Back to Journals » Drug Design, Development and Therapy » Volume 17
Comparison of Remimazolam Tosilate and Etomidate on Hemodynamics in Cardiac Surgery: A Randomised Controlled Trial
Authors Hu B , Zhang M, Wu Z, Zhang X, Zou X, Tan L, Song T, Li X
Received 19 December 2022
Accepted for publication 2 February 2023
Published 8 February 2023 Volume 2023:17 Pages 381—388
DOI https://doi.org/10.2147/DDDT.S401969
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Bailong Hu,1,2,* Mei Zhang,2,* Zhen Wu,2,* Xiaoyuan Zhang,2,* Xiaohua Zou,1,2 Li Tan,1 Tao Song,1 Xingyu Li1
1Department of Anesthesiology, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou Province, People’s Republic of China; 2College of Anesthesiology, Guizhou Medical University, Guiyang, Guizhou Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaohua Zou, Email [email protected]
Background: Remimazolam tosilate (RT) is a new ultrashort-acting γ-aminobutyric acid subtype A (GABAA) agonist, with the characteristics of rapid onset and offset, minimal cardiorespiratory depression. Currently, few studies have compared the effect of RT and etomidate on hemodynamics during anesthesia induction. Here, we aimed to compare the hemodynamic effects of different doses of RT and etomidate for anesthesia induction in patients undergoing cardiac surgeries.
Methods: Patients were recruited from January to September 2022 in this single-center, prospective, randomized, double-blind trial. A total of 117 patients undergoing selective valve replacement surgery were randomly divided into low-dose RT (0.2 mg/kg) group (group LR), high-dose RT (0.3 mg/kg) group (group HR), or etomidate (1.5 mg/kg) group (group E), respectively. The primary outcome was hemodynamic fluctuations (mean arterial pressure fluctuation value [∆MAP]; heart rate fluctuation value [∆HR]) during anesthesia induction. Secondary outcomes included the incidence of adverse drug reactions (injection pain and myoclonus) and adverse cardiovascular events, vital signs at different time points and the cumulative doses of vasoactive drugs.
Results: The hemodynamic fluctuations (∆MAP) in group LR and group E were significantly lower than that in group HR. In addition, the incidence of hypotension and the cumulative norepinephrine doses in group E and group LR were also significantly lower than that in group HR. Furthermore, the incidence of injection pain and myoclonus in group LR and group HR were less frequently recorded compared with group E. There were no significant differences in terms of ∆HR, tachycardia, hypertension, severe bradycardia, vital signs at different time points, lactic acid and blood glucose between both groups.
Conclusion: Compared with etomidate, low-dose RT (0.2mg/kg) can not only provide stable hemodynamic parameters but also cause fewer adverse reactions when used for anesthesia induction in patients with cardiac disease.
Keywords: remimazolam tosilate, etomidate, hemodynamics, cardiac surgery
Introduction
Patients with cardiovascular disease are more likely to experience hypotension during induction of general anesthesia due to their poor cardiovascular function reserve. The superior hemodynamic properties of etomidate have made it a preferred sedative for the induction of general anesthesia in critically ill patients.1–3 However, etomidate may result in serious side-effects, namely adrenal cortex function depression,4,5 myoclonus,6,7 and pain on injection,8,9 which limited its widespread use in clinical practice. Choosing an effective and safe intravenous anesthetic for general anesthesia is still one of the key issues for anesthesiologists.
Remimazolam tosilate (RT), a new ultra-short acting benzodiazepine agent, has the characteristics of fast onset and metabolism, slight inhibition on respiratory and cardiovascular systems.10,11 Growing studies have demonstrated that RT could be safely and effectively used for digestive endoscopy, bronchoscopy, induction and maintenance of general anesthesia, and even for high-risk patients.12–15 At present, there are no clinical studies comparing the RT and etomidate on hemodynamics during anesthesia induction in patients undergoing cardiac surgery. The purpose of this study is to investigate the effects of different doses of RT and etomidate on hemodynamics in patients undergoing cardiac valve replacement during anesthesia induction.
Methods
Study Design
The study was a single-center, prospective, randomized, double-blinded, controlled trial to evaluate the effect of different doses of RT and etomidate on hemodynamics change during anesthesia induction in patients underwent valve replacement surgery. The study has been approved by the Chinese Ethics Committee of Registering Clinical Trials (Ref: ChiECRCT20210524) and registered in the Chinese Clinical Trial Registry (ChiCTR2100052535). The protocol was published in Trials.16 The study was also conducted according to the principles of the Declaration of Helsinki, and written informed consent was obtained from all patients before the enrollment. The flowchart detailing the study design is illustrated in Figure 1.
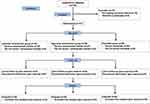 |
Figure 1 Consort flow diagram of the trial design. |
Patients aged 18–65 years, with an American Society of Anesthesiologists (ASA) grade I–III, who were scheduled for elective valve replacement surgery from January to November 2022 were recruited in this study. Exclusion criteria included the following: patients with (1) severe cardiovascular instability (acute heart failure, myocardial infarction, etc); (2) uncontrolled or poorly controlled hypertension; (3) a history of renal or hepatic dysfunction; (4) myasthenia gravis or mental disorders disease; (5) known allergic or contraindicated to benzodiazepines, opioids and etomidate; (6) a history of alcohol abuse or addicted to opioids or benzodiazepines; and (7) expected difficult airway.
All selected patients were randomly divided into the low-dose RT group (group LR), high-dose RT group (group HR) or etomidate group (group E) using a computer-generated random number with a 1:1:1 allocation ratio prior to the induction. Opaque envelopes were made according to the randomization sequence and opened by a nurse who was not involved in the study. Before anesthesia induction, a nurse who was not involved in this study prepared the required experimental drugs in equal volumes into identical syringes, and then covered all the syringes with masking tape to conceal the information of the experimental drugs. In addition, the surgeons, nurses, patients, anesthesiologists and outcome observers were also unaware of the group assignment throughout the study.
Interventions
All patients were routinely fasted 8h before surgery. Standard monitoring including electrocardiogram (ECG), peripheral oxygen saturation (SPO2), invasive arterial blood pressure (IBP), noninvasive blood pressure (NIBP) and bispectral index (BIS) were routinely performed after the patient’s arrival to the operating room. Ringer lactate (10 mL/kg) was carried out via a peripheral 20-gauge intravenous catheter. After preoxygenation, patients in group LR, group HR and group E were induced with an initial dose of RT (0.2 mg/kg), RT (0.3 mg/kg) or etomidate (0.3 mg/kg), respectively. Then, 0.5 µg/kg of sufentanil and 0.6 mg/kg of rocuronium were injected when BIS value was below 60. Tracheal intubation was performed after meeting the condition. Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP) and heart rate (HR) were recorded at baseline (T0), 1 min after the induction of anesthesia (T1), 3 min after the induction of anesthesia (T2), immediately at tracheal intubation (T3), 1 min after tracheal intubation (T4), 3 min after tracheal intubation (T5), and 5 min after tracheal intubation (T6).
Hypotension was defined as a decrease less than 20% from baseline in MAP or a fall below 60 mmHg lasting at least 1min, 50 µg norepinephrine or more was given until the MAP returned to normal range once hypotension occurred. Severe bradycardia was considered as HR decline below 45 beats per min, and 0.25 mg atropine was given and could be added repeatedly according to patient’s HR. When MAP rose above 120 mmHg, appropriate nitroglycerin was given, and once HR rose above 120 bpm, appropriate esmolol was administered. Adverse cardiovascular events (hypotension, severe bradycardia, etc), adverse drug reactions (injection pain and myoclonus), the cumulative doses of vasoactive drugs, blood glucose values as well as vital signs at different time points were recorded.
Outcome Assessment
The primary outcomes was the hemodynamic fluctuations during anesthesia induction (∆MAP, the difference between maximum or minimum MAP and baseline; ∆HR, the difference between maximum or minimum HR and baseline). Secondary outcomes include the incidence of adverse drug reactions (injection pain and myoclonus) and adverse cardiovascular events, vital signs at different time points, the cumulative doses of vasoactive drugs, lactic acid and glucose values.
Statistical Analysis
We calculated the sample size based on the ∆MAP. In our pilot study, the ∆MAP (mean ± SD) in group E, group LR and group HR were 13.6 ± 7.3 mmHg, 15.5 ± 7.5 mmHg and 20.8 ± 8.1 mmHg, respectively. Based on our pilot data and relevant literature,17 assuming a αlevel of 0.05 and a power of 0.90, we estimated that 31 patients per group would be needed. As a result, we finally included 39 patients in each group, taking into consideration the 20% dropout rate.
Analysis of the data was performed using SPSS 22.0 software (SPSS Inc, Chicago, IL, USA). The Shapiro–Wilk test was used to determine the normality of the distribution. Data with normal distribution (age, height, weight, BMI, ∆MAP, ∆HR, blood glucose and lactic acid values) were presented as mean±standard deviation and compared by one-way analysis of variance (ANOVA). Vital signs at each time point were analyzed by repeated-measures ANOVA. Post hoc tests were performed using a Bonferroni method. Data with abnormal distribution (norepinephrine use) were presented as median (25th and 75th percentile) and compared using Kruskal–Wallis test. Categorical data (gender, ASA grade, surgical procedure, incidence of adverse reactions) were expressed as n (%) and compared using a Fisher’s exact test or Chi square test. A p value <0.05 was considered statistically significant.
Results
A total of 135 patients were recruited for study participation. Among them, four patients refused to participate; five patients had expected difficult airways and nine patients had hemodynamic instability. As a result, 117 patients were finally analyzed, as shown in Figure 1. Patients’ demographics and baseline characteristics are summarized in Table 1. There was no significant difference between three groups in terms of age, BMI, sex, ASA classification, procedure time (P > 0.05; Table 1).
 |
Table 1 Demographic and Baseline Characteristics of Patients |
The hemodynamic fluctuations (∆MAP) in group LR and group E were significantly lower than that in group HR (19.67±8.9, 18.45±7.83 and 24.85±10.12 mmHg, respectively; P<0.05, Table 2). In addition, the incidence of hypotension and the cumulative norepinephrine doses in group LR and group E were also significantly lower than that in group HR. Furthermore, the incidence of injection pain and myoclonus in group LR and group HR were less frequently recorded compared with group E (P<0.05; Table 3). There were no significant differences in terms of ∆HR, tachycardia, hypertension, severe bradycardia, vital signs at different time points and blood lactic acid and glucose between both groups (Table 4 and Figure 2).
 |
Table 2 Hemodynamic Fluctuations Between the Three Groups |
 |
Table 3 Comparison of Adverse Reactions Between the Three Groups |
 |
Table 4 Comparison of Blood Lactic Acid and Glucose Between the Three Groups |
 |
Figure 2 Vital signs at different time points between the three groups. (A) MAP at different time points between the three groups; (B) HR at different time points between the three groups. |
Discussion
We compared the effects of different doses of RT and etomidate on hemodynamics in patients who underwent valve replacement surgery in the present study. The results showed that anesthesia induction with RT (0.2 mg/kg) in patients with heart disease existed excellent characteristics of stable haemodynamic and fewer adverse reactions compared with etomidate.
Patients with cardiac disease are associated with a higher risk of hypotension during anesthesia induction due to their impaired cardiovascular function reserve.18 How to maintain the stability of hemodynamics during anesthesia induction is one of the important issues that anesthesiologists have been concerned about for a long time. Etomidate is commonly used for induction of anesthesia in patients with hemodynamic instability owing to its low risk of hypotension.19,20 However, adrenocortical function inhibition is one of the most important adverse effects of etomidate, which limits its clinical application to some extent.19,21,22
RT, a new ultra-short-acting type of GABAA receptor agonist, has been widely used in procedural sedation and general anesthesia due to its promising properties, including a rapid onset, short recovery time and stable hemodynamics.23,24 Our results showed that the hemodynamic fluctuation (∆MAP) in group LR and group E were significantly lower than that in group HR, while the ∆HR between the three groups were noted no significant difference. Although the average difference of ∆MAP between the three groups are only a few mmHg (19.67 vs 18.45 vs 24.85 mmHg, respectively), these minor hemodynamic changes may affect the balance between myocardial oxygen supply and demand, especially for patients with potential myocardial ischemia risk. Besides, the margin of 3–5 mmHg is often considered as the minimal clinically important difference value for blood pressure.25,26 Therefore, minimizing hemodynamic changes during anesthesia induction has been one of the important issues that anesthesiologists pay attention to. In addition, the incidence of hypotension and the cumulative use of vasoactive drugs in group LR and group E were also significantly reduced compared with group HR. Hypotension is a common side effect after general anesthesia induction, and even a slight drop in blood pressure could increase the risk of organ damage, especially in patients with cardiac disease.27,28 Our results indicated that reduction of MAP fluctuation was closely related to the reduction of hypotension. The more stable the MAP fluctuation during the anesthesia induction period, the more beneficial it is for patients with cardiac disease.
Tang et al demonstrated that remimazolam could reduce hemodynamic fluctuations by influencing the stress response and enhancing myocardial contractility.29 Similarly, Qiu et al revealed that remimazolam could reduce hypotension might be related to its less effect on cardiac output and systemic vascular resistance.30 Our study results suggest that anesthesia induction with low-dose RT (0.2mg/kg) has potential advantages in patients undergoing cardiac surgery, which its hemodynamic effect is comparable to that of etomidate. Liu et al showed that the effect of RT (0.3mg/kg) on hemodynamic fluctuation was significantly smaller than that of propofol in patients who underwent cardiac surgery.17 In another study, Zhang et al demonstrated that the influence of RT (0.2–0.4mg/kg) on circulation was also smaller than that of propofol in elderly patients who underwent hip surgery.31 Chae et al recommended that optimal ED95 doses of RT for induction in patients aged <40, 60–80, and >80 years were 0.25–0.33, 0.19–0.25, and 0.14–0.19 mg/kg, respectively.32 Nevertheless, considering the cardiac function of patients, we suggest that the induction with 0.2mg/kg of RT is relatively safe for patients with cardiac disease according to our results. However, further studies are needed to determine the optimal dose of RT for the less cardiovascular depressant and side effect in cardiac surgery.
Myoclonus is one of the common adverse reactions of etomidate, which can result in serious consequence in patients with poor cardiovascular reserve.33,34 In our study, the incidence of myoclonus in group E was nearly 30%, which was similar to the rate reported by Liu et al.7 On the contrary, myoclonus occurred in only two people in either the low-dose RT group or the high-dose RT group. Additionally, the severity of myoclonus were also reduced in the group LR and group HR than that in the etomidate group. These results suggest that RT may have potential advantages in avoiding such adverse reactions. Although the exact mechanism of myoclonus induced by etomidate is still unclear, it is reported that the mechanism of etomidate myoclonus may be related to the inhibition of GABA neurons, which makes the pathway associated with skeletal muscle more sensitive.35,36 Although RT also acts on the central GABA receptor, the incidence of myoclonus caused by RT was rare, and the specific mechanism may need to clarify in future.
Moreover, similar to previous studies,37,38 injection pain was frequently recorded in the etomidate group. Injection pain is another major adverse reaction of etomidate, with an incidence from 4% to 80%.39 In our study, the incidence of injection pain in the etomidate group was 14.3%, while the incidence of injection pain in group LR and group HR were both reduced. As the structure of RT is different from the phenol structure of propofol, RT may have less stimulation on blood vessels.
However, there are still several limitations in our study. First, this was a single-center study with a relatively small sample size, larger sample size studies are needed to determine the effect of RT on hemodynamics in the future. Second, we did not monitor other cardiac functional indicators such as cardiac output, vascular resistance, stroke volume, etc, so it could not well explain the effect and mechanism of RT on hemodynamics. Third, patients with severe cardiovascular diseases were excluded in this study, so the conclusion cannot be applied to more elderly or fragile patients. Further clinical trials are needed to address the above issues.
Conclusions
Compared with etomidate, low-dose RT (0.2mg/kg) can not only provide stable hemodynamic parameters but also have fewer adverse reactions when used for anesthesia induction in patients with cardiac disease. RT may be a suitable alternative sedative agent for such patients due to its non-inferior efficacy and higher safety profile.
Abbreviations
MAP, mean arterial pressure; HR, heart rate; GABAA, γ-aminobutyric acid subtype A; ASA, American Society of Anesthesiologists; NIBP, noninvasive blood pressure; SPO2, peripheral oxygen saturation; BIS, bispectral index; IBP, invasive arterial blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; CPB, cardiopulmonary bypass.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the privacy policy but are available from the corresponding authors on reasonable requests.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported in part by the Natural Science Foundation of China (No. 82160951, 82160224), Fund of Yunyan District Science and Technology department (yunkehezi[2021]10), Found of University Student Innovation and Entrepreneurship Training Program of Guizhou Provincial (S202210660085), the Science and Technology Fund of Guizhou Provincial Health Department (qiankehepingtairencai[2018]5779-52), the Fund of Guizhou Provincial Natural Science Foundation (qiankehejichu[2020]1Y298), the Cultivate project 2021 for National Natural Science Foundation of China, Affiliated Hospital of Guizhou Medical University (gyfynsfc-2021-35), the Fund of Guiyang Science and Technology department([2019]9-1-24), the Health and Family Planning Commission of Guizhou Province (gzwkj2021-273), the Fund of Guizhou Provincial Education Department (qianjiaoheKYzi[2021]182).
Disclosure
Bailong Hu, Mei Zhang, Zhen Wu and Xiaoyuan Zhang are co-first authors for this study. The authors declare that they have no competing interests for this work.
References
1. Song JC, Lu ZJ, Jiao YF, et al. Etomidate anesthesia during ERCP caused more stable haemodynamic responses compared with propofol: a randomized clinical trial. Int J Med Sci. 2015;12:559–565. doi:10.7150/ijms.11521
2. Yao YT, He LX, Fang NX, Ma J. Anesthetic induction with etomidate in cardiac surgical patients: a prisma-compliant systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2021;35:1073–1085. doi:10.1053/j.jvca.2020.11.068
3. Hannam JA, Mitchell SJ, Cumin D, et al. Haemodynamic profiles of etomidate vs propofol for induction of anaesthesia: a randomised controlled trial in patients undergoing cardiac surgery. Br J Anaesth. 2019;122:198–205. doi:10.1016/j.bja.2018.09.027
4. Albert SG, Ariyan S, Rather A. The effect of etomidate on adrenal function in critical illness: a systematic review. Intensive Care Med. 2011;37:901–910. doi:10.1007/s00134-011-2160-1
5. Kaushal RP, Vatal A, Pathak R. Effect of etomidate and propofol induction on hemodynamic and endocrine response in patients undergoing coronary artery bypass grafting/mitral valve and aortic valve replacement surgery on cardiopulmonary bypass. Ann Card Anaesth. 2015;18:172–178. doi:10.4103/0971-9784.154470
6. Miao S, Zou L, Wang G, Wang X, Liu S, Shi M. Effect of dexmedetomidine on etomidate-induced myoclonus: a randomized, double-blind controlled trial. Drug Des Devel Ther. 2019;13:1803–1808. doi:10.2147/DDDT.S194456
7. Liu J, Liu R, Meng C, et al. Propofol decreases etomidate-related myoclonus in gastroscopy. Medicine. 2017;96:e7212. doi:10.1097/MD.0000000000007212
8. Chen L, Liang X, Tan X, Wen H, Jiang J, Li Y. Safety and efficacy of combined use of propofol and etomidate for sedation during gastroscopy: systematic review and meta-analysis. Medicine. 2019;98:e15712. doi:10.1097/MD.0000000000015712
9. Komatsu R, You J, Mascha EJ, Sessler DI, Kasuya Y, Turan A. Anesthetic induction with etomidate, rather than propofol, is associated with increased 30-day mortality and cardiovascular morbidity after noncardiac surgery. Anesth Analg. 2013;117:1329–1337. doi:10.1213/ANE.0b013e318299a516
10. Chen SH, Yuan TM, Zhang J, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, Phase III trial. J Gastroenterol Hepatol. 2021;36:474–481. doi:10.1111/jgh.15188
11. Liu X, Ding B, Shi F, et al. The efficacy and safety of remimazolam tosilate versus etomidate-propofol in elderly outpatients undergoing colonoscopy: a prospective, randomized, single-blind, non-inferiority trial. Drug Des Devel Ther. 2021;15:4675–4685. doi:10.2147/DDDT.S339535
12. Guo J, Qian Y, Zhang X, Han S, Shi Q, Xu J. Remimazolam tosilate compared with propofol for gastrointestinal endoscopy in elderly patients: a prospective, randomized and controlled study. BMC Anesthesiol. 2022;22:180. doi:10.1186/s12871-022-01713-6
13. Cao Y, Chi P, Zhou C, Lv W, Quan Z, Xue FS. remimazolam tosilate sedation with adjuvant sufentanil in Chinese patients with liver cirrhosis undergoing gastroscopy: a randomized controlled study. Med Sci Monit. 2022;28:e936580. doi:10.12659/MSM.936580
14. Tan Y, Ouyang W, Tang Y, Fang N, Fang C, Quan C. Effect of remimazolam tosilate on early cognitive function in elderly patients undergoing upper gastrointestinal endoscopy. J Gastroenterol Hepatol. 2022;37:576–583. doi:10.1111/jgh.15761
15. Zhao TY, Chen D, Sun H, et al. Moderate sedation with single-dose remimazolam tosilate in elderly male patients undergoing transurethral resection of the prostate with spinal anesthesia: a prospective, single-arm, single-centre clinical trial. BMC Anesthesiol. 2022;22:247. doi:10.1186/s12871-022-01788-1
16. Hu B, Zhou H, Zou X, et al. Effect of remimazolam tosilate versus etomidate on hemodynamics in patients undergoing valve replacement surgery: study protocol for a randomized controlled trial. Trials. 2022;23(1):992. doi:10.1186/s13063-022-06962-x
17. Liu T, Lai T, Chen J, et al. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: a randomized, double-blind, controlled trial. Pharmacol Res Perspect. 2021;9:e00851. doi:10.1002/prp2.851
18. Baradari AG, Alipour A, Habibi MR, Rashidaei S, Emami ZA. A randomized clinical trial comparing hemodynamic responses to ketamine-propofol combination (ketofol) versus etomidate during anesthesia induction in patients with left ventricular dysfunction undergoing coronary artery bypass graft surgery. Arch Med Sci. 2017;13:1102–1110. doi:10.5114/aoms.2016.63193
19. Matchett G, Gasanova I, Riccio CA, et al. Etomidate versus ketamine for emergency endotracheal intubation: a randomized clinical trial. Intensive Care Med. 2022;48:78–91. doi:10.1007/s00134-021-06577-x
20. Zgola K, Kulakowski P, Czepiel A, et al. Haemodynamic effects of etomidate, propofol and electrical shock in patients undergoing implantable cardioverter-defibrillator testing. Kardiol Pol. 2014;72:707–715. doi:10.5603/KP.a2014.0086
21. Valk BI, Struys M. Etomidate and its analogs: a review of pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2021;60:1253–1269. doi:10.1007/s40262-021-01038-6
22. Gu WJ, Wang F, Tang L, Liu JC. Single-dose etomidate does not increase mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled trials and observational studies. Chest. 2015;147:335–346. doi:10.1378/chest.14-1012
23. Chen X, Xin D, Xu G, Zhao J, Lv Q. The efficacy and safety of remimazolam tosilate versus dexmedetomidine in outpatients undergoing flexible bronchoscopy: a prospective, randomized, blind, non-inferiority trial. Front Pharmacol. 2022;13:902065. doi:10.3389/fphar.2022.902065
24. Liu M, Sun Y, Zhou L, Feng K, Wang T, Feng X. The median effective dose and bispectral index of remimazolam tosilate for anesthesia induction in elderly patients: an up-and-down sequential allocation trial. Clin Interv Aging. 2022;17:837–843. doi:10.2147/CIA.S364222
25. FDA. Guidance for Industry. Non-inferiority clinical trials; 2010. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf.
26. Gosho M. Non-inferiority margins employed in clinical trials in Japan. J Clin Pharm Ther. 2015;40:289–298. doi:10.1111/jcpt.12268
27. Wesselink EM, Kappen TH, Torn HM, Slooter A, van Klei WA. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121:706–721. doi:10.1016/j.bja.2018.04.036
28. Sessler DI, Bloomstone JA, Aronson S, et al. Perioperative quality initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:563–574. doi:10.1016/j.bja.2019.01.013
29. Tang F, Yi JM, Gong HY, et al. Remimazolam benzenesulfonate anesthesia effectiveness in cardiac surgery patients under general anesthesia. World J Clin Cases. 2021;9:10595–10603. doi:10.12998/wjcc.v9.i34.10595
30. Qiu Y, Gu W, Zhao M, Zhang Y, Wu J. The hemodynamic stability of remimazolam compared with propofol in patients undergoing endoscopic submucosal dissection: a randomized trial. Front Med. 2022;9:938940. doi:10.3389/fmed.2022.938940
31. Zhang J, Wang X, Zhang Q, Wang Z, Zhu S. Application effects of remimazolam and propofol on elderly patients undergoing Hip replacement. BMC Anesthesiol. 2022;22:118. doi:10.1186/s12871-022-01641-5
32. Chae D, Kim HC, Song Y, Choi YS, Han DW. Pharmacodynamic analysis of intravenous bolus remimazolam for loss of consciousness in patients undergoing general anaesthesia: a randomised, prospective, double-blind study. Br J Anaesth. 2022;129:49–57. doi:10.1016/j.bja.2022.02.040
33. Hueter L, Schwarzkopf K, Simon M, Bredle D, Fritz H. Pretreatment with sufentanil reduces myoclonus after etomidate. Acta Anaesthesiol Scand. 2003;47:482–484. doi:10.1034/j.1399-6576.2003.00081.x
34. Zhou C, Zhu Y, Liu Z, Ruan L. Effect of pretreatment with midazolam on etomidate-induced myoclonus: a meta-analysis. J Int Med Res. 2017;45:399–406. doi:10.1177/0300060516682882
35. Gancher S, Laxer KD, Krieger W. Activation of epileptogenic activity by etomidate. Anesthesiology. 1984;61:616–618. doi:10.1097/00000542-198411000-00029
36. Wang W, Lv J, Wang Q, Yang L, Yu W. Oxycodone for prevention of etomidate-induced myoclonus: a randomized double-blind controlled trial. J Int Med Res. 2018;46:1839–1845. doi:10.1177/0300060518761788
37. Nyman Y, Von Hofsten K, Palm C, Eksborg S, Lonnqvist PA. Etomidate-Lipuro is associated with considerably less injection pain in children compared with propofol with added lidocaine. Br J Anaesth. 2006;97:536–539. doi:10.1093/bja/ael187
38. Coghill JC. Pain on injection with droperidol and prevention of pain with etomidate. Anaesthesia. 1986;41:214–215. doi:10.1111/j.1365-2044.1986.tb13191.x
39. Brown PM, Moss E. Reduction of pain on injection of etomidate. Anaesthesia. 1981;36:814–816. doi:10.1111/j.1365-2044.1981.tb08823.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
