced salt solution through the drainage tube and valve using a cannula. The primed implant was inserted into the pocket between the rectus muscles and sutured to the sclera. The leading edge was measured at approximately 8 mm from the limbus. The drainage tube was trimmed and inserted through a 23-gauge needle track into the anterior chamber at the limbus. The drainage tube was covered with donor sclera, cornea, or pericardium. The conjunctiva was closed. Minor variations of the surgical technique were at the discretion of the surgeon.
Postoperatively, patients were treated with topical corticosteroids and antibiotics, tapered over approximately six weeks. Scheduled follow-up visits were at 1 day, 1 month, 3 months, 6 months, 9 months, and 12 months postoperatively. Unscheduled visits were made according to the assessment of the surgeon. Data collected in the postoperative visits were visual acuity, intraocular pressure, slit lamp exam, gonioscopy (at 3, 6, 9, and 12 months only), fundus exam, adverse events and complications, and systemic and ocular medications. Glaucoma medication was added as necessary to achieve target intraocular pressure.
Surgical success was defined as 5 mmHg ≤ intraocular pressure ≤21 mmHg (with or without glaucoma medications), with no loss of light perception and no additional glaucoma procedures. An additional analysis of success based on percent lowering of intraocular pressure was performed, with success defined as 30% lowering of intraocular pressure from baseline (with or without medications), without loss of light perception and without additional glaucoma procedures. Bleb needling was not considered as an additional glaucoma procedure. Hypertensive phase was defined as intraocular pressure more than 21 mmHg during the first three months postoperatively. Primary endpoints of the study were the mean intraocular pressure and surgical success of the M4 group compared with the FP7 group at 12 months postoperatively. Secondary endpoints were comparisons of the mean glaucoma medications and complications in the M4 and FP7 groups.
The sample size was calculated with a power of 80% to detect a 1.5 mmHg in intraocular pressure with a standard deviation of 3 mmHg. Demographic and pre-operative data were compared with Chi-square test for homogeneity or Fisher’s exact test, and independent-samples t-test or Mann Whitney U. Statistical significance of the differences of mean intraocular pressure and mean number of medications were determined using independent t-test. Kaplan-Meier survival analysis and log-rank test (Mantel-Cox) were performed to compare the cumulative probability of survival between the two groups. Proportions were compared using the comparison of proportions test (z test). Statistical analyses were done using SPSS version 25 (SPSS Inc., Chicago, IL).
Data sharing statement: deidentified participant data and the study protocol may be requested from the corresponding author up to 36 months following article publication. Proposals for use of deidentified data require approval by both an independent review committee at the University of Virginia and the University of Virginia IRB.
Results
A total of 52 eyes from 52 patients were enrolled in the study, including 26 treated with the model M4 and 26 treated with the model FP7 Ahmed Glaucoma Valve. The demographic and preoperative data (Table 1) showed no significant difference in population distribution with respect to surgical eye laterality, gender, age, ethnicity, lens status, glaucoma diagnosis and previous glaucoma surgery (P>0.05). Eight patients were screen failures due to failure to meet inclusion and exclusion criteria. The number of patients analyzed was 52 at day 1, 48 at month 6, and 41 at month 12, due to removal of failures.
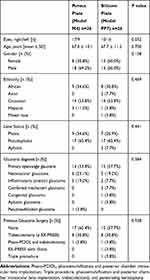 |
Table 1 Demographic and Preoperative Data for Patients Treated with the Porous Plate (Model M4) and Silicone Plate (Model FP7) Ahmed Glaucoma Valves |
Intraoperatively, tube placement was in the anterior chamber in 25 (96.2%) and 24 (92.3%), in the sulcus in 1 (3.8%) and 1 (3.8%), and in the pars plana in 0 (0%) and 1 (3.8%) in eyes treated with the model M4 and model FP7 plates, respectively (P = 1.0). The patch graft material used to cover the tube was cornea in 10 (38.5%) and 8 (30.8%), sclera in 10 (38.5%) and 11 (42.3%), pericardium in 4 (15.4%) and 5 (19.2%), and none (scleral tunnel) in 2 (7.7%) and 2 (7.7%) in eyes treated with the model M4 and model FP7 plates, respectively (P = 0.948).
Figure 1 shows the mean intraocular pressure at all time points for both groups. Preoperative mean intraocular pressure was similar at 33.79 ± 10.5 mmHg and 29.91 ± 6.6 mmHg for the M4 and FP7 groups, respectively (p = 0.118). Both the M4 and FP7 groups had significant reductions in mean intraocular pressure at all time points during the study (P > 0.05 for all time points), including 12 months postoperatively (P < 0.0001 for both the M4 and FP7 groups). Although the intraocular pressure was lower during the early postoperative period and higher from months 3 to 12, the differences in mean intraocular pressure between groups were not statistically significant at any time point. A hypertensive phase occurred in 15 (57.7%) and 13 (50.0%) of the M4 group and the FP7 group, respectively (P = 0.578, Table 2).
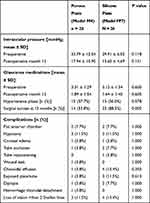 |
Table 2 Comparison of Efficacy and Complications in Patients Treated with the Porous Plate (Model M4) and Silicone Plate (Model FP7) Ahmed Glaucoma Valves |
The mean number of medications was reduced in both the M4 group and the FP7 group at all time points after surgery (P < 0.05). At 12 months after surgery, the mean number of medications was significantly lower in both groups (P = 0.009 in the M4 group and P < 0.0001 in the FP7 group). As shown in Figure 2, the reduction of mean number of medications was similar in both groups at all time points. There was no statistically significant difference in the mean number of glaucoma medications between the two groups at any time points.
The cumulative probability of success (Figure 3) was significantly lower after implantation of the model M4 compared with the Model FP7 Ahmed Glaucoma Valve (P = 0.005, log-rank test). In this analysis, success was defined as 5 mmHg ≤ intraocular pressure ≤ 21 mmHg (with or without glaucoma medications), with no loss of light perception and no additional glaucoma procedures. The reasons for failure were intraocular pressure > 21 mmHg in 6 (23.1%) and 1 (3.8%), intraocular pressure < 5 mmHg in 2 (7.7%) and 0 (0%), additional glaucoma surgery in 3 (11.5%) and 2 (7.7%), and loss of light perception vision in 1 (3.8%) and 0 (0%) after implantation of the model M4 and model FP7 Ahmed Glaucoma Valve, respectively. The majority of the failures in the model M4 group were due to intraocular pressure >21 mmHg. Glaucoma diagnosis of failures included primary open-angle glaucoma, neovascular glaucoma, uveitic glaucoma, and pseudoexfoliation glaucoma, which showed no significant differences between the model M4 and the FP7 groups (P = 1.00). Prior surgery in failures included trabeculectomy and cataract surgery, which showed no significant differences between groups (P = 0.604).
The baseline intraocular pressure was not statistically significantly different comparing the M4 and FP7 groups (33.8 ± 10.5 mmHg and 29.9 ± 6.6 mmHg, respectively, P = 0.118). We evaluated the two groups for surgical success using an alternative definition for success, based on percent reduction of intraocular pressure from baseline. In this analysis, success was defined as 30% lowering of intraocular pressure from baseline (with or without medications), without loss of light perception and without additional glaucoma procedures (Figure 4). Kaplan–Meier curve cumulative probability of success was performed in patients treated with model M4 and model FP7 Ahmed Glaucoma Valve implantation. Using this definition of success, the surgical success of treatment with model FP7 was significantly higher compared with model M4 implantation (P = 0.014), which was similar to the finding using the definition of success using 5 mmHg ≤ intraocular pressure ≤21 mmHg (with or without glaucoma medications), with no loss of light perception and no additional glaucoma procedures.
As shown in Table 2, there were no significant differences of postoperative complications in the Ahmed Glaucoma Valve model M4 and FP7 groups. Four patients developed shallow to flat anterior chambers that required reformation of the anterior chamber on postoperative day 7. One patient from the FP7 group with flat anterior chamber and lens-cornea touch developed lens opacification and underwent cataract extraction 8 months after Ahmed Glaucoma Valve implantation. Four patients developed exposed tube or plate and were treated with conjunctivoplasty and patch graft. A patient from the M4 group developed hemorrhagic choroidal detachment on postoperative day 7, and was treated with drainage of choroidals and pars plana vitrectomy one month after Ahmed Glaucoma Valve implantation, with recovery of preoperative vision by 6 months. The most common complication for both groups was loss of more than 2 Snellen lines of vision that was observed in 3 patients from the M4 group and 4 patients from the FP7 group.
Discussion
This study compared the efficacy and safety of the porous polyethylene model M4 with the silicone model FP7 Ahmed Glaucoma Valves. In experimental studies, different glaucoma drainage implant plate materials may influence capsule formation and intraocular pressure control after glaucoma drainage implants.16,17 In clinical studies, different glaucoma drainage implant plate materials have been associated with significant differences of mean intraocular pressure in some5,18,19 but not all20,21 studies. In this randomized, prospective clinical trial, we compared the clinical outcomes after implantation of the porous polyethylene plate (model M4) with the silicone plate (model FP7) Ahmed Glaucoma Valves. We found differences in surgical success after Ahmed Glaucoma Valve implantation, with significantly lower success in the porous plate (M4) group compared with the silicone plate (FP7) group, with the majority of failures in the M4 group due to intraocular pressure >21 mmHg.
In our study, we found differences in surgical success using two Ahmed Glaucoma Valve plates that differed in the materials used for their construction. Our results found significantly lower surgical success after implantation of the porous plate device compared with the silicone plate Ahmed Glaucoma Valve. Nine of twelve failures (75%) in the porous plate (model M4) group occurred due to intraocular pressure >21 or additional glaucoma surgery required for increased intraocular pressure. We also found a trend towards increased intraocular pressure after the early postoperative period in the model M4 group compared with the model FP7 group. Our surgical failures showed no significant differences in glaucoma diagnosis or previous surgical history. A previous retrospective comparative study showed a trends toward increasing intraocular pressure and increasing failure rate after implantation of model M4 (porous plate), S2 (polypropylene), and FP7 (silicone), which were not statistically significantly different.10 Another retrospective non-comparative study showed a trend toward higher intraocular pressure after model M4 compared with model S2 implantation, but a survival analysis of surgical success was not performed.13 In previous non-comparative retrospective studies of the model M4 implant, the relatively high failure rates were consistent with those found in our study.12,14
While we did not directly measure the capsule around the plate, the hypertensive phase and surgical success analyses provided clinical outcomes related to encapsulation around the plate. In previous retrospective studies, mitigated or no hypertensive phase was observed after model M4 Ahmed Glaucoma Valve implantation.10,14 Different variables may influence fibrous capsule formation and the hypertensive phase after glaucoma drainage implant surgery, including hydrostatic pressure and chemical mediators in the aqueous humor that may influence the fibrotic response.22,23 Aqueous suppression during the early postoperative period may reduce the hypertensive phase after Ahmed Glaucoma Valve implantation.24 In our randomized, prospective comparative study, hypertensive phase was not significantly different after implantation of the porous plate (model M4) or the silicone plate (model FP7) Ahmed Glaucoma Valves.
Integration of fibrovascular tissue into the porous plate could lead to differences in bleb morphology and clinical outcome measures. Differences in bleb morphology have been observed after implantation of the model M4 Ahmed Glaucoma Valve, with a low profile bleb presumably due to less aqueous in the cyst around the plate.10,12 Direct examination of the capsule during surgical explantation of 4 model M4 Ahmed glaucoma valves has been described,11 indicating that integration of fibrovascular tissue does occur. Variables that may influence tissue integration in the porous polyethylene plate are poorly understood, but may include time after surgery, pore size of the material, increased capsule-plate contact with aqueous suppression or flow restriction, and other variables. Cases of exposure of the model M4 posterior tube and the anterior corner of the plate have been described.11 In our study, we did not observe any significant differences in complications after model M4 and model FP7 Ahmed Glaucoma Valve implantation.
The failure rate may have been higher in the M4 group compared with the FP7 group for several reasons. Cvintal et al suggested that adhesions between the M4 plate and Tenon’s capsule may prevent the formation of a diffuse filtration bleb, and inadequate tissue integration in the porous material may form a thick-walled bleb with higher outflow resistance and increased intraocular pressure.12 It is possible that less surface area was available for absorption of aqueous than anticipated with the porous material. Also, the plates are manufactured using polyethylene (model M4) and silicone (model FP7), which may differ in their biocompatibility, rigidity, inflammatory response, and other variables that may influence implant surgical outcomes. Use of differing materials has been associated with different clinical outcomes in previous studies of glaucoma drainage implants.5,16–19
A strength of our study is the randomized, prospective, comparative design. However, this study has limitations that may have influenced the results. Physicians were not masked to the treatment group during the postoperative period, the number of patients was relatively small, and the follow-up period was limited. Other differences between the plates, including the higher profile and the absence of fenestration holes in the M4 implant, could account for the differences we observed. Despite these limitations, we were able to demonstrate a significantly lower success rate after model M4 implantation, with the majority of failures in this group due to intraocular pressure >21 mmHg and additional glaucoma surgery required for increased intraocular pressure.
Conclusions
Intraocular pressure reduction was similar after implantation of the model M4 and model FP7 Ahmed Glaucoma Valves, with a trend toward increasing intraocular pressure after treatment with the model M4. The success rate was significantly lower after implantation of the porous polyethylene plate (model M4) compared with the silicone plate (model FP7). The majority of the failures in the model M4 group were due to increased intraocular pressure. Complications after surgery were similar in the two groups. Production of the model M4 was voluntarily discontinued by the manufacturer, although the device is approved by the Food and Drug Administration (FDA). Results from this study indicate that glaucoma drainage implant plate material may influence clinical outcomes.
Acknowledgments
Ahmed Glaucoma Devices were provided by New World Medical (Rancho Cucamonga, CA) for study implantation. There was no financial grant support provided for this investigator-initiated study. No company wrote or influenced the wording of the manuscript. Clinical trial registration (http://clinicaltrials.gov) identifier NCT01883856, with the study start date listed as the date of IRB approval at the sponsoring institution (University of Virginia) and the submission (registration) date listed after completion of IRB approval and site training completion of the other sites in this multicenter study.
Disclosure
Dr. Roa and Dr. Netland have no proprietary interest or conflict of interest related to the devices described in this study. Dr Costa received personal fees from Alcon and Iridex; grants, personal fees from Allergan, outside the submitted work. Dr. Sarkisian received grant support from and/or was a consultant/advisor for Alcon, Allergan, Bausch & Lomb, Beaver-Visitec International, Inc., Katena Products, Inc, Ocular Science, Omeros, Santen, Inc., Sight Sciences, and Glaukos; consulting fees from New World Medical, Alcon, Sight Sciences, and Glaukos; speaker honoraria from Alcon; and has equity ownership in Sight Sciences and Ocular Science, unrelated to this work. Dr Al-Aswad reports grants from New World Medical, during the conduct of the study; grants from Topcon, owns shares from Globechek, and personal fees from Aries and Topcon, outside the submitted work. Dr. Ahmed received consulting fees from Alcon, Allergan, New World Medical, and Glaukos; and speaker honoraria from Alcon, Allergan, and New World Medical. Dr. Moster received consulting fees from Alcon, Allergan, Bausch and Lomb, Glaukos, Aerie, and Qura; and speaker honoraria from Alcon, Allergan, Aerie, and Bausch and Lomb. The authors report no other conflicts of interest in this work.
References
1. Ashburn FS, Netland PA. The evolution of glaucoma drainage implants. J Ophthalmic Vis Res. 2018;13:498–500. doi:10.4103/jovr.jovr_26_18
2. Arora KS, Robin AL, Corcoran KJ, Corcoran SL, Ramulu PY. Use of various glaucoma surgeries and procedures in Medicare beneficiaries from 1994 to 2012. Ophthalmology. 2015;122(8):1615–1624. doi:10.1016/j.ophtha.2015.04.015
3. Netland PA. The Ahmed glaucoma valve in neovascular glaucoma (An AOS Thesis).. Trans Am Ophthalmol Soc. 2009;107:325–342.
4. Heuer DK, Lloyd MA, Abrams DA, et al. Which is better? One or two? A randomized clinical trial of single-plate versus double-plate Molteno implantation for glaucomas in aphakia and pseudophakia. Ophthalmology. 1992;99(10):1512–1519. doi:10.1016/S0161-6420(92)31772-5
5. Ishida K, Netland PA, Costa VP, Shiroma L, Khan B, Ahmed IIK. Comparison of polypropylene and silicone Ahmed glaucoma valves. Ophthalmology. 2006;113(8):1320–1326. doi:10.1016/j.ophtha.2006.04.020
6. Netland PA. Ahmed Glaucoma valve model M4. In: Samples JR, Ahmed IIK, editors. Surgical Innovations in Glaucoma. New York, NY: Springer; 2014:223–225.
7. DeCroos FC, Ahmad S, Kondo Y, et al. Expanded polytetrafluoroethylene membrane alters tissue response to implanted Ahmed glaucoma valve. Curr Eye Res. 2009;34(7):562–567. doi:10.1080/02713680902963167
8. DeCroos FC, Kondo Y, Mordes D, et al. In vitro fluid dynamics of the Ahmed glaucoma valve modified with expanded polytetrafluoroethylene. Curr Eye Res. 2011;36(2):112–117. doi:10.3109/02713683.2010.512115
9. Dozier C, Allingham RR, Asrani S, et al. Quantifying outflow resistance of a modified glaucoma valve in vivo. Invest Ophthalmol Vis Sci. 2006;47:E–abstract 32.
10. Kim J, Allingham RR, Hall J, Klitzman B, Stinnett S, Asrani S. Clinical experience with a novel glaucoma drainage implant. J Glaucoma. 2014;23:e91–e97. doi:10.1097/IJG.0b013e3182955d73
11. Hu WD, Pro MJ, Fudemberg SJ, Moster MR. Explantation of the novel Ahmed glaucoma valve M4 implant. J Glaucoma. 2015;24(2):e1–e4. doi:10.1097/IJG.0000000000000192
12. Cvintal V, Moster MR, Shyu AP, et al. Initial experience with the new Ahmed glaucoma valve model M4: short term results. J Glaucoma. 2016;25(5):e475–e480. doi:10.1097/IJG.0000000000000324
13. Gil-Carrasco F, Jiménez-Román J, Turati-Acosta M, Bello-López Portillo H, Isida-Llerandi CG. Comparative study of the safety and efficacy of the Ahmed glaucoma valve model M4 (high density porous polyethylene) and the model S2 (polypropylene) in patients with neovascular glaucoma. Arch Soc Esp Oftalmol. 2016;91:409–414. doi:10.1016/j.oftal.2016.02.009
14. Sluch I, Gudgel B, Dvorak J, et al. Clinical Experience with the M4 Ahmed glaucoma drainage implant. J Curr Glaucoma Pract. 2017;11(3):92–96. doi:10.5005/jp-journals-10028-1231
15. Boyle JWIV, JR M, PA N. Surgical technique 3 (Ahmed glaucoma valve drainage implant). In: Shaarawy TM, Sherwood MB, Hitchings RA, Crowston JG, editors. Glaucoma.
16. Ayyala RS, Harman LE, Michelini-Norris B, et al. Comparison of different biomaterials for glaucoma drainage devices. Arch Ophthalmol. 1999;117:233–236. doi:10.1001/archopht.117.2.233
17. Ayyala RS, Michelini-Norris B, Flores A, Haller E, Margo CE. Comparison of different biomaterials for glaucoma drainage devices: part 2. Arch Ophthalmol. 2000;118:1081–1084. doi:10.1001/archopht.118.8.1081
18. Hinkle DM, Zurakowski D, Ayyala RS. A Comparison of the Polypropylene Plate Ahmed ™ Glaucoma Valve to the Silicone Plate Ahmed ™ Glaucoma Flexible Valve. Eur J Ophthalmol. 2007;17:696–701. doi:10.1177/112067210701700502
19. Mackenzie PJ, Schertzer RM, Isbister CM. Comparison of silicone and polypropylene Ahmed glaucoma valves: two-year follow-up. Can J Ophthalmol. 2007;42(2):227–232. doi:10.3129/canjophthalmol
20. Law SK, Nguyen A, Coleman AL, Caprioli J. Comparison of safety and efficacy between silicone and polypropylene Ahmed glaucoma valves in refractory glaucoma. Ophthalmology. 2005;112(9):1514–1520. doi:10.1016/j.ophtha.2005.04.012
21. Brasil MVOM, Rockwood EJ, Smith SD. Comparison of silicone and polypropylene Ahmed glaucoma valve implants. J Glaucoma. 2007;16(1):36–41. doi:10.1097/01.ijg.0000243477.82779.31
22. Molteno ACB, Fucik M, Dempster AG, Bevin TH. Otago Glaucoma surgery outcome study: factors controlling capsule fibrosis around Molteno implants with histopathological correlation. Ophthalmology. 2003;110(11):2198–2206. doi:10.1016/S0161-6420(03)00803-0
23. McCluskey P, Molteno A, Wakefield D, Di Girolamo N. Otago Glaucoma surgery outcome study: the pattern of expression of MMPs and TIMPs in Bleb capsules surrounding Molteno implants. Invest Ophthalmol Vis Sci. 2009;50(5):2161–2164. doi:10.1167/iovs.08-2063
24. Law SK, Kornmann HL, Giaconi JA, Kwong A, Tran E, Caprioli J. Early aqueous suppressant therapy on hypertensive phase following glaucoma drainage device procedure: a randomized prospective trial. J Glaucoma. 2016;25(3):248–257. doi:10.1097/IJG.0000000000000131
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




